Abstract
The activation of hepatic stellate cells (HSCs) is a cue to initiate liver fibrosis. Activated stellate cells acquire contractile activity similar to pericytes and myofibroblasts in other organs by inducing the contractile machinery of cytoskeletons such as smooth muscle α-actin (α-SMA), a well-known marker of activated stellate cells, and actin-binding proteins. We further show herein the expression of tropomyosin in rat HSCs in the course of their activation during primary culture and liver tissue damaged by thioacetamide intoxication. In immunoblot analysis, tropomyosin became detectable in an early stage of the primary culture of rat stellate cells in a manner similar to the expression of α-SMA and platelet-derived growth factor receptor-β. Tropomyosin was found to be colocalized with α-SMA on fluorescent immunocytochemistry. At the liver tissue level, an increased expression of tropomyosin was observed by immunoblot analysis and immunohistochemistry along the septum of fibrosis, where α-SMA was enriched. These results strongly suggest that tropomyosin is a new marker of activated stellate cells and may serve as a useful diagnostic marker of liver fibrosis.
Keywords: Actin, Cell contraction, Vitamin A, Liver injury, Liver sinusoid
Introduction
Hepatic stellate cells (HSCs) play a key role in liver fibrogenesis regardless of the pathogenesis [1–4]. In response to local tissue damage and hepatocyte necrosis, HSCs undergo activation characterized by the proliferation, migration, contraction, secretion of several profibrogenic mediators such as cytokines, growth factors, chemokines, and tissue inhibitors of matrix metalloproteinases, and generation of extracellular matrix (ECM) materials such as type I collagen. HSC activation thus contributes to scar formation in chronically injured liver tissue.
One of the indicators of activated HSCs is smooth muscle α-actin (α-SMA), the actin isoform typical of smooth muscle cell differentiation [5–10]. Similar to the expression of γ-actin, α-SMA was first demonstrated in primary-cultured rat HSCs in the course of the culture-dependent HSC activation process [5]. α-SMA-positive HSCs are also seen along the fibrotic septum of chronically damaged livers of rodent models, in which ECM proteins such as type I collagen and fibronectin are dominantly produced and deposited [11, 12]. Furthermore, in the human liver, the augmented expression of α-SMA has been documented in and around the area of hepatocyte damage [13, 14].
Tropomyosin is one of the actin-associated proteins, present in virtually all eukaryotic cells, and modulates the interaction between actin and myosin to stabilize the actin-filament structure. Tropomyosin assembles into an α-helical coiled heterodimer composed of an α-chain and a β-chain, with each molecule interacting with six or seven monomers of actin [15, 16]. Tropomyosin regulates the contractility of striated muscle by blocking myosin-binding sites on actin in the relaxed state. On activation, tropomyosin moves away from these sites [17]. X-ray studies have suggested that the initiation of smooth muscle contraction leads to the movement of tropomyosin in a manner similar to that in striated muscle [18]. Although one recent study has shown the expression and organization of actin filaments and tropomyosin in the cloned hepatic GRX cell line [19], tropomyosin expression patterns in primary cultured HSCs and the fibrotic liver remain to be studied.
This study aimed to demonstrate the presence of tropomyosin in HSCs and clarify the dynamics of its expression pattern during HSC activation. Herein, we report in detail the expression pattern of tropomyosin in cultured rat HSCs and fibrotic liver tissue induced in rats by thioacetamide (TAA) administration, and further demonstrate the network of α-SMA filaments and tropomyosin in primary HSC cultures.
Materials and methods
Materials
Collagenase was purchased from Wako Pure Chemical Co. (Osaka, Japan). Pronase E was obtained from Merck (Damstadt, Germany). Mouse monoclonal IgG2a antibody against α-SMA, mouse monoclonal IgG1 antibody against tropomyosin, Dulbecco’s modified Eagle’s medium (DMEM), TAA, and fetal bovine serum (FBS) were purchased from Sigma Chemical Co. (Saint Louis, MO, USA). Rabbit polyclonal IgG antibodies against human platelet-derived growth factor receptor-β (PDGFR-β) that reacts with rat PDGFR-β were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated secondary antibodies against mouse and rabbit immunoglobulins were obtained from Dako. Alexa Fluor 488 goat antimouse IgG2a antibodies and Alexa Fluor 594 goat antimouse IgG1 antibodies were from Molecular Probes (Eugene, OR, USA). ECL immunoblotting detection reagent was purchased from Amersham Pharmacia Biotech (Buckinghamshire, England). Immobilon P membranes were from Millipore Corp. (Bedford, MA, USA). Cell culture inserts were from Falcon (Beckton Dickinson, Franklin Lakes, NJ, USA). All the other reagents were purchased from Sigma Chemical Co. or Wako Pure Chemical Co.
Animals
Pathogen-free male Wistar rats (12-week-old, body weight 200–220 g) were obtained from SLC (Shizuoka, Japan). Animals were housed at a constant temperature and supplied with laboratory chow and water ad libitum. The protocol of the experiments was approved by the Animal Research Committee of Osaka City University (Guide for Animal Experiments, Osaka City University).
Induction of liver fibrosis
Liver fibrosis was induced in rats (n = 3) by the intraperitoneal injection of TAA (40 mg/body weight) dissolved in 2 ml of saline twice a week for up to 10 weeks. Control rats (n = 3) were given 2 ml of saline during the same period.
Preparation of HSCs
HSCs were isolated from male Wistar rats, as previously described in detail [20]. Isolated HSCs were suspended in DMEM supplemented with 10% FBS at a cell density of 5 × 105 cells/ml, and 1.5 ml of the cell suspension was introduced into a 35-mm cell tissue culture plate (Falon, 3003). After the culture had continued for the indicated number of days, the cells were fixed in 4% paraformaldehyde solution for immunocytochemistry or lysed for immunoblotting.
Immunoblotting
Protein samples (10 μg) were subjected to SDS-PAGE and then transferred onto Immobilon P membranes. After blocking, the membranes were treated with primary antibodies and then with peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized using the ECL system (Amersham Pharmacia Biotech) and documented by LAS 1000 (Fuji Photo Film, Kanagawa, Japan). The density of bands was analyzed using a BIO-RAD GS-700 densitometer. Experiments were repeated thrice using samples obtained from HSCs isolated from different rats.
Immunohistochemistry and collagen staining
Immunohistochemistry was performed using the methods described in detail elsewhere [21]. After the development of fibrosis, rats were anesthetized and laparotomized. The liver was perfused with phosphate-buffered saline (PBS) and then perfusion-fixed with 4% formaldehyde, dehydrated, and embedded in Polybed. Sections were cut at a thickness of 5 μm and stained for 1 h in 0.1% (w/v) Sirius red (Direct Red 80, Aldrich, Milwaukee, WI, USA) [22]. Double immunostaining analysis was carried out using methods described previously [22]. After blocking with 5% bovine serum albumin/PBS, they were incubated overnight with primary antibodies in the medium. They were then incubated with both 20 μg/ml Alexa Fluor 488 goat antimouse IgG2a antibody and 20 μg/ml Alexa Fluor 594 goat anti-mouse IgG1 antibody for 2 h. Specimens were counterstained for nuclei with DAPI. The sections were observed under an LSM510 confocal laser scanning microscope (Carl Zeiss, Germany).
Cultured HSCs on glass microscope slides were fixed with 3.7% formaldehyde for 30 min at room temperature. After washing thrice with PBS containing 0.1% Triton X-100, the fixed cells were incubated with anti-α-SMA antibody and anti-tropomyosin antibody for 1 h at room temperature and successively with FITC-labeled goat antimouse IgG1 (Alexa Fluor 488) and rhodamine-labeled goat antimouse IgG2a (Alexa Fluor 594) for 1 h at room temperature. After washing, the specimens were observed under an LSM510 confocal laser scanning microscope (Carl Zeiss, Germany). Experiments were repeated thrice using samples obtained from HSCs isolated from different rats.
Immunohistochemical analysis of human liver samples
One specimen obtained by resection during surgery from subjects with normal liver function was used as a control. Informed written consent was obtained from all patients at the time of their liver biopsy, and the study was conducted in conformance with the Helsinki Declaration. The diagnosis of liver cirrhosis was established on the basis of the clinical and histopathological features. Immunohistochemistry was performed according to the methods described above.
Results
Tropomyosin expression in primary cultured HSCs
We investigated the expression of tropomyosin in primary-cultured HSCs as a model because HSC culture precisely resembles the in vivo cellular phenotypic change of HSCs from a vitamin A-storing quiescent phenotype to an activated and myofibroblastic phenotype in response to inflammatory stimuli [23]. Freshly isolated and plated HSCs resembled lipocytes, extended branching cytoplasmic processes, and enclosed multiple droplets that contained retinol (Fig. 1Aa). After culturing for 3 days, the cells expanded their cell body with enlarged processes and nuclei, and the size of fat droplets decreased (Fig. 1Ab). By day 7, most of the cells had lost their fat droplets, spread more prominently, and appeared “myofibroblastic” (Fig. 1Ac). These observations are in good agreement with previous reports [1, 6, 21].
Fig. 1.
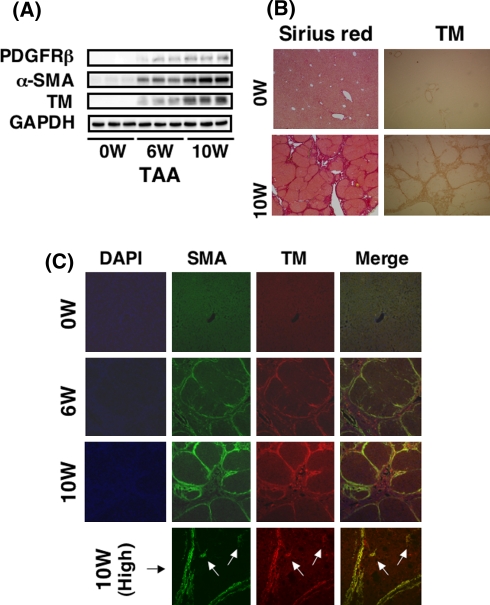
Tropomyosin expression in primary cultured HSCs. A The cell morphology of isolated and cultured HSCs was observed under phase-contrast microscopy everyday, monitored, and digitally recorded. a Day 1. HSCs attached to the plate enclose droplets that contain vitamin A. b Day 3. HSCs start extending their processes. c Day 7. HSCs showed enlarged cell body and lose their droplets (magnification, ×200). B The expression of tropomyosin in cultured HSCs was determined by immunoblot and immunocytochemistry. Whole-cell homogenates were subjected to SDS-PAGE, transferred onto the membrane, and successively immunoreacted with PDGFR-β, α-SMA, or tropomyosin. Note that tropomyosin is induced in HSCs time dependently after starting the culture in a manner similar to the expression of PDGFR-β and α-SMA. Representative data from three independent preparations are presented here. C Immunocytochemistry of tropomyosin and α-SMA. Cultured HSCs on days 1, 5, and 10 were fixed in 4% paraformaldehyde and subjected to immunocytochemistry, as described in section “Materials and methods”. Note that tropomyosin appears on day 5 and becomes prominent on day 10. Tropomyosin colocalizes and generates stress fibers with α-SMA (magnification ×200)
As shown in Fig. 1B, immunoblot analysis showed that bands for tropomyosin at molecular weights of 36–39 kDa [24] were invisible in freshly isolated HSCs, started to appear in them after being cultured for 3 days, and thereafter increased in a time-dependent manner. The relative level of tropomyosin evaluated by densitometric normalization against GAPDH gradually increased with culture prolongation (data not shown). The multiple bands detected are considered to be related to post-transcriptional modification, particularly N-terminal acetylation, of the protein, as described elsewhere [25]. Tropomyosin induction took place in a similar manner to that of α-SMA and PDGFR-β (Fig. 1B). These observations were further confirmed by immunocytochemistry. α-SMA became readily detectable on day 1 and was uniformly distributed in the cytoplasm. HSCs cultured for more than 5 days exhibited a flattened and stretched morphology with developed stress fibers, which consisted of α-SMA (Fig. 1C). Tropomyosin was negligible on day 1, but was visible and co-localized with α-SMA at day 5, and then exhibited prominent stress fibers crossing the cytoplasm together with α-SMA bundles (Fig. 1C).
Expression of tropomyosin in fibrotic livers
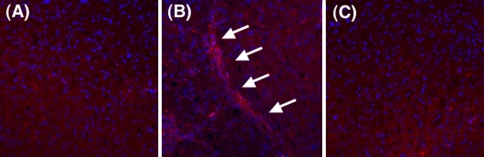
Tropomyosin induction in culture-activated HSCs prompted us to investigate its expression in liver tissue. As shown in Fig. 2A, total protein extracted from rat livers treated with TAA for 6 or 10 weeks cross-hybridized to α-SMA and tropomyosin antibodies, whereas virtually no hybridization was observed in the total extraction from an intact rat liver. This result indicates that tropomyosin is not ubiquitously expressed in liver-constituent cells such as hepatocytes, Kupffer cells, and endothelial cells. The level of tropomyosin in the liver homogenate increased in a time-dependent manner after TAA administration, similar to the induction of PDGFR-β and α-SMA. Collagen deposition was prominent in the liver after a 10-week TAA administration, as shown on Sirius red staining. Tropomyosin was found to be present along the fibrotic septum, although it was rarely seen in the intact liver (Fig. 2B). Double immunostaining of α-SMA and tropomyosin confirmed that these two proteins co-existed in and around the fibrotic septum and were hardly present in “pseudolobular” parenchyma where no fibrosis was obvious (Fig. 2C). However, activated HSCs that were present close to the septum and positive for α-SMA also expressed tropomyosin (Fig. 2C, high), indicating both activated HSCs and septum-forming myofibroblasts ubiquitously expressed tropomyosin. A strong linear-pattern expression of these proteins at the site between the septum and the parenchyma was notable. Tropomyosin expression was rarely observed in intact human liver, while it was localized along the fibrotic septum in human cirrhosis (Fig. 3).
Fig. 2.
Expression of tropomyosin in fibrotic livers. The expression of tropomyosin in the fibrotic liver was determined by immunoblot and immunohistochemistry. A Whole-liver homogenates were subjected to SDS-PAGE, transferred onto the membrane, and successively immunoreacted with PDGFR-β, α-SMA, or tropomyosin. Note that tropomyosin is induced in the liver of rats treated with TAA time dependently after starting injection in a similar manner to the expression of PDGFR-β and α-SMA. B Histology. Prominent liver fibrosis is observed in the liver of rats treated with TAA for 10 weeks by Sirius red staining. Tropomyosin expression is clear along the septa. C Fluorescent immunohistochemistry of tropomyosin and α-SMA. Double immunostaining was performed in the liver of rats treated with TAA for 6 and 10 weeks. Note that both proteins always colocalize and are expressed strongly at the site between noninjurious parenchyma and septa (magnification ×100). Activated HSCs (arrows) that were present close to the septum and positive for α-SMA also expressed tropomyosin (magnification ×400)
Fig. 3.
Expression of tropomyosin in human livers. The expression of tropomyosin in the human liver was determined by immunohistochemistry. A Intact human liver. B Cirrhosis caused by hepatitis C infection. C Negative control stained without antitropomyosin antibody (magnification ×200)
Discussion
Activated HSCs express α-SMA as contractile machinery. Although accumulated studies have evaluated the importance of α-SMA and other cytoskeletons filaments on HSC contraction, there has been no report on the expression of tropomyosin, which is one of the components of actin filaments and calcium-binding proteins and plays a major role in the contraction process of smooth muscle, in HSCs and fibrotic liver tissue. The key molecular function of tropomyosin is to shield and unshield the binding site of myosin to actin [15, 16]. Thus, tropomyosin is speculated to play a pivotal role also in the contraction of HSCs. In the present study, we report for the first time that the expression of tropomyosin is found predominantly in activated HSCs and to be as high as α-SMA. These observations suggest that tropomyosin is a regulatory protein which counteracts or triggers the contraction of HSCs due to its calcium-binding status. In fact, HSC contraction is reportedly induced by endothelin-1, angiotensin II, and thrombin, which are all intracellular calcium inducers [23].
HSCs are considered to be more contractile at pre-sinusoidal terminal portal venules in the injured liver, as indicated by the high-level expression of endothelin-1 receptors [26]. HSCs localized at this site also generate endothelin-1 and angiotensin-II, that are responsible molecules for portal hypertension, a pathological process induced by constriction of the hepatic vasculature [27, 28]. As a consequence, tropomyosin expressed at the site between the septum and the parenchyma is speculated to play a positive regulatory role in actin–myosin association and contribute to presinusoidal portal rigidity.
In conclusion, the present findings indicate that tropomyosin could be a novel marker for activated HSCs in vivo as well as in culture and be utilizable for a clinical diagnosis of liver fibrosis.
Acknowledgment
This work was supported by the Grants-in-Aid from the Japan Society for the Promotion of Science to NK.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- ECM
Extracellular matrix
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HSC
Hepatic stellate cell
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PDGFR-β
Platelet-derived growth factor receptor-β
- TAA
Thioacetamide
- α-SMA
Smooth muscle-α actin
References
- 1.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 3.Okuyama H, Shimahara Y, Kawada N. The hepatic stellate cell in the post-genomic era. Histol Histopathol. 2002;17:487–495. doi: 10.14670/HH-17.487. [DOI] [PubMed] [Google Scholar]
- 4.Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- 5.Ramadori G, Veit T, Schwögler S, Dienes HP, Knittel T, Rieder H, et al. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- 6.Kawada N, Klein H, Decker K. Eicosanoid-mediated contractility of hepatic stellate cells. Biochem J. 1992;285:367–371. doi: 10.1042/bj2850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 8.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 10.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:452–1473. doi: 10.1002/hep.1840160623. [DOI] [PubMed] [Google Scholar]
- 12.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381–1388. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaida I, Nagatomi A, Hironaka K, Uchida K, Okita K. Quantitative analysis of liver fibrosis and stellate cell changes in patients with chronic hepatitis C after interferon therapy. Am J Gastroenterol. 1999;94:489–496. doi: 10.1111/j.1572-0241.1999.884_m.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinelli AL, Ramalho LN, Zucoloto S. Hepatic stellate cells in hepatitis C patients: relationship with liver iron deposits and severity of liver disease. J Gastroenterol Hepatol. 2004;19:91–98. doi: 10.1111/j.1440-1746.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock-DeGregori SE, Varnell TA. Tropomyosin has discrete actin-binding sites with sevenfold and fourteenfold periodicities. J Mol Biol. 1990;214:885–896. doi: 10.1016/0022-2836(90)90343-K. [DOI] [PubMed] [Google Scholar]
- 16.McLachlan AD, Stewart M. The troponin binding region of tropomyosin. Evidence for a site near residues 197 to 127. J Mol Biol. 1976;106:1017–1022. doi: 10.1016/0022-2836(76)90349-1. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer SS. The regulatory switch of the muscle thin filament: Ca21 or myosin heads? J Muscle Res Cell Motil. 1994;15:232–236. doi: 10.1007/BF00123476. [DOI] [PubMed] [Google Scholar]
- 18.Vibert PJ, Haselgrove JC, Lowy J, Poulsen FR. Structural changes in actin-containing filaments of muscle. J Mol Biol. 1972;71:757–767. doi: 10.1016/S0022-2836(72)80036-6. [DOI] [PubMed] [Google Scholar]
- 19.Mermelstein CS, Guma FC, Mello TG, Fortuna VA, Guaragna RM, Costa ML, et al. Induction of the lipocyte phenotype in murine hepatic stellate cells: reorganisation of the actin cytoskeleton. Cell Tissue Res. 2001;306:75–83. doi: 10.1007/s004410100428. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, et al. Proteome analysis of rat hepatic stellate cells. Hepatology. 2000;32:268–277. doi: 10.1053/jhep.2000.9322. [DOI] [PubMed] [Google Scholar]
- 21.Nakatani K, Okuyama H, Shimahara Y, Saeki S, Kim DH, Nakajima Y, et al. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab Invest. 2004;84:91–101. doi: 10.1038/sj.labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani K, Seki S, Kawada N, Kitada T, Yamada T, Sakaguchi H, et al. Expression of SPARC by activated hepatic stellate cells and its correlation with the stages of fibrogenesis in human chronic hepatitis. Virchows Arch. 2002;441:466–474. doi: 10.1007/s00428-002-0631-z. [DOI] [PubMed] [Google Scholar]
- 23.Kawada N. The hepatic perisinusoidal stellate cell. Histol Histopathol. 1997;12:1069–1080. [PubMed] [Google Scholar]
- 24.Somara S, Bitar KN. Tropomyosin interacts with phosphorylated HSP27 in agonist-induced contraction of smooth muscle. Am J Physiol Cell Physiol. 2004;286:C1290–C1301. doi: 10.1152/ajpcell.00458.2003. [DOI] [PubMed] [Google Scholar]
- 25.Gondo K, Ueno T, Sakamoto M, Sakisaka S, Sata M, Tanikawa K. The endothelin-1 binding site in rat liver tissue: light- and electron-microscopic autoradiographic studies. Gastroenterology. 1993;104:1745–1749. doi: 10.1016/0016-5085(93)90654-u. [DOI] [PubMed] [Google Scholar]
- 26.Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233–240. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- 27.Pinzani M, Milani S, Franco R, Grappone C, Caligiuri A, Gentilini A, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 28.Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal. 2005;7:1346–1355. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]