Abstract
Background
Serologic assays that identify herpes simplex type 2 (HSV-2) type-specific antibodies have been commercially available for more than a decade. Greater acceptance of these tests is hindered by uncertainty regarding their performance in real-world clinical settings.
Objectives
The primary objective was to compare the test characteristics of the Focus HerpeSelect® Express Assay (EA) versus the Focus HerpeSelect® enzyme linked immunoassay (ELISA) for detection of HSV-2 type-specific antibodies among pregnant women enrolled from 3 geographic sites with varying prevalences of HSV-2 infection. A second objective was to evaluate the performance of a HSV-2 testing strategy in which EA screens and ELISA confirms HSV-2 serodiagnosis.
Study Design
We enrolled 399 pregnant women from Atlanta, GA, Moorestown, NJ, and Pittsburgh, PA into this cross-sectional investigation. Capillary whole blood was obtained from study participants, and evaluated for the presence of type-specific HSV-2 antibodies using the EA. Serum samples were also obtained from all study participants for subsequent identification of HSV-2 type-specific antibodies using both ELISA and the Focus Immunoblot assays.
Results
We observed 96.2 % agreement between results obtained with EA and ELISA. Overall, when compared to ELISA results, the sensitivity of EA for detection of HSV-2 type-specific antibodies was 94.2% and the specificity was 97.1%. Using Immunoblot results as our standard for performance calculations, the positive predictive value (PPV) of HSV-2 serodiagnosis increased from 91.7% to 98.2% when ELISA was used to confirm EA testing.
Conclusions
EA provides similar results to ELISA for the identification of HSV-2 type-specific antibodies among pregnant women. As use of the point-of-care EA in conjunction with confirmatory ELISA testing improves the PPV of HSV-2 serodiagnosis compared to the use of EA or ELISA testing alone, validation of this diagnostic algorithm in other at-risk populations may be warranted.
Keywords: HSV-2 serodiagnosis, point-of-care testing, pregnancy
INTRODUCTION
The number of individuals infected with genital herpes simplex virus type 2 (HSV-2) has reached epidemic proportions1. Infection is considered to be life long, and intermittent reactivation of the virus from latency is associated with substantial morbidity. For example, intermittent genital tract reactivation can produce painful ulceration of epithelial and mucosal tissues2. Most individuals with genital herpes, however, are unaware of their infection, and sexual transmission of HSV-2 is often the result of subclinical reactivation of the virus3. Considerable evidence suggests that HSV-2 infection facilitates both sexual transmission and acquisition of HIV4, 5, while maternal-fetal transmission of the virus, also frequently asymptomatic; can cause severe and permanent neurological damage to the neonate6, 7. Because of heightened awareness of the adverse sequelae associated with both symptomatic and asymptomatic HSV-2 reactivation, the identification of individuals with genital herpes infection has been recommended8.
The advent of HSV type-specific serologic tests has greatly facilitated this identification. Previously available serologic assays, based on crude antigen preparations, were unreliable diagnostic tools as infection with HSV-1 or HSV-2 generated indistinguishable antibody responses9. Despite the high degree of serologic cross-reactivity between HSV-1 and HSV-2, an envelope glycoprotein (g), gG, was identified as antigenically distinct between the two viruses10. gG type-specific serologic assays therefore can accurately differentiate between infections with HSV-1 (gG1) or HSV-2 (gG2), and are increasingly used in research and clinical settings to identify HSV-2 infected individuals.
Available type-specific gG2 serologic products or services include Western Blot (WB) testing, immunoblot (IB) strips, enzyme linked immunoassays (ELISA), and point of care (POC) membrane tests11–14. WB has been considered the gold standard for serodiagnosis of HSV infection, but it is an expensive and labor-intensive test whose results may be difficult to interpret15. In comparison to WB, ELISA and POC tests are less costly and easier to perform, and are therefore better suited for high-volume diagnostic screening. Although numerous investigations have demonstrated that type-specific ELISAs are highly accurate for the serodiagnosis of HSV-2 infection in populations where the prevalence of disease is high, their positive predictive value suffers when used in populations with lower prevalence of infection16. As delineated by the Centers for Disease Control and Prevention, there remains a pressing need for studies that determine the “real world” performance of type-specific HSV tests in populations with variable burdens of disease17. The primary objective in this investigation was to compare the ability of the Focus HerpeSelect® Express Assay (EA) POC test (Focus Diagnostics, Cypress, CA) to the Focus HerpeSelect® ELISA to identify the presence of HSV-2 type-specific antibodies in a cohort of pregnant women enrolled from 3 sites with varying prevalences of disease. Our secondary objective was to evaluate the performance characteristics of a HSV-2 serodiagnostic algorithm where EA and ELISA type-specific tests were used, respectively, to screen for and confirm the presence of HSV-2 serum antibodies.
METHODS
Study participants
A total of 399 pregnant women aged 17–57 years (mean age = 27.5 ± 6.0 years) were enrolled from November 2006 to March 2007 from three clinical sites in the United States. 160 of the study participants were enrolled from Atlanta, GA; 120 from Moorestown, NJ; and 119 from Pittsburgh, PA. Samples were collected with informed consent from study participants and with the approval of the institutional review boards at each clinical site.
Serologic methods
The presence of HSV-2 type-specific antibodies was determined using the EA POC test. EA is a rapid lateral flow test that uses purified native gG2 antigen bound to a nitrocellulose membrane to detect type-specific HSV-2 antibodies. Capillary whole blood obtained by finger stick was immediately added to the EA device according to kit instructions (see package insert). Positive results were those in which the test strip was colored red or pink, while negative results were identified by the absence of color along the test strip. Positive and negative control serum was tested daily by research staff at each recruitment site to ensure kits were performing appropriately.
Serum samples were concurrently obtained from all study participants, and stored at −20° C prior to further HSV-2 type-specific antibody testing at Focus Diagnostics’ Reference Laboratory (Cypress, CA). ELISA and IB assays were performed on the collected sera according to kit instructions without knowledge of the prior POC test results. Using the same criteria provided by the package insert, ELISA index values < 0.9 were considered negative, those > 1.1 were considered positive, and index values 0.9 – 1.1 (inclusive) were considered equivocal results. For the purposes of this investigation, equivocal ELISA results were not re-tested.
Statistical Methods
Results from 395 of the 399 total study participants enrolled were utilized in this investigation. Three women with equivocal ELISA results were excluded as was one woman for whom IB results were unavailable. All statistical procedures were performed using SPSS statistical software (release 14.0.1) (SPSS Inc., Chicago, IL). When data satisfied conditions of normal distribution differences between groups were analyzed by the Student’s t test, while the Mann Whitney test and the test of binomial proportions were used to analyze data not satisfying conditions of normal distribution. IB was used as the standard for performance calculations that included sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
RESULTS
Table 1 shows the concordance between EA and ELISA for the detection of HSV-2 type-specific antibodies in the full study cohort. Based on the results obtained from EA POC testing, the overall prevalence of HSV-2 infection was 30.6% (121/395), while the prevalences of infection at the Atlanta, GA; Moorestown, NJ; and Pittsburgh, PA enrollment sites were 47.5%, 8.4%, and 30.2%, respectively. Concordant results were obtained for 380 of the 395 (96.2%) total evaluable serologic pairs (Table 1). Discordant results consisted of 8 women identified as HSV-2 seropositive by EA who were HSV-2 seronegative by ELISA, and 7 women identified as HSV-2 seronegative by EA who were HSV-2 seropositive by ELISA. Using ELISA as the standard for diagnosis, the sensitivity of EA for detection of HSV-2 type-specific antibodies among pregnant women was 94.2%, specificity was 97.1%, PPV was 93.4%, and NPV was 97.4% (Table 1).
Table 1.
Comparison of HSV-2 serodiagnosis by EA and ELISA among pregnant women enrolled from 3 U.S. sites (n = 395) a
| Outcome of tests (EA/ELISA) |
Concordance | Sensitivity | Specificity | PPV | NPV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | n | −/− | −/+ | +/− | +/+ | (%) | (%) | (%) | (%) | (%) |
| Atlanta, GA | 160 | 81 | 3 | 2 | 74 | 96.9% | 96.1% | 97.6% | 97.4% | 96.4% |
| Moorestown, NJ | 119 | 107 | 2 | 1 | 9 | 97.5% | 81.8% | 99.1% | 90.0% | 98.2% |
| Pittsburgh, PA | 116 | 79 | 2 | 5 | 30 | 94.0% | 93.8% | 94.0% | 85.7% | 97.5% |
| Total | 395 | 267 | 7 | 8 | 113 | 96.2% | 94.2% | 97.1% | 93.4% | 97.4% |
Adjusted to exclude study participants with equivocal ELISA results (n = 3) or with unavailable IB results (n = 1).
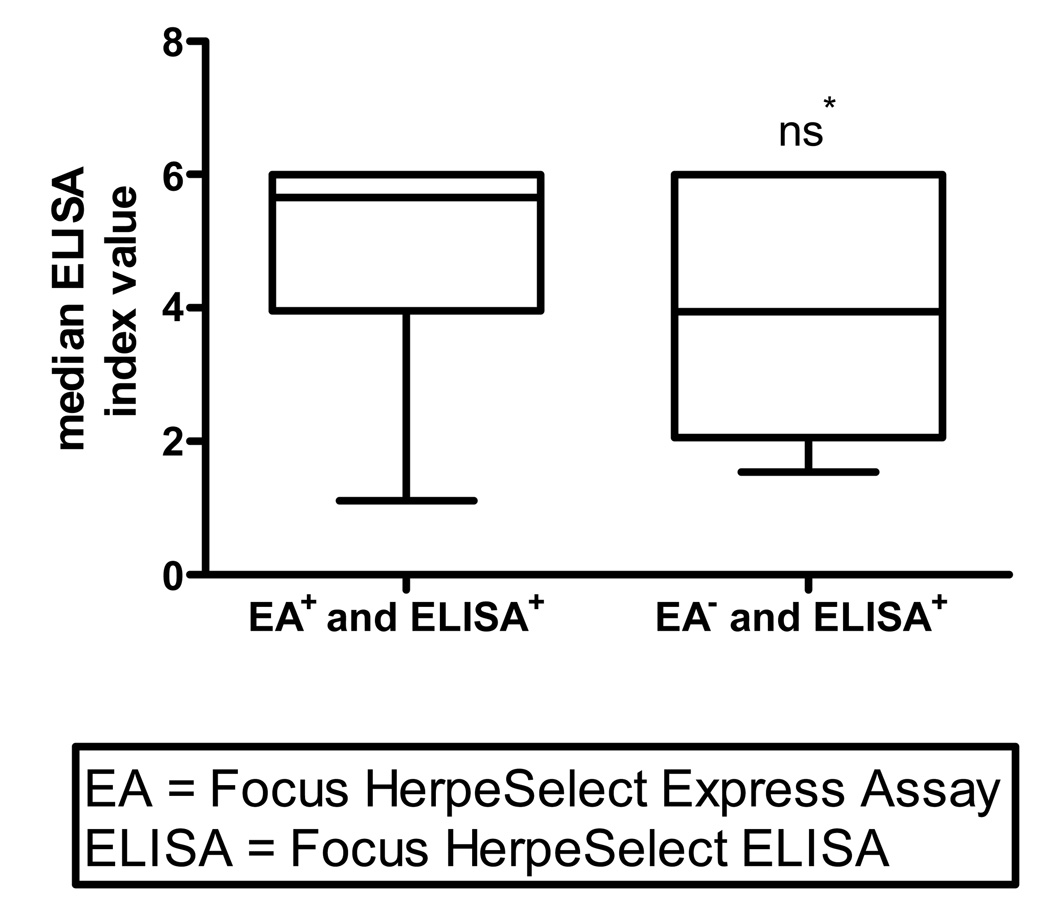
The Focus ELISA used in this investigation has been reported more likely to provide false positive results, in comparison to WB, when serum index values are in the low positive range (index values 1.1 – 3.5) 18, 19. Further analysis of the data was therefore performed to discern if lower positive index values increased the likelihood for discordancy between EA and ELISA results. Although there was a tendency for women identified as HSV-2 seropositive by both EA and ELISA to have higher median positive index values that women EA seronegative but ELISA seropositive, the relationship did not reach statistical significance (5.6 vs. 3.9; P = 0.15) (Figure 1). Discordant results were also not significantly associated with the site of enrollment, age of the study participant, or gestational age at the time of testing (data not shown).
Figure 1. Median ELISA index values for women with concordant EA and ELISA positive (n = 113) results versus women identified as EA negative and ELISA positive (n = 7).
* Mean positive ELISA index value for women identified as HSV-2 seropositive by both EA and ELISA was not statistically different than the mean positive index value for women identified as EA seronegative and ELISA seropositive (5.6 vs. 3.9; P= 0.15).
Mathematical modeling has demonstrated that among populations with variable estimated prevalences of HSV-2 infection, the PPV for serodiagnosis of disease was improved when a type-specific POC test was used to confirm positive results obtained with an ELISA20. Because our investigation included the results from three clinical sites where HSV-2 seroprevalences ranged widely from less than 10% to nearly half of women enrolled, we recognized an opportunity to evaluate the “real world” performance characteristics of a testing algorithm in which ELISA was used instead to confirm initial POC testing. Employing IB as the standard for performance calculations, use of ELISA to confirm EA POC results did not alter sensitivity of HSV-2 serodiagnosis (94.9%), but diagnostic specificity did increase from 96.4% to 99.3% (P = 0.02) (Table 2). Combined testing also improved the PPV for HSV-2 serodiagnosis from 91.7% to 98.2% (P = 0.02), without sacrifice of the NPV (Table 2).
Table 2.
Performance characteristics for the serodiagnosis of HSV-2 infection in a cohort of pregnant women enrolled from three sites in the United States (EA and ELISA versus EA or ELISA alone) a
| Test | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| EA | 94.9% | 96.4% | 91.7% | 97.8% |
| ELISA | 99.1% | 98.6% | 96.7% | 99.6% |
| EA+ELISA | 94.9% | 99.3% | 98.2% | 97.9% |
IB used as the diagnostic standard for performance calculations
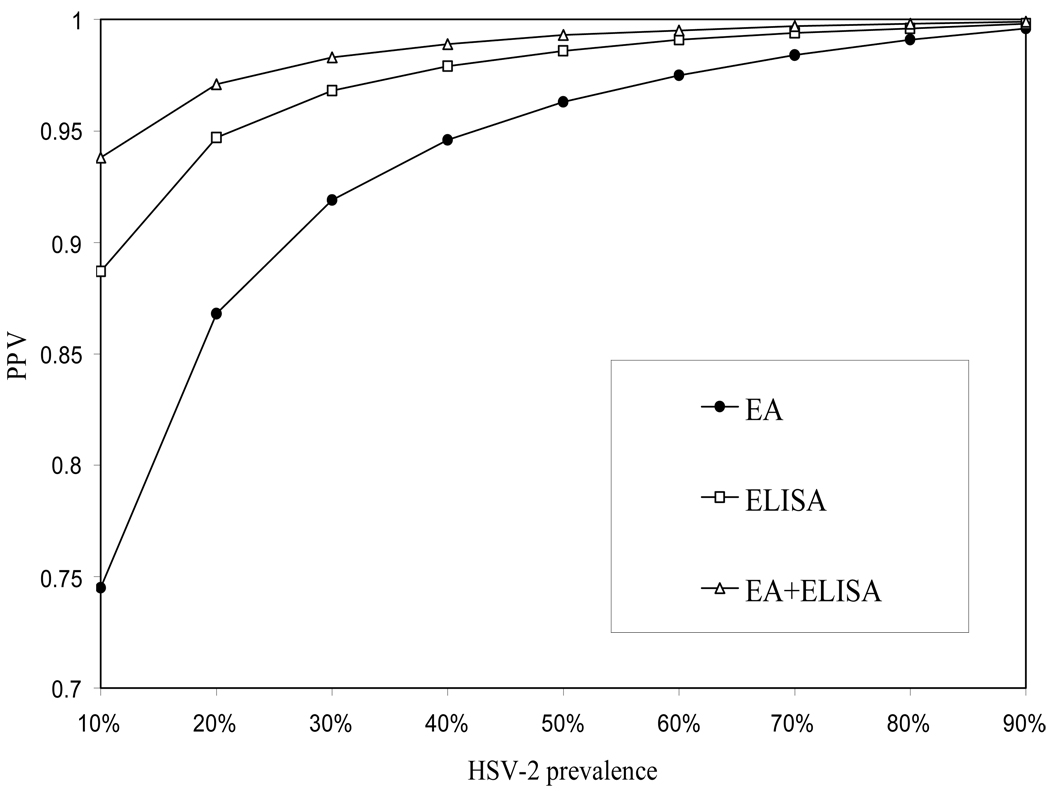
Finally, we used the results from our investigation to calculate the expected PPV among populations of varying prevalences of HSV-2 infection if ELISA was used to confirm initial EA POC testing. As demonstrated in the figure, use of this algorithm would improve the PPV of HSV-2 serodiagnosis in all theoretical populations examined. As expected, the PPV was most dramatically improved, compared to EA testing alone, in those populations where the prevalence of HSV-2 infection was lowest. Furthermore, the PPV of a HSV-2 serodiagnosis obtained by initial POC EA screening and follow-up ELISA testing among populations with prevalence of HSV-2 infection similar to those seen among women at higher risk for sexually transmitted infection21, was calculated to be ≥ 98% (Figure 2).
Figure 2. Use of EA in conjunction with ELISA improves the positive predictive value of HSV-2 serodiagnosis compared to EA or ELISA testing alonea.
Estimated positive predictive value for the serodiagnosis of HSV-2 infection when ELISA confirms point-of-care EA results among populations with varying prevalences of HSV-2 infection
a IB used as the standard for performance calculations
DISCUSSION
Our study indicates a high concordance between results for the serodiagnosis of HSV-2 infection among pregnant women from populations with variable prevalences of disease with use of the Focus EA and ELISA. ELISA was previously shown to exhibit high concordance with results achieved with WB testing22, regarded by many as the gold standard for the serologic diagnosis of HSV-2 infection, and has been cleared by the U.S. Food and Drug Administration for the detection of gG2 IgG antibodies in the sera of sexually active adults and expectant mothers. Our findings demonstrate that the Focus EA represents another reliable and commercially available test for HSV-2 serodiagnosis. Advantages of the EA over other available tests include its ease of use; ability to use capillary whole blood; and the rapidity with which results are obtained. These characteristics would expedite same-day diagnosis and counseling of individuals with HSV-2 infection.
Our investigation also indicates that compared to use of EA alone, testing with both EA and ELISA may improve the accuracy of HSV-2 serodiagnosis, particularly among populations with lower prevalences of infection. Moreover, the epidemiology of HSV-2 infection suggests there would be multiple benefits from an improved diagnostic algorithm. For example, most individuals who are latently infected with HSV-2 are asymptomatic, and identification of type-specific serum antibodies remains the only practical way to identify these individuals. Because a portion of individuals diagnosed with genital herpes infection can be expected to initiate daily antiviral suppressive therapy, it is important from a public health perspective to ensure that inaccurate diagnosis of disease does not result in unnecessary use of these medications. Similarly, a better diagnostic algorithm for HSV-2 serodiagnosis could help minimize the frequency with which an inaccurate diagnosis of genital herpes incorrectly labels individuals as infected with a life-long sexually transmitted disease. Sexual relationships between HSV-2 seronegative and asymptomatic HSV-2 infected individuals may also benefit from the construction of improved diagnostic algorithms, as identification of HSV-2 infected individuals may result in behavioral changes, such as consistent condom use, that reduce the efficiency of viral transmission. As most cases of neonatal herpes are the result of acquisition of third trimester infections 23, accurate HSV-2 serodiagnosis during the first or second trimester would better characterize the nature of the risk a pregnant woman has for peripartum transmission of the virus. Our investigation suggests that combined EA and ELISA testing would improve both the specificity and the PPV for the diagnosis of HSV-2 infection without any sacrifice in the sensitivity or the NPV of results.24
Although our investigation demonstrates that EA accurately identifies the presence of type-specific HSV-2 antibodies in expectant mothers of any gestational stage, it was not designed to comment on the most appropriate use of these tests in this population. While screening all pregnant women for HSV-2 infection is not universally endorsed 25, 26, some support has been given for serologic testing of pregnant women and their partners as a means to identify those at greatest risk for HSV-2 acquisition during pregnancy 27, 28. Another limitation of our study was its inability to delineate reasons for discordant EA and ELISA test results. Although women identified as EA seronegative/ ELISA seropositive tended to have lower median positive index values than women with concordant results, the exact proportion of women in the former group falsely identified as HSV-2 seropositive by ELISA is uncertain. Thus, determination of the validity of the sequential use of EA and ELISA for improved accuracy of HSV-2 serodiagnosis is limited by our inability to compare the results to an accepted gold standard such as WB. Of note, the Focus IB, our standard for performance calculations in this investigation, possesses similar performance characteristics as WB for the serodiagnosis of HSV-2.15 Stronger endorsement of the diagnostic algorithm introduced in this investigation is further limited by our inability to determine how a requirement for both finger stick (EA) and venipucture (ELISA) would affect its acceptance by patients. It will also be necessary to determine if the higher costs associated with the use of an additional serologic test are offset by the benefits associated with more accurate serodiagnosis of HSV-2 infection. Finally, HSV-2 serodiagnosis remains an important component of genital herpes management programs, so further research will be needed to evaluate the performance characteristics of any potential diagnostic algorithm and to better delineate the specific populations most likely to benefit from improved serologic testing.
Acknowledgements
B Leyland and MR Kennedy contributed equally to the work. Funding for this investigation was provided by Focus Diagnostics (Cypress, CA). The authors thank Leslie A. Meyn M.S., Harold C. Wiesenfeld, M.D., Glenn M. Updike, M.D., Wayne R. Hogrefe, Ph.D., Heather Pham, and Megan Burgess from Focus Diagnostics, the study participants, and the participating clinical and laboratory research teams.
Footnotes
Conflicts of Interest
None.
References
- 1.Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mertz GJ, Benedetti J, Ashley R, Selke S, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 5.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317:1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- 7.Brown ZA, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 8.Corey L, Handsfield HH. Genital herpes and public health: addressing a global problem. JAMA. 2000;283:791–794. doi: 10.1001/jama.283.6.791. [DOI] [PubMed] [Google Scholar]
- 9.Ashley R, Cent A, Maggs V, Nahmias A, Corey L. Inability of enzyme immunoassays to discriminate between infections with herpes simplex virus types 1 and 2. Ann Intern Med. 1991;115:520–526. doi: 10.7326/0003-4819-115-7-520. [DOI] [PubMed] [Google Scholar]
- 10.Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wutzler P, Doerr HW, Färber I, Eichhorn U, Helbig B, Sauerbrei A, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations-relevance for the incidence of genital herpes. J Med Virol. 2000;61:201–207. doi: 10.1002/(sici)1096-9071(200006)61:2<201::aid-jmv5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Prince HE, Ernst CE, Hogrefe WR. Evaluation of an enzyme immunoassay system for measuring herpes simplex virus (HSV) type 1-specific and HSV type 2-specific IgG antibodies. J Clin Lab Anal. 2000;14:13–16. doi: 10.1002/(SICI)1098-2825(2000)14:1<13::AID-JCLA3>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley RL, Eagleton M, Pfeiffer N. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J Clin Microbiol. 1999;37:1632–1633. doi: 10.1128/jcm.37.5.1632-1633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley RL. Sorting out the new HSV type specific antibody tests. Sex Transm Infect. 2001;77:232–237. doi: 10.1136/sti.77.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark HD, Nanda JP, Roberts J, Rompalo A, Melendez JH, Zenilman J. Performance of focus ELISA tests for HSV-1 and HSV-2 antibodies among university students with no history of genital herpes. Sex Transm Dis. 2007;34:681–685. doi: 10.1097/01.olq.0000258307.18831.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handsfield HH, Stone KM, Wasserheit JN. Prevention agenda for genital herpes. Sex Transm Dis. 1999;26:228–231. doi: 10.1097/00007435-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10:530–536. doi: 10.1111/j.1469-0691.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogrefe W, Su X, Song J, Ashley R, Kong L. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J Clin Microbiol. 2002;40:3635–3640. doi: 10.1128/JCM.40.10.3635-3640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow RA, Friedrich D, Meier A, Corey L. Use of “biokit HSV-2 Rapid Assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis. 2005;5:84–90. doi: 10.1186/1471-2334-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherpes TL, Wiesenfeld HC, Melan MA, Kant JA, Cosentino LA, Meyn LA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33:747–752. doi: 10.1097/01.olq.0000218869.52753.c7. [DOI] [PubMed] [Google Scholar]
- 22.Ribes JA, Hayes M, Smith A, Winters JL, Baker DJ. Comparative performance of herpes simplex virus type 2-specific serologic assays from Meridian Diagnostics and MRL diagnostics. J Clin Microbiol. 2001;39:3740–3742. doi: 10.1128/JCM.39.10.3740-3742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 24.Prober CG, Corey L, Brown ZA, et al. The management of pregnancies complicated by by genital infections with herpes simplex virus. Clin Infect Dis. 1992;15:1031–1038. doi: 10.1093/clind/15.6.1031. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:6–8. [Google Scholar]
- 26.Guerry SL, Bauer HM, Klausner JD, Branagan B, Kerndt PR, Allen BG, et al. Recommendations for the selective use of herpes simplex virus type 2 serological tests. Clin Infect Dis. 2005;40:38–45. doi: 10.1086/426438. [DOI] [PubMed] [Google Scholar]
- 27.Gardella C, Brown Z, Wald A, Selke S, Zeh J, Morrow RA, et al. Risk factors for herpes simplex virus transmission to pregnant women: a couples study. Am J Obstet Gynecol. 2005;193:1891–1899. doi: 10.1016/j.ajog.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay MK. HSV neutralizing antibodies further refinement in preventing neonatal herpes infection. Am J Obstet Gynecol. 2006;195:4–6. doi: 10.1016/j.ajog.2006.02.014. [DOI] [PubMed] [Google Scholar]