Abstract
Liver fibrosis is a common pathway leading to cirrhosis, which is the final result of injury to the liver. Accurate assessment of the degree of fibrosis is important clinically, especially when treatments aimed at reversing fibrosis are being evolved. Liver biopsy has been considered to be the “gold standard” to assess fibrosis. However, liver biopsy being invasive and, in many instances, not favored by patients or physicians, alternative approaches to assess liver fibrosis have assumed great importance. Moreover, therapies aimed at reversing the liver fibrosis have also been tried lately with variable results. Till now, there has been no consensus on various clinical, pathological, and radiological aspects of liver fibrosis. The Asian Pacific Association for the Study of the Liver set up a working party on liver fibrosis in 2007, with a mandate to develop consensus guidelines on various aspects of liver fibrosis relevant to disease patterns and clinical practice in the Asia-Pacific region. The process for the development of these consensus guidelines involved the following: review of all available published literature by a core group of experts; proposal of consensus statements by the experts; discussion of the contentious issues; and unanimous approval of the consensus statements after discussion. The Oxford System of evidence-based approach was adopted for developing the consensus statements using the level of evidence from 1 (highest) to 5 (lowest) and grade of recommendation from A (strongest) to D (weakest). The consensus statements are presented in this review.
Keywords: Cirrhosis, Liver biopsy, Noninvasive tools, HVPG, Ultrasound
Introduction
The Asian Pacific Association for the Study of the Liver (APASL) set up a working party on liver fibrosis in 2007, with a mandate to develop consensus guidelines on various clinical, pathological, and radiological aspects of liver fibrosis relevant to disease patterns and clinical practice in the Asia-Pacific region. The process for the development of these consensus guidelines contained the following steps: (1) Review of all available published literature by a core group of experts (hepatologists, internists, immunologists, molecular biologists, radiologists, and medical statisticians), predominantly from the Asia-Pacific region. (2) Then, the experts were requested to write short reviews on specific areas covering hepatic fibrosis and propose consensus statements according to the Oxford System, which were circulated among all the experts. (3) Discussion of the consensus statements during a 1-day meeting at the APASL Single Topic Conference “Liver Fibrosis With and Without Hepatitis C or B” held from January 30 to February 1, 2008, at Cairo, Egypt. (4) Each consensus statement was then subjected to voting by all participants, and only those statements that were unanimously approved by the experts were accepted. The experts adopted the Oxford System for developing an evidence-based approach. They assessed the level of existing evidence and accordingly ranked the recommendations (i.e., level of evidence from 1 [highest] to 5 [lowest]; grade of recommendation from A [strongest] to D [weakest]).
The consensus statements are presented in this review. A brief background note has been added to explain in more detail the genesis of the consensus statements.
Liver biopsy
Liver biopsy: technique
Liver biopsy is still considered to be the procedure of choice to assess the amount of fibrosis in the tissue. It is, however, an invasive procedure and should be performed by a trained, experienced physician so that an adequate liver biopsy sample is available for the pathologist to interpret the lesions. Liver biopsy techniques over the years have undergone many improvements and changes, and it is still considered to be the gold standard, even in this era of serologic markers, better imaging techniques, and advanced molecular techniques for diagnosis and quantification of hepatitis viruses [1]. Liver biopsies are also nowadays very often indicated in a transplantation setup.
Liver biopsy is an invasive procedure with risks of morbidity and mortality, hence the patient should be counseled and informed about the usefulness of the liver biopsy, as well as the possible complications associated with it. It is important to know the contraindications to liver biopsy. The preprocedure requirements for percutaneous liver biopsy are given in Table 1.
Table 1.
Preprocedure requirements for percutaneous liver biopsy
| 1 | Indications and contraindications of percutaneous liver biopsy should be reconfirmed in the patient |
| 2 | Informed consent should be obtained |
| 3 | Nonsteroidal anti-inflammatory drugs and salicylates should be withheld 1 week prior to and after liver biopsy |
| 4 | For patients on anticoagulants, stop oral anticoagulants at least 72 h before the biopsy and start heparin and oral anticoagulants 24 h and 48–72 h, respectively, after biopsy. Patients with a prothrombin time prolonged to more than 4 s should be given 2–3 units of FFP, and for those with a platelet count of less than 60,000 cells/cumm should be given platelet-rich plasma infusions |
| 5 | In patients with chronic renal failure, biopsy should be done after dialysis and with minimum use of heparin on the day of biopsy |
| 6 | Patient’s blood group should be known and facilities for blood and FFP transfusion must always be available |
| 7 | Patient should be fasting for 12 h before the biopsy |
| 8 | An intravenous catheter should be fixed |
| 9 | Patient should be trained to hold breath for a few seconds in expiration |
| 10 | Patient should be given intravenous meperidine and midazolam to allay anxiety and pain before the procedure |
FFP fresh frozen plasma
Consensus statements
Percutaneous liver biopsy is an invasive procedure, but major complications are very rare (1a).
Indication and contraindications of liver biopsy should be clearly established (1a, A).
Patient should be properly prepared. Premedication and informed consent are must (1a, A).
Image-guided liver biopsy is strongly recommended (3, C).
If there is coagulopathy or thrombocytopenia <100,000/cumm or ascites, a transjugular liver biopsy (TJLB) is advised (2a, B).
However, further study is needed to standardize the methodology of TJLB and validate its relevance in routine clinical practice (2b, B).
Liver biopsy: sample size and quality
The sampling variability of liver fibrosis has been poorly investigated. This issue is relevant because the liver core biopsy specimen represents only a very limited part of the whole liver and fibrosis is heterogeneously distributed. In order to avoid these caveats and limit the risk for false evaluation, the use of a biopsy specimen of sufficient length and sufficient number of portal tracts is usually recommended [2].
Several previous studies regarding sample size and liver biopsy did not account for the current semiquantitative scoring systems. A satisfactory length of liver biopsy was reported to range from 1 to 4 cm and a sample 1.5-cm long and/or containing four to six portal tracts has been considered acceptable [3]. There is another interesting study in which the impact of liver biopsy size on histologic evaluation of chronic viral hepatitis was evaluated; the authors concluded that liver biopsy size strongly influences the grading and staging of chronic viral hepatitis. A biopsy specimen of 1.5-cm length and 1.4-mm thickness gives sufficient information regarding the histological features of liver biopsy, and the use of fine-needle aspiration biopsies in this setting should be discouraged [4].
Consensus statements
Biopsy needle should be at least 16 G (3a, C).
Preferable core length should be longer than 15 mm or contain at least ten portal tracts. A repeat pass should be done, if biopsy length is <1 cm (1a, A).
Liver biopsy: interobserver differences and expertise
Grading and staging of liver biopsies in patients with chronic hepatitis remain the “gold standard”; however, it is influenced by variability in scoring systems, sampling, observer agreement, and expertise. This sampling variability is most probably due to
uneven distribution of lesions in liver parenchyma;
size of the sample (depends on type and size of needle used);
number of passes in the liver; and
interobserver variability (least likely cause).
Ratziu et al. [5] studied biopsies in 51 patients with nonalcoholic fatty liver disease (NAFLD), and they found that histological lesions of nonalcoholic steatohepatitis (NASH) are unevenly distributed throughout the liver parenchyma; therefore, sampling error of liver biopsy can result in substantial misdiagnosis and staging inaccuracies. Grizzi et al. [6] derived samples from six to eight different parts of livers removed from 12 patients with cirrhosis undergoing orthotopic liver transplantation. They assessed the sampling variability using computer-aided, fractal-corrected measures of fibrosis in liver biopsies. They found a high degree of intersample variability in the measurements of the surface and wrinkledness of fibrosis, but the intersample variability of Hurst’s exponent was low [6]. Dioguardi et al. [7] measured digitized histological biopsy sections taken from 209 patients with chronic hepatitis C virus (HCV) infection with different grade of fibrosis or cirrhosis by means of a new, rapid, user-friendly, fully computer-aided method, based on an international system of measurement that is meter rectified and uses fractal principles. Skripenova et al. [8] in 2007 studied 60 patients with chronic HCV infection, and they showed a difference of one grade or one stage in 30% of paired liver biopsies taken from left and right lobes.
Consensus statements
Liver biopsy should be performed only by experts with minimum training of 50 biopsies under supervision (1a, A).
Liver biopsy: quantification of fibrosis
The first histological classification was published in 1968 [9], which was essentially a qualitative classification of liver biopsy. The authors coined the terminology “chronic persistent and chronic aggressive hepatitis.” Knodell et al. [10] in 1981 introduced semiquantitative and reproducible histological scoring of liver biopsies. Lesions were assigned weighted numeric values, which resulted in a score termed as histology activity index (HAI). The HAI comprised three categories for necroinflammation and one for fibrosis, with points for the severity of the lesion in each category. The sum of points constituted the final score, or HAI. The French METAVIR Cooperative Study Group [11] proposed a comprehensive but complex system for the histologic evaluation of hepatitis C. The final score reflects the combined ratings for focal lobular necrosis, portal inflammation, piecemeal necrosis, and bridging necrosis. Modification of the Knodell HAI, commonly referred to as the Ishak system [12], provides consecutive scores for well-defined lesions within four separate categories that are added together for the activity grade.
Studies to validate the results of liver biopsy reporting showed that there was reproducibility on the fibrosis score when different scoring systems were used, but less reproducibility was seen on the necroinflammatory scores. Reading of necroinflammatory lesions is more reproducible with the Scheuer scale than with the Knodell HAI [13]. Recently, an automated analyzer was used to quantify the fibrosis, which seems an intelligent approach that attempts to utilize the current semiquantitative methods of liver fibrosis assessment to turn them into real quantitative ones with significant reduction in variability and subjectivity [14].
Consensus statements
Quantitation of fibrosis using image analysis may be a reproducible technique, with little interobserver variation, as well as, should be considered for investigational studies of liver fibrosis (3a, C).
Noninvasive diagnosis of liver fibrosis
Aspartate aminotransferase-platelet ratio index
The aspartate aminotransferase (AST)-platelet ratio index (APRI) is proposed as a simple and noninvasive predictor in the evaluation of liver fibrosis status. It has several advantages. First, it is readily available because AST and platelets counts are part of the routine tests in managing patients with chronic HCV infection. No additional blood tests or cost is needed. Second, it is easy to compute, without the use of complicated formula. In fact, clinicians could simply work out the value without the use of a calculator. Third, and more importantly, it is backed by sound pathogenesis. More advanced state of fibrosis is associated with lower level of platelets through lower production of thrombopoietin, as well as higher portal hypertension and enhanced pooling and sequestration of platelets in the spleen [15].
However, we ought to be aware of the limitations of APRI. First, APRI was originally derived in a group of patients with chronic HCV infection. Its usefulness in other forms of chronic liver diseases remains uncertain. Two studies performed by Chun-Tao Wai and Kim on patients with chronic hepatitis B virus (HBV) infection showed a poor correlation between liver histology and APRI, with the area under receiver operating characteristic curves (AUROC) being <0.70. Another study by Lieber et al. [16] also showed poor ARPI with AUROC being <0.70 in patients with alcoholic liver disease.
In addition, as in the original article, 19% of patients with cirrhosis and 49% of patients with significant fibrosis could not be accurately predicted. Hence, further studies are needed to improve prediction of histology in this group of patients. Future studies should focus on how to optimize its predictive value in combination with other noninvasive markers.
FibroTest
FibroTest was first described for patients with HCV infection in 2001 and is licensed to BioPredictive (Paris, France). This test uses five serum markers, Apo A1, Hap, α-2-M, γ-glutamyl transpeptidase (γGT) activity, and bilirubin, together with the age and sex of the patient to calculate a score [17]. In the original report, FibroTest scores from 0 to 0.10 provided 100% negative predictive value for the absence of significant fibrosis (defined as F2, F3, or F4 by METAVIR score), whereas scores from 0.60 to 1.00 had a more than 90% positive predictive value for significant fibrosis for patients with HCV infection. Scores from 0.11 to 0.59 were indeterminate and liver biopsy was recommended. In an independent validation of FibroTest, the negative predictive value of a score lower than 0.10 was 85% and the positive predictive value of a score higher than 0.60 was 78%.
FibroTest has also been applied to detect liver fibrosis in patients with chronic HBV infection. For application in NAFLD, FibroTest has been modified and presented as NASH test by including the following additional parameters: height, weight, serum triglycerides, cholesterol, and both AST and ALT.
The European liver fibrosis test
The European liver fibrosis (ELF) test combines three serum biomarkers, which have been shown to correlate to the level of liver fibrosis as assessed by liver biopsy. These biomarkers include hyaluronic acid (HA), procollagen III amino terminal peptide, and tissue inhibitor of metalloproteinase 1. The algorithm measures each of these markers by immunoassay to create an ELF score [18].
Unlike previous studies that focused predominately or exclusively on patients with chronic HCV infection, this study examined a variety of liver diseases; also, all fibrosis stages were adequately represented. An algorithm was developed, which detected moderate or advanced fibrosis (Scheuer stages 3 and 4) with a sensitivity of 90% and the absence of fibrosis (Scheuer stages 0–2) with a negative predictive value of 92%. The test appeared to be suitable for NAFLD and alcoholic liver disease but not for patients with HCV infection.
FIBROSpect
FIBROSpect II was first described [19] for patients with HCV infection in 2004 and is licensed by Prometheus Laboratories (San Diego, CA, USA). FIBROSpect uses three serum markers, α-2-M, HA, and TIMP, to calculate a score. When applied to 696 patients with HCV infection, a score lower than 0.36 excluded significant fibrosis with a negative predictive value of 76% and a score higher than 0.36 detected significant fibrosis with a positive predictive value of 74% [20].
Hepascore
Hepascore requires the measurement of serum bilirubin, γGT activity, α-2-M, and HA levels. Hepascore ranges from 0.00 to 1.00 and is calculated from the results of these four analyses and the age and sex of the patient. Hepascore has been validated in patients with HCV infection, where a score of 0.50 or above provided a positive predictive value of 88% for significant fibrosis (METAVIR score of F2 or above) and a score of <0.5 had a negative predictive value of 95% for the absence of advanced fibrosis (METAVIR score of F3 or above) [21].
FibroMeter
This is a new serum model that is claimed to outperform other models. The six parameters required to calculate FibroMeter are platelets, PT index, AST, α-2-M, HA, and urea levels [22].
Breath tests for assessment of liver fibrosis
13C-breath tests for the study of liver function have been developed to noninvasively quantitate the residual liver function in patients with various degrees of liver fibrosis. Sequential studies that were performed over the years using various 13C-breath test substrates showed that increasing degrees of liver fibrosis are paralleled by concomitant modifications in 13C-breath test results [23]. Promising results that breath tests might be able to replace percutaneous liver biopsy in certain patients with chronic HCV infection before interferon therapy need further confirmation. Breath tests seem to be superior to the Child-Pugh classification in predicting long-term prognosis [24]. Further studies should evaluate the diagnostic yield of 13C-breath test, in particular clinical situations, such as in patients with normal static parameters of liver function, in following the effects of therapeutic regimen, the decision of optimal transplant timing, or to test the residual organ function before planning a resection of the liver.
FibroFast
A simple noninvasive score (FibroFast) was developed and evaluated on the basis of several simple blood biomarkers (ALT:AST ratio, albumin, alkaline phosphatase, and platelets count) that can be easily used by clinicians to predict severe fibrosis or cirrhosis in patients with chronic HCV infection [25]. The validation of 1,067 cases from several international centers (Egypt, Italy, Brazil, Romania, and UAE) showed that the sensitivity of FibroFast was 61.5%, specificity 81.1%, positive predictive value 59%, and negative predictive value 82.6%. New cut-off scores of FibroFast were developed that allow the diagnosis of cirrhosis (F4) and F0–F3 with the highest possible accuracy (>95%). FibroFast with the new two cut-off scores could be an alternative to liver biopsy in about one-third of the patients, with sensitivity 95% and specificity 95% [26].
Consensus statements
Noninvasive tests are useful for identifying only those patients with no fibrosis or with extreme levels of fibrosis (1a, A).
Staging of liver fibrosis in the intermediate range cannot be satisfactorily predicted by any of the available tests (1a, A).
A stepwise algorithm incorporating noninvasive markers of fibrosis may reduce the number of liver biopsies by about 30% (1a, A).
Imaging of liver fibrosis
Abdominal ultrasound
Abdominal ultrasound (US) is a simple imaging technique for almost all the cases of chronic liver disease. Many investigators [26] have examined its role in diagnosis of hepatic fibrosis and differentiating chronic hepatitis from liver cirrhosis. An US evaluation of the liver fibrosis stage of chronic liver disease has been performed by assessing various US factors such as the liver size, the bluntness of the liver edge, the coarseness of the liver parenchyma, nodularity of the liver surface, the size of the lymph nodes around the hepatic artery, the irregularity and narrowness of the inferior vena cava, portal vein velocity, or spleen size [27].
The sonographic pattern for schistosomiasis periportal fibrosis is characteristic and is not mimicked by other hepatic diseases. Schistosomiasis could be separated from cirrhosis, as well as from combined lesions. In case of discordance, sonography gives a more accurate diagnosis and grading of schistosomal hepatic fibrosis [28]. It has been repeatedly demonstrated that gallbladder wall thickening is associated with periportal fibrosis in the absence of a calculous cholecystitis.
The fibrosis index (FI) is a new index obtained to differentiate cirrhosis from chronic hepatitis, portal vein peak velocity (PVPV), hepatic artery resistive index (HARI):
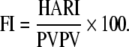 |
The FI is higher in cirrhotic patients than patients with chronic hepatitis; the value of 3.6 as a cut-off is considered the best value in differentiating chronic hepatitis from cirrhosis with 96% accuracy. FI is an appropriate noninvasive test for diagnosing liver cirrhosis, and its use will decrease the need for liver biopsy [29].
An ultrasonographic scoring system grading periportal fibrosis, portal vein diameter, spleen size, and portosystemic anastomoses was evaluated as a predictor of esophageal varices and proved useful in predicting the presence of esophageal varices [30].
In conclusion, although ultrasonographic data proved reliable in differentiating cirrhosis from milder stages of fibrosis, their diagnostic values have not been definitely clarified, as documented by the wide range of sensitivity and specificity rates. The fibrosis test (FI) calculated from Doppler parameters is considered to provide the best value in differentiating chronic hepatitis from cirrhosis with 96% accuracy, and it can decrease the need for liver biopsy.
Consensus statements
Although ultrasonographic data proved reliable in differentiating cirrhosis from milder stages of fibrosis, diagnostic value has not been definitely clarified, as documented by the wide range of sensitivity and specificity rates (1b, A).
The FI calculated from Doppler parameters is promising and needs to be validated (2b, B).
Microbubble US
US contrast agents have been introduced into clinical practice in the 1990s. The agents consist of microbubbles smaller than red blood cells, and they act as an intravascular space enhancer. These agents have the following properties: nontoxic, injectable intravenously, capable of passing through the capillary, and stable in the circulation.
Levovist, a galactose-based microbubble agent, is the first US contrast agent that could be used by peripheral venous injection. Albrecht et al. [31] analyzed hepatic vein transit time using Levovist, and found that much earlier onset of enhancement and peak enhancement (maximum microbubble concentration in the hepatic vein) in patients with cirrhosis than controls or patients with noncirrhotic diffuse liver disease. Arrival time of <24 s was 100% sensitive and 96% specific for the diagnosis of cirrhosis. They concluded that measurement of the arrival time of the bolus allows discrimination of patients with cirrhosis from controls and patients with noncirrhotic diffuse liver disease, and it has the potential as a simple and noninvasive test for cirrhosis. However, patients with liver metastases show a “left shift” of the time-intensity curves similar to that in cirrhotic patients, and this is a limitation of this method. On the basis of these backgrounds, Kaneko et al. [32] compared the signal intensity of liver parenchyma in liver-specific phase with the degree of fibrosis on histopathological findings. Significant inverse correlation was found between the signal intensity of the liver parenchyma and the hepatica FI, which is the ratio of fibrosis area to visual field area. They concluded that contrast-enhanced US may be useful for the assessment of hepatic fibrosis.
Although contrast-enhanced US may be useful for the evaluation of hepatic fibrosis, this method is based on the observation of postvascular static phase. So, we have to recognize that the results acquired from this technique represent indirect diagnostic aspect for hepatic fibrosis. Noncontrast-enhanced US may be a goal of noninvasive assessment for hepatic fibrosis as a direct observation method for fibrosis.
Consensus statements
Contrast-enhanced US with microbubble contrast agent may be promising as an indirect assessment tool for hepatic fibrosis (2b, B).
Noncontrast-enhanced US is expected to be a direct assessment tool for hepatic fibrosis (2b, B).
FibroScan
FibroScan (transient elastography or liver stiffness measurement) is a noninvasive test that is based on the physics of transient elastography to assess liver fibrosis. It is a noninvasive test, and no adverse effects have been reported. A specialized US transducer placed over the liver transmits mild amplitude, low-frequency vibration. The vibration creates an elastic shear wave that moves through the underlying liver tissue. Its velocity is measured using a pulse-echo US. Shear waves propagate more quickly in stiff tissue and liver stiffness increases with increased fibrosis. The machine validates that the measurement is through the liver and the procedure is performed by obtaining multiple validated measurements in each patient, reducing sampling errors. FibroScan takes less than 5 min to perform and produces immediate, operator-independent results expressed in kiloPascals (kPa) [33]. The depth of measurement from the skin surface is between 25 and 65 mm, limiting its use in obese patients, and morbid obesity or narrow intercostal spaces prevent its use in 5–8% of patients. However, newer probes are being developed for obese patients or those who have narrow intercostal spaces.
Several studies evaluated the accuracy of FibroScan, blood tests, or combinations compared with liver biopsy [34]. Most include patients with HCV infection, one includes patients with chronic liver disease of any origin, one includes patients with biliary cirrhosis due to primary biliary cirrhosis or primary sclerosing cholangitis, and one includes only those patients who are coinfected with HIV and HCV. These studies show that FibroScan results are reproducible across operators and time [35]. All the studies report that FibroScan’s diagnostic performance is good, indicating that it agrees perfectly with liver biopsy.
Two recent meta-analyses [36, 37] assessed the utility of FibroScan in evaluating liver fibrosis. They showed that for patients with stage IV fibrosis (cirrhosis), the pooled estimates for sensitivity were 87%, specificity 91%, positive likelihood ratio 11.7, and negative likelihood ratio 0.14. Their analysis concluded that transient elastography is a clinically useful test for detecting cirrhosis. Shaheen et al. [37] analyzed studies published for both FibroScan and Fibrotest for detecting cirrhosis, the summary area under the curves for FibroScan was 0.95 (0.87–0.99) and for FibroTest was 0.90. Both had less sensitivity for differentiating minor degrees of fibrosis.
Consensus statements
Clinical utility of FibroScan techniques would be proven by further studies in large number of patients (2b, B).
Hepatic venous pressure gradient
Methodology and technique
Histology remains the gold standard to assess fibrosis of the liver, but hepatic venous pressure gradient (HVPG) has also been explored for the same. Samonakis et al. [38] evaluated patients with recurrent HCV infection after liver transplantation to assess whether HVPG correlates with liver histology, particularly fibrosis. They concluded that HVPG correlates with fibrosis and its progression. In another study, FibroTest was found to correlate with the presence and degree of portal hypertension. A strong relationship between liver stiffness measurement and HVPG measurements was found in the study [39].
HVPG measurement is done after overnight fasting, under conscious sedation, and vital sign monitoring (including heart rate, arterial blood pressures, digital oxygen saturation, and electrocardiogram) under local anesthesia and aseptic conditions [40, 41]. The time required in this procedure ranges from 10 to 20 min. The rate of successful hepatic catheterization is >95%. Although HVPG is an easy and simple technique, accurate measurements require specific training. In order to achieve results that are highly reliable and comparable from center to center, meticulous attention to details is required [40, 42].
Safety and complications of HVPG
HVPG has been shown to be very safe and the rate of successful hepatic vein catheterization is >95% [43]. No reports of serious complications have been published in the medical literature [44]. Samonakis et al. [38] reported one episode of supraventricular tachycardia, which disappeared with repositioning of the guide wire [38]. A number of observations over the past 5 years have led to a greater appreciation of how HVPG measurements could be used in the management of the patient with liver disease and the hepatology community may begin to use this technique in the same manner as they use liver biopsy. Clearly, the time for using the HVPG more broadly has arrived and its utility in the management of patients with liver disease will only be limited by our lack of interest in using this old technique in new ways [45].
Can HVPG be recommended as a routine surrogate marker of fibrosis?
Accurate measurement of disease progression is difficult in different stages of chronic liver disease. Indeed, a correct and reliable assessment of the stage of chronic liver disease and fibrosis has relevant implications for assessing the effectiveness of the current therapeutic regimens, predicting the natural course, and development of complications. The disease progression from chronic hepatitis to cirrhosis of the liver is associated with structural and biological changes responsible for an increase in portal pressure [46].
Liver biopsy has been considered the gold standard for evaluation of stage of hepatic fibrosis. It is, however, invasive, occasionally accompanied by complications, and is not acceptable by all patients [47].
The HVPG measurement reflects the status of a significant portion of the liver, and it is not prone to sampling error as in the case of liver biopsy. It accurately reflects portal pressure, and if precisely measured, has a very low variability [44]. HVPG reflects the interaction between hepatic vascular resistance and blood flow, and, as such, is thought to closely indicate disease severity.
The HVPG measurement has several advantages compared with liver biopsy and can be complementary to liver biopsy. It is important to note that balloon-catheter measurements of HVPG are not subject to sampling errors [43]. Moreover, HVPG measurements are reproducible in experienced hands and the risks of the procedure are very low. HVPG has been shown to correlate well with the severity of advanced liver disease in HCV infection [38]. It has been even shown to be helpful in assessing reversal of fibrosis following antiviral therapy [48]. Recently, a correlation between HVPG and histologic stages of liver disease has been shown in a study in patients with chronic liver disease due to HBV infection [49]. HVPG can be a good alternative as well as additive to liver biopsy in these patients.
Consensus statements
HVPG measurement is a relatively simple procedure (2b).
HVPG is a safe procedure with a very low complication rate in experienced hands (2b).
HVPG measures both the irreversible and reversible components of portal hypertension. It is a dynamic marker of disease progression, especially precirrhotic stage (3a).
HVPG closely correlates with the degree of advanced fibrosis and can be recommended as a marker of fibrosis (2a, B).
The etiology of cirrhosis does not significantly influence HVPG levels (3a).
Therapy of liver fibrosis
Fibrosis as an end point in the treatment of HCV infection
Evidence obtained from the available studies is promising and suggest that fibrosis can be a reasonable target for the treatment of chronic HCV infection. Studies have shown that interferon has an antifibrotic effect, and there is sufficient information to indicate that treatment of patients with chronic HCV infection reduces the incidence of hepatocellular carcinoma. This effect is more pronounced in patients who achieve a sustained virologic response. Moreover, there is evidence that specific antiviral therapy can reduce progress of hepatic fibrosis in patients with nonsustained virologic response [50, 51]. In future, a study population enrolling a homogenous group of HCV and NASH patients at high risk for fibrosis progression compared with those who are not would be ideal to substantiate treatment differences in both groups. Preventing progression of fibrosis and development of cirrhosis in HCV patients will not only save money, but also enhance the quality of life of the patient. The need for a liver biopsy to initiate and monitor the treatment is the main hurdle to this objective at the present time. The development of reliable surrogate markers of fibrosis in the future may solve this problem [52].
Effect of silymarin on hepatic fibrosis for patients with chronic liver disease
Silymarin is an extract of milk thistle (MT), Silybum marianum (L) Gaertneri, which has been used as a medical remedy since the time of ancient Greece, and is widely used as an alternative medication [53]. Silymarin is the collective name for the flavonolignans (silybin or silibinin, silydianin, silychristin) extracted from the MT. These extracts have been shown to protect animals against various hepatotoxins, including acetaminophen [54], radiation [55], and iron overload.
The Cochrane hepatobiliary group [56] has published a systematic review along with meta-analysis of randomized clinical trails on the effects of MT for alcoholic and/or hepatitis B or C liver diseases. The review included 26 references, 13 of them were randomized trials and these assessed MT in 915 patients. MT versus placebo or no intervention for a median duration of 6 months was evaluated in these studies; MT had no significant effects on complications of liver disease or liver histology. MT showed a significant effect on AST and ALT levels, whereas it did not significantly influence prothrombin or serum albumin. There were no significant effects of MT on liver biopsy findings in the only trial reporting this outcome [57]. This systematic Cochrane review criticized the methodological quality of most studies reviewed and recommended adequate randomized control trials on silymarin versus placebo.
In a recent review by Stickel and Schuppan [58] on the use of herbal medicine in the treatment of liver disease, the authors reported that a major problem of most clinical trials on silymarin has been the definition of end points such as progression of fibrosis and such pitfalls might even have led to missing a true treatment effect. Whether higher doses of silymarin (i.e., 840 mg/day) are more effective in attenuating fibrosis progression in chronic viral HCV infection is currently tested in a randomized controlled multicenter trial, but, so far, owing to the lack of firm clinical evidence, silymarin has no effect on hepatic fibrosis.
Consensus statements
There is no evidence that silymarin has a significant effect on hepatic fibrosis in patients with chronic liver disease (1b).
The effect of higher doses of silymarin on hepatic fibrosis may need large, randomized, multicenter controlled trials (B).
Liver fibrosis can be considered as an end point parameter in the treatment of chronic hepatitis, provided it is adequately quantitated before and after the therapy (1b, A).
More evidence is needed to prove that treatment of chronic hepatitis significantly reduces the rate of progression of liver fibrosis (1b, A).
Reversibility of hepatic fibrosis
The reversibility of cirrhosis, which earlier was thought to be irrefutable, is now a hotbed for debate. It is now understood that hepatic fibrogenesis is a dynamic, continuously evolving process. Thus, the laying down and resorption of collagen or extracellular matrix can both occur at different time periods. Hepatic fibrosis can therefore decrease with appropriate therapy, or by removing the cause.
The issue remains regarding the reversibility of cirrhosis and this depends on the definition of cirrhosis. Popper and Zak [59] described cirrhosis as being characterized by an increase in the extracellular matrix, parenchymal changes, and has important functional consequences in contrast to simple fibrosis, which may be devoid of significant functional effects. They further highlighted this difference in a communication published in 1968, in which they defined cirrhosis as a process characterized by a clinically, functionally, and pathologically significant disturbance of hepatic circulation [60].
The pathology of cirrhosis involves not only diffuse fibrosis, but also regeneration, altered lobular architecture, and altered vascular relationships. Thus, the normal liver architecture is converted to structurally abnormal nodules with a resultant liver dysfunction and portal hypertension [61]. The questions that thus need answering are as follows:
Does mature collagen reabsorb?
Can the altered architecture return to normal/near normal?
Can the functional and hemodynamic changes revert?
Thus, most evidence that we have pertains to diminishing of the fibrosis component of developing cirrhosis. However, evidence of reversal of established cirrhosis with its clinical ramifications is scant. A lot of evidence comes from animal studies [62] in which the development of cirrhosis or fibrosis is of short duration and cannot be considered as an exact reproduction of human cirrhosis. In a landmark article by Wanless et al. [63], they described one case and gave evidence of the possible mechanisms, although they too indicated that there is no accumulated clinical evidence to support the view that cirrhosis regresses.
The controversy about the reversal of fibrosis component of cirrhosis is only one aspect in the arguments on the reversibility of cirrhosis. There is evidence that other architectural alterations have greater significance. It has been shown in experiments that, once regenerative nodules develop, they continue to grow or persist even when the stimulus is removed. Thus, this aspect of cirrhosis cannot be reversed. Popper and Elias [64] have elaborately described the anastomotic connections among branches of the portal vein, hepatic artery, and hepatic vein, which develop in cirrhosis and may cause a tendency to progression of the cirrhotic process. It is also known that the micronodular pattern of cirrhosis is seen early in the course of the disease and larger nodules develop later. These larger nodules may give the false impression of decreased fibrosis on needle biopsy. In the Laennec system of grading cirrhosis, mild cirrhosis has thin septae, whereas moderate to severe grades have thicker septae. Thick septae are usually vascularized and probably more resistant to resorption. Pérez-Tamayo [65] in 1979 showed that experimental cirrhosis induced in mice by carbon tetrachloride or low-protein diet with 20% alcohol could show regression of reticular fibers and improvement in hepatocellular carcinoma after the agents were discontinued. Mature collagen and nodular regeneration, however, showed no significant regression.
There are reports of improvement of hepatic fibrosis with colchicine treatment; however, there are also reports to the contrary [66]. Similarly, although there is an occasional study reporting decrease in hepatic fibrosis in autoimmune hepatitis, a case series showed the development of cirrhosis to be associated with less response to therapy [67].
There is, however, hope for the optimistic with evidence related to reversal of hepatic fibrosis [68].
Consensus statements
Hepatic fibrosis as assessed by tissue pathology can regress especially after specific treatment (1a).
There is not enough evidence to suggest that cirrhosis is reversible; however, remodeling can occur (3a).
Fibrosis regression is not synonymous with cirrhosis reversal (1a).
Liver architecture may not return to normal even after specific treatment (1a).
Hepatic function may not return to normal even after specific treatment (3a).
Vascular shunts when established do not reverse even after specific treatment (2a).
Appendix 1
Egyptians: Abdelrhman Elzayadi, Abdelhamid Abaza, Abdelkhalek Hamed, Abdelrhman Zekry, Abueldahab Elsahly, Ahmed Eldorry, Bahaa Abass, Basma Samir, Emad Barakat, Eman Rewisha, Fayza Azzam, Hassan Elshenawy, Inas El-Korimi, Khaled Hemedah, Helmy Abaza, Khaled Zalata, Mazen Naga, Magdy Atta, Mamdouh Gabr, Mohamed Amer Afefy, Mohamed Elateek, Mohamed El-Khashab, Mohamed Sharaf Eldin, Mohsen Maher, Mostafa Elawady, Mostafa Gabr, Omar Haikal, Rasheed Bahgat, Sherif Abdelfattah, Taghreed Gaafar, Tawheed Mowafy, Yousry Taher.
International: Abdelatif Charqawi (Morocco), Abdul-Naser Elzouki (Libya), Ali Elsaid (UAE), Hasnain Ali Shah (Pakistan), Mamun-Al-Mahtab (Bangladesh), Nizar Zein (USA), Salem Awad (UAE).
Footnotes
The members of Jury of the APASL Consensus Development Meeting are given in Appendix 1.
References
- 1.Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis. 2006;15:149–159. [PubMed] [Google Scholar]
- 2.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/S0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 3.Hølund B, Poulsen H, Schlichting P. Reproducibility of liver biopsy diagnosis in relation to the size of the specimen. Scand J Gastroenterol. 1980;15:329–335. doi: 10.3109/00365528009181479. [DOI] [PubMed] [Google Scholar]
- 4.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/S0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, LIDO Study Group et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Grizzi F, Russo C, Franceschini B, Rocco M, Torri V, Morenghi E, et al. Sampling variability of computer-aided fractal-corrected measures of liver fibrosis in needle biopsy specimens. World J Gastroenterol. 2006;12:7660–7665. doi: 10.3748/wjg.v12.i47.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dioguardi N, Grizzi F, Franceschini B, Bossi P, Russo C. Liver fibrosis and tissue architectural change measurement using fractal-rectified metrics and Hurst’s exponent. World J Gastroenterol. 2006;12:2187–2194. doi: 10.3748/wjg.v12.i14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skripenova S, Trainer TD, Krawitt EL, Blaszyk H. Variability of grade and stage in simultaneous paired liver biopsies in patients with hepatitis C. J Clin Pathol. 2007;60:321–324. doi: 10.1136/jcp.2005.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groote J, Desmet VJ, Gedigk P, Korb G, Popper H, Poulsen H, et al. A classification of chronic hepatitis. Lancet. 1968;2:626–628. doi: 10.1016/s0140-6736(68)90710-1. [DOI] [PubMed] [Google Scholar]
- 10.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 11.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 12.Ishak K, Baptista A, Bianchi L, Callea F, Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 13.Westin J, Lagging LM, Wejstål R, Norkrans G, Dhillon AP. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver. 1999;19:183–187. doi: 10.1111/j.1478-3231.1999.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Matalka II, Al-Jarrah OM, Manasrah TM. Quantitative assessment of liver fibrosis: a novel automated image analysis method. Liver Int. 2006;26:1054–1064. doi: 10.1111/j.1478-3231.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 15.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006;101:1500–1508. doi: 10.1111/j.1572-0241.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 17.Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Myers RP, Thabut D, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg WM. Rating fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:357–360. doi: 10.1016/S0168-8278(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 19.Poordad FF. FIBROSpect II: a potential noninvasive test to assess hepatic fibrosis. Expert Rev Mol Diagn. 2004;4:593–537. doi: 10.1586/14737159.4.5.593. [DOI] [PubMed] [Google Scholar]
- 20.Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, et al. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 22.Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 23.Festi D, Capodicasa S, Sandri L, Colaiocco-Ferrante L, Staniscia T, Vitacolonna E, et al. Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: comparison with Child-Pugh score and serum bile acid levels. World J Gastroenterol. 2005;11:142–148. doi: 10.3748/wjg.v11.i1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campli C, Angelini G, Armuzzi A, Nardo B, Zocco MA, Candelli M, et al. Quantitative evaluation of liver function by the methionine and aminopyrine breath tests in the early stages of liver transplantation. Eur J Gastroenterol Hepatol. 2003;15:727–732. doi: 10.1097/00042737-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Attallah AM, Shiha GE, Omran MM, Zalata KR. A discriminant score based on four routine laboratory blood tests for accurate diagnosis of severe fibrosis and/or liver cirrhosis in Egyptian patients with chronic hepatitis C. Hepatol Res. 2006;34:163–169. doi: 10.1016/j.hepres.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Iacobellis A, Fusilli S, Mangia A, Clemente R, Festa V, Giacobbe A, et al. Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther. 2005;22:769–774. doi: 10.1111/j.1365-2036.2005.02633.x. [DOI] [PubMed] [Google Scholar]
- 27.Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S57–S64. doi: 10.1002/hep.1840360708. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Wahab MF, Esmat G, Farrag A, el-Boraey YA, Strickland GT. Grading of hepatic schistosomiasis by the use of ultrasonography. Am J Trop Med Hyg. 1992;46:403–408. doi: 10.4269/ajtmh.1992.46.403. [DOI] [PubMed] [Google Scholar]
- 29.Liu CH, Lin JW, Tsai FC, Yang PM, Lai MY, Chen JH, et al. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int. 2006;26:1087–1094. doi: 10.1111/j.1478-3231.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 30.Richter J, Correia Dacal AR, Vergetti Siqueira JG, Poggensee G, Mannsmann U, Deelder A, et al. Sonographic prediction of variceal bleeding in patients with liver fibrosis due to Schistosoma mansoni. Trop Med Int Health. 1998;3:728–735. doi: 10.1046/j.1365-3156.1998.00285.x. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, et al. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579–1583. doi: 10.1016/S0140-6736(98)06373-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko T, Teshigawara O, Sugimoto H, Hirota M, Inoue S, Takeda S, et al. Signal intensity of the liver parenchyma in microbubble contrast agent in the late liver phase reflects advanced fibrosis of the liver. Liver Int. 2005;25:288–293. doi: 10.1111/j.1478-3231.2005.01025.x. [DOI] [PubMed] [Google Scholar]
- 33.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Castéra L, Vergniol J, Foucher J, Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 36.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 38.Samonakis DN, Cholongitas E, Thalheimer U, Kalambokis G, Quaglia A, Triantos CK, et al. Hepatic venous pressure gradient to assess fibrosis and its progression after liver transplantation for HCV cirrhosis. Liver Transpl. 2007;13:1305–1311. doi: 10.1002/lt.21227. [DOI] [PubMed] [Google Scholar]
- 39.Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74. [PubMed] [Google Scholar]
- 41.Groszmann R, Vorobioff JD, Gao H. Measurement of portal pressure: when, how, and why to do it. Clin Liver Dis. 2006;10:499–512. doi: 10.1016/j.cld.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 43.Groszmann RJ, Glickman M, Blei AT, Storer E, Conn HO. Wedged and free hepatic venous pressure measured with a balloon catheter. Gastroenterology. 1979;76:253–258. [PubMed] [Google Scholar]
- 44.Huet PM, Pomier-Layrargues G. The hepatic venous pressure gradient: “remixed and revisited”. Hepatology. 2004;39:295–298. doi: 10.1002/hep.20070. [DOI] [PubMed] [Google Scholar]
- 45.Boyer TD. Wedged hepatic vein pressure (WHVP): ready for prime time. Hepatology. 2006;43:405–406. doi: 10.1002/hep.21118. [DOI] [PubMed] [Google Scholar]
- 46.Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999;19:397–410. doi: 10.1055/s-2007-1007128. [DOI] [PubMed] [Google Scholar]
- 47.Poynard T, Ratziu V, Bedossa P. Appropriateness of liver biopsy. Can J Gastroenterol. 2000;14:543–548. doi: 10.1155/2000/107982. [DOI] [PubMed] [Google Scholar]
- 48.Rincon D, Ripoll C, Lo Iacono O, Salcedo M, Catalina MV, Alvarez E, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–2274. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 49.Kumar M, Kumar A, Hissar S, Jain P, Rastogi A, Kumar D, et al. Hepatic venous pressure gradient as a predictor of fibrosis in chronic liver disease because of hepatitis B virus. Liver Int. 2008;28:690–698. doi: 10.1111/j.1478-3231.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 50.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132(7):517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 51.Veldt BJ, Saracco G, Boyer N, Camma C, Bellobuono A, Hopf U, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–1508. doi: 10.1136/gut.2003.038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, et al. Meta-analysis HCV (on) individual patients’ data Study Group relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 54.Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–442. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 55.Hakov’a H, Misúrová E. The effect of silymarin and gamma radiation on nucleic acids in rat organs. J Pharm Pharmacol. 1993;45:910–912. doi: 10.1111/j.2042-7158.1993.tb05619.x. [DOI] [PubMed] [Google Scholar]
- 56.Rambaldi A, Jacobs BP, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases. Cochrane Database Syst Rev 2007;(4):CD003620 [DOI] [PMC free article] [PubMed]
- 57.Trinchet JC, Coste T, Lévy VG, Vivet F, Duchatelle V, Legendre C, et al. Treatment of alcoholic hepatitis with silymarin A double-blind comparative study in 116 patients. Gastroenterol Clin Biol. 1989;13:120–124. [PubMed] [Google Scholar]
- 58.Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Popper H, Zak FG. Pathologic aspects of cirrhosis. Am J Med. 1958;24:593–619. doi: 10.1016/0002-9343(58)90298-5. [DOI] [PubMed] [Google Scholar]
- 60.Agarwal BL. Cirrhosis of the liver. BMJ. 1968;2:561–562. doi: 10.1136/bmj.2.5604.561-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang YH, Shi MN, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Therapeutic effect of interleukin–10 on CCl4-induced hepatic fibrosis in rats. World J Gastroenterol. 2006;12:1386–1391. doi: 10.3748/wjg.v12.i9.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 64.Popper H, Elias H. Histogenesis of hepatic cirrhosis studied by the three-dimensional approach. Am J Pathol. 1955;31:405–441. [PMC free article] [PubMed] [Google Scholar]
- 65.Pérez-Tamayo R. Cirrhosis of the liver: a reversible disease? Pathol Annu. 1979;14:183–213. [PubMed] [Google Scholar]
- 66.Wang YJ, Lee SD, Hsieh MC, Lin HC, Lee FY, Tsay SH, et al. A double-blind randomized controlled trial of colchicine in patients with hepatitis B virus-related postnecrotic cirrhosis. J Hepatol. 1994;21:872–877. doi: 10.1016/S0168-8278(94)80252-1. [DOI] [PubMed] [Google Scholar]
- 67.Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510–1516. doi: 10.1111/j.1572-0241.2004.30457.x. [DOI] [PubMed] [Google Scholar]
- 68.Muddu AK, Guha IN, Elsharkawy AM, Mann DA. Resolving fibrosis in the diseased liver: translating the scientific promise to the clinic. Int J Biochem Cell Biol. 2007;39:695–714. doi: 10.1016/j.biocel.2006.10.006. [DOI] [PubMed] [Google Scholar]


