Abstract
Pemphigus foliaceus (PF) is an organ-specific autoimmune skin disease characterized by subcorneal epidermal cell detachment (acantholysis) and pathogenic autoantibodies against desmoglein 1. The mechanism responsible for pemphigus autoantibody-induced epidermal injury is not fully understood. In this study we used the IgG passive transfer mouse model of PF to investigate the relevance of the apoptotic mechanisms in pemphigus pathogenesis. TUNEL-positive epidermal cells and increased oligonucleosomes in the epidermal cytosolic fractions were detected in the diseased mice. Time course study reveals that TUNEL-positive epidermal cells appear prior to intraepidermal blisters. Moreover, the pro-apoptotic factor Bax was up-regulated at the earlier time points (2 and 4 h) while the anti-apoptotic factor Bcl-xl was down-regulated at the later time points (6, 8, and 20 h) post PF IgG injection by Western blot analysis. The active forms of caspase-3 and -6 were detected at the later time period (6, 8, and 20 h). Administration of Ac-DEVD-cmk, a peptide-based caspase-3/7 inhibitor, protected mice from developing intraepidermal blisters and clinical disease induced by PF IgG. The same protective effect was also observed using a broad-spectrum caspase inhibitor, Bok-D-fmk. Collectively, these findings show that biochemical events of apoptosis are provoked in the epidermis of mice injected with PF autoantibodies. Caspase activation may contribute to acantholytic blister formation in PF.
Keywords: skin, autoimmune, autoantibody, apoptosis
INTRODUCTION
Pemphigus foliaceus (PF) and pemphigus vulgaris (PV) are the two classical forms of pemphigus characterized by detachment of epidermal cells known as acantholysis and anti-epidermal autoantibodies (1, 2). Whereas PF displays intraepidermal blisters at the granular layer and autoantibodies to the desmosomal glycoproteins desmoglein 1 (Dsg1), PV exhibits intraepidermal blisters just above the basal cells and autoantibodies to Dsg3 (3, 4). The IgG fraction of the patients’ sera is pathogenic, as demonstrated by passive transfer experiments in neonatal mice (5, 6). Moreover, affinity-purified anti-Dsg1 and anti-Dsg3 IgG from patients’ sera are also pathogenic (7, 8). There are other autoantibodies detected in the sera of pemphigus patients, for example, autoantibodies against acetylcholine receptors (9) and E-cadherin (10). The pathogenic role of these autoantibodies remains to be determined.
The molecular mechanism responsible for the acantholysis induced by pemphigus autoantibodies has been a subject of intensive investigation in recent years. It has been generally believed that binding of pemphigus autoantibodies to the ectodomain of Dsg3 or Dsg1 impairs the adhesive function of these molecules thus causing acantholysis (11, 12). However, recent single-molecule atomic force microscopy studies show that in a cell-free system PF IgG does not inhibit homophilic trans-interaction of Dsg1 molecules by steric hindrance (13), but PV IgG directly inhibit Dsg3-mediated transinteraction (14). Cell culture studies has shown that following the binding of PV autoantibodies to the cell surface, the antigen-antibody complex is internalized into cytoplasmic vesicles and subsequently fused with lysosomes (15, 16). The autoantibody-mediated Dsg3 or Dag1 internalization and depletion from the cell surface is accompanied by impaired desmosomal assembly/disassembly and decreased cell adhesiveness (16–21).
Besides the two possible mechanisms-the steric hindrance and desmoglein depletion from the cell surface, an increased body of evidence has demonstrated that pemphigus autoantibodies are able to trigger intracellular events that may indirectly lead to acantholysis by yet unclear mechanisms (22–30). Additionally, it has been hypothesized that keratinocyte apoptosis, in response to binding of pemphigus autoantibodies, results in pemphigus acantholysis (31–35). In vitro cell culture studies have shown that PV IgG or serum induces apoptosis (31, 32, 34–36). The pro-apoptotic changes are evident by various measurements, including annexin V binding, Hoechst 33342 staining, TUNEL labeling, DNA laddering, oligonucleosome formation, caspase activation, up-regulation of pro-apoptotic proteins (Fas, FasL, Bax, p53), and down-regulation of anti-apoptotic proteins such as Bcl-2 and FLIP-l. Since most cell types are more susceptible to apoptosis in vitro than in vivo, demonstration of epidermal cell apoptotic response to pemphigus autoantibodies in an in vivo model is required to establish the pathogenic relevance of these mechanisms. In this study, using the passive transfer mouse model of PF, we provide the first evidence that a pro-apoptotic response in the epidermal cells occurs during the development of experimental PF. Moreover, we show that administration of caspase inhibitors abolishes PF IgG-induced intraepidermal blisters and clinical disease in mice.
MATERIAL AND METHODS
Pemphigus IgG preparation
Sera from two patients with classic clinical and histological features of PF were used for IgG preparation. The two sera contain IgG autoantibodies against the epidermal intercellular substance, showing the indirect immunofluorescense titer of 1:320 for PF1 and 1:640 for PF2. Both sera contained IgG autoantibodies to Dsg1 as determined by immunoprecipitation and ELISA. A normal donor serum with a negative anti-epidermal autoantibody titer was included as a control. The IgG fraction was prepared from the sera by ammonium sulfate precipitation followed by extensive dialysis.
Animal
Breeding pairs of BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained at the Division of Laboratory of Animal Medicine Facility, University of North Carolina at Chapel Hill (UNC-CH). Neonatal BALB/c mice (1 to 2 days old with body weight between 1.4 to 1.6 g) were used for IgG passive transfer experiments. Animal care and animal experiments were approved by the Institutional Animal Care and Use Committee at UNC-CH and were in accordance with NIH guidelines.
Induction of experimental PF
Mouse models of PF were induced by IgG passive transfer experiments using a modified protocol originally described (5, 6). The IgG isolated from PF1 and PF2 sera were used individually for disease induction. Briefly, various doses of PF IgG in a total volume of 50 µl were administrated to neonatal mice by a single subcutaneous (s.c) injection in the dorsal area. Twenty hours after IgG injection, the extent of skin disease in the dorsal area of the animals was evaluated and scored on a scale of 0 to 3+ as described previously (37). IgG from PF1 and PF2 sera induced the same types of blisters clinically and histologically. The minimal dose of PF1 or PF2 IgG that produced skin lesions equal or larger than 2+ was used for the rest of the experiments. For time course studies, animals (two animals per time point) were sacrificed at various time points (up to 24 h) post IgG injection. Skin samples from the dorsal area of each animal were harvested for H&E staining, TUNEL assay, and Western blotting.
Inhibitor administration
Peptide-based caspase inhibitors Ac-Asp-Glue-Val-Asp-Ch2Cl (Ac-DEVD-cmk) and Boc-Asp(OMe)-CH2F (Boc-D-fmk), purchased from Calbiochem (San Diego, CA), were dissolved in dimethylsulfoxide and diluted in TBS-Ca++ buffer before use. The inhibitor (0.034–6.8 µg/g body weight) or vehicle in 50 µl total volume was administrated to mice by two subcutaneous injections. The first was given 1.5 h before the IgG injection and the second was delivered concomitantly with IgG injection. The same dose of pathogenic PF IgG was used for comparison of the effects of the inhibitor and the vehicle treatment.
Terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (TUNEL) assay
The experimental mouse skin samples were fixed in 10% buffered formalin and embedded in paraffin. Paraffin sections were subjected to TUNEL assay using the PopDETEK Green kit (Enzo Life Sciences, Farmingdale, NY) according to the manufacture’s instructions. Briefly, following deparaffinization and rehydration, sections were treated with proteinase K and incubated with reaction mixture containing terminal deoxynucleotidyl transferase and fluorescence-conjugated dUTP for 1 h at 37 °C. The labeled DNA was examined under a fluorescence microscope.
ELISA-based nuclearsome assay
Epidermal sheets were obtained from mouse skin pieces and used for the nucleosome detection assay using an ELISA kit from Roche Molecular Biochemicals (Mannheim, Germany) according to the manufacture’s protocol.
Western blotting
Mouse skin proteins were extracted by homogenization. RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, and 0.1% SDS ) containing 2 mM PMSF and proteinase inhibitor cocktail (Sigma) was used for Bax, Bcl-xl, and caspase expression. SDS buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, and 10% Glycerol) was used for detection of cleaved acinus. Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Blots were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 for 1 hour. Blots were then incubated with primary antibodies overnight at 4 °C followed by incubation with appropriate secondary antibodies conjugated with horseradish peroxidase. Chemiluminescene was performed with Supersignal reagents (Pierce Biotechnology, Rockford, IL). The primary antibodies used include antibodies to Bax, Bcl-xl, cleaved caspase-3 and caspase-6 (Cell Signaling Technology), and to cleaved acinus (Santa Cruz).
Statistics
The data were expressed as mean ± SD and were analyzed using the Student’s t test. A p< 0.05 was considered statistically significant.
RESULTS
PF IgG administrated in vivo induced DNA fragmentation in the epidermal cells
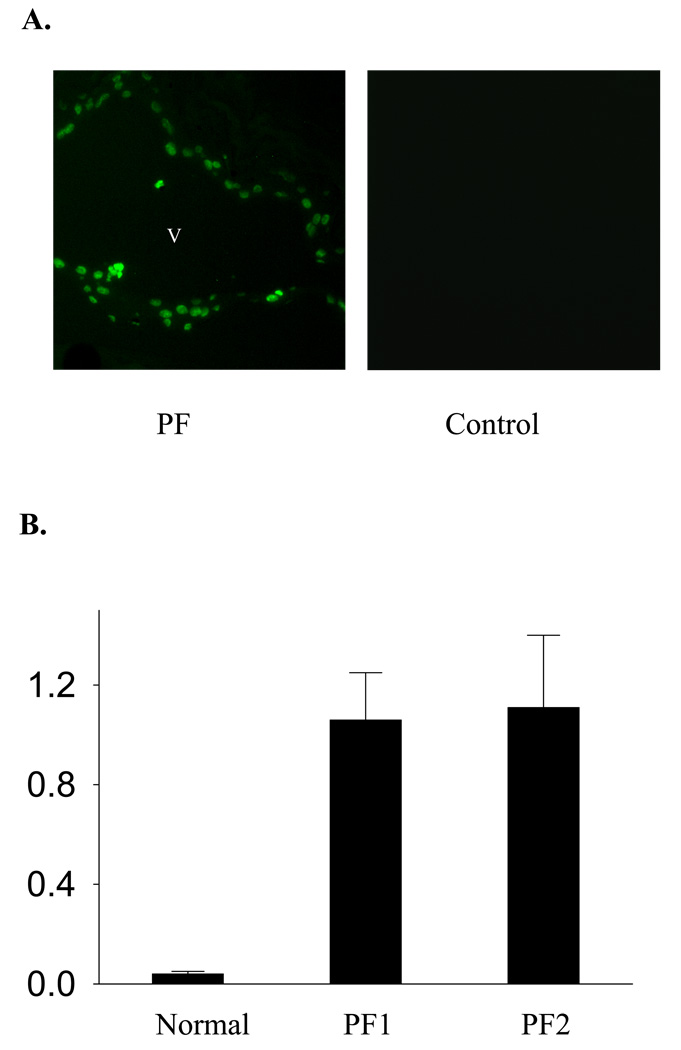
To evaluate whether PF autoantibodies are able to induce an apoptotic response in vivo, we first conducted the IgG passive transfer experiments and then examined the epidermal cell DNA fragmentation by TUNEL staining. Twenty hours after IgG injection, TUNEL-positive epidermal cells were detected along the roof and the floor of the intraepidermal vesicle in the skin sections from mice (n=6) injected with PF IgG isolated from PF1 or PF2 sera, but not from control mice injected with normal human IgG (n=3). Representative results are shown in Figure 1A To verify the induction of DNA fragmentation by PF IgG, we further examined the presence of oligonucleosomes in the cytoplasmic fractions of lysates from epidermis of mice injected with IgG using an ELISA-based assay. We found that PF IgG significantly increased the oligonucleosomes in these fractions, compared with the lysates of epidermis of mice injected with normal human IgG (Figure 1B).
Figure 1. Induction of DNA fragmentation in the mouse epidermal cells following PF IgG passive transfer.
(A) TUNEL staining. Neonatal mice were injected (s.c.) with pathogenic PF or normal human IgG. Skin samples were obtained 20 h post IgG injections and subjected to TUNEL staining. Positive TUNEL labeling was revealed in the epidermis from mice (n=6) injected with IgG from PF1 or PF2, but not from control mice (n=3) injected with the normal human IgG. Representative results are shown. v: vesicle. (B) Oligonucleosome releasing assay. DNA oligonucleosomes presented in the cytoplasmic fractions of mouse epidermal sheets obtained from control mice (n=2) and PF mice (n=2) were analyzed with an ELISA-based assay for presence of the histone-DNA complex.
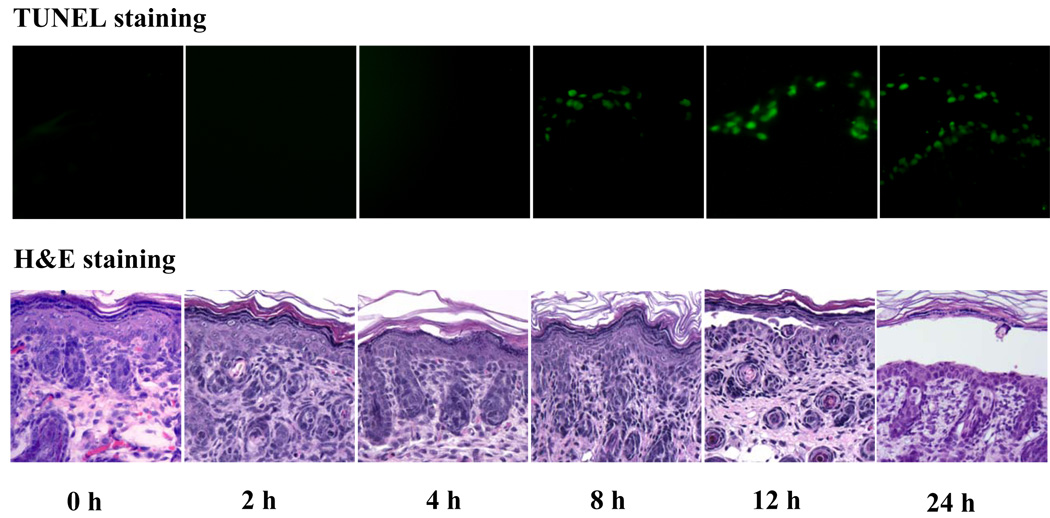
We then performed time course studies to determine the temporal relationship between the appearance of TUNEL-positive cells and the onset of histological blisters induced by PF IgG. PF1 IgG was injected into mice subcutaneously. At various time points post IgG injection, mice (n=2, each time point) were terminated. Skin specimens from the injected area of each animal were taken for TUNEL and histological examination. Figure 2 shows the representative results of TUNEL and H&E staining on the same set of mouse skin specimens obtained at various time points following IgG injections. TUNEL-positive epidermal cells were first detected at 8 h post PF IgG injection, whereas initial histological blisters appeared at 12 h. The number of TUNEL-positive cells increased at 12 h and remained steadily at 24 h, when clinical disease was fully developed. Consistent results were observed on the other set of samples from the duplicated mice. In addition, the experiment (n=2, each time point) was repeated using the second patient IgG. Similarly, IgG from PF2 also induced positive TUNEL epidermal cells from 8 h afterward and limited histological blisters at 12 h and extensive blisters at 24 h (data not shown). These results demonstrate that the appearance of TUNEL-positive epidermal cells precedes the onset of histological blisters.
Figure 2. Temporal relationship between TUNEL-positive cells and histological blisters.
Neonatal mice (n=2, each time point) were injected (s.c.) with pathogenic PF1 IgG and terminated at various time points post IgG injection. Skin specimens were obtained and subjected to TUNEL labeling and H&E staining. As shown in these representative results, TUNEL-positive cells were first detected at 8 h, whereas initial histological cleft was revealed at 12 h post IgG injection. Similar temporal relationship between TUNEL-positive cells and histological blisters was also observed using IgG isolated from PF2 serum.
Up-and down-regulation of apoptotic regulators
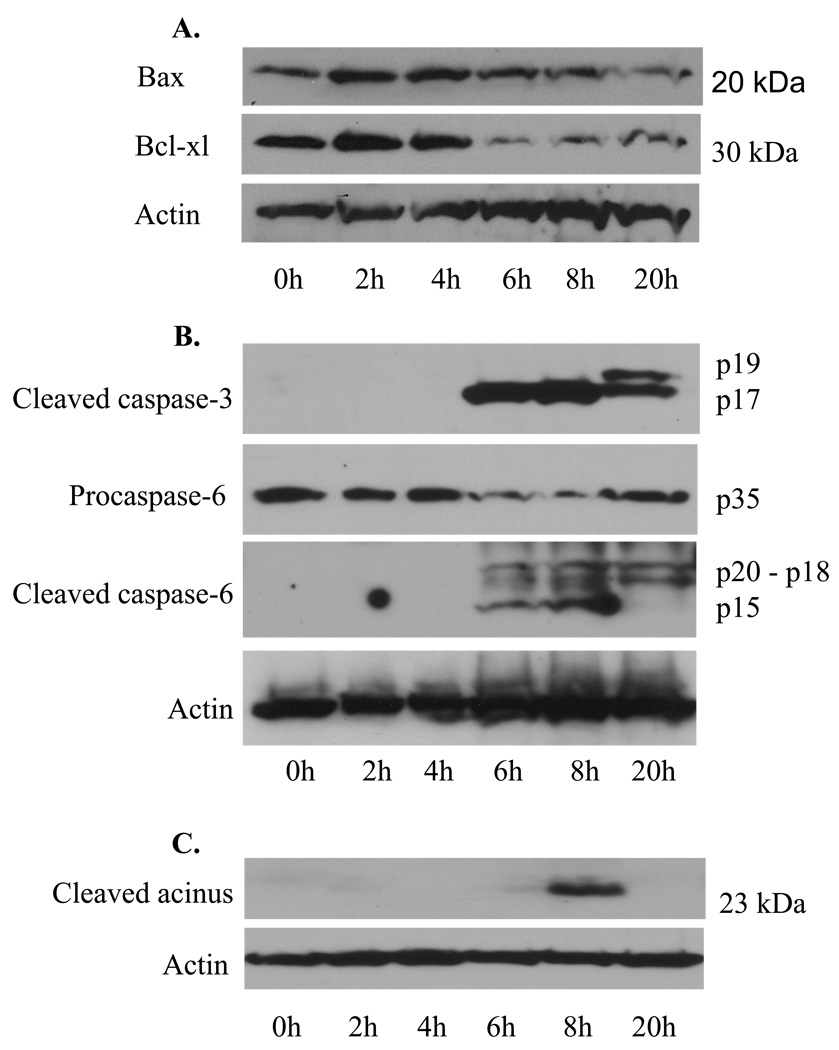
We next performed Western blot analysis to examine the expression of the pro-apoptotic factor Bax and the anti-apoptotic factor Bcl-xl in the skin extracts of mice injected with PF1 IgG. We found that administration of PF1 IgG slightly up-regulated the expression of Bax at 2 and 4 h and remarkably down-regulated the expression of Bcl-xl at 6, 8 and 20 h (Figure 3A). These findings further support that a pro-apoptotic response is provoked in the skin cells of mice injected with PF1 IgG.
Figure 3. Expression of apoptotic mediators during the development of experimental PF.
At various time points post PF1 IgG injection, mouse skin specimens were harvested, pooled (n=2, each time point), and extracted. The extracts were subjected to Western blot analysis using a polyclonal antibody for Bax, a monoclonal antibody to Bcl-xl (A), a monoclonal antibody specific for the cleaved caspase-3, a polyclonal antibody for cspase-6 (cleaved and procaspase-6) (B), or a polyclonal antibody against the cleaved acinus (23 kDa) (C). β-actin was detected for protein loading controls. As shown, the level of pro-apoptotic factor Bax was slightly increased at 2 and 4 h, whereas the anti-apoptotic factor Bcl-xl expression was reduced markedly at 6, 8, and 20 h. The cleaved (activated) of caspase-3 and -6 were detected by 6, 8, and 20 h after IgG injection. Acinus cleavage was revealed transiently at 8 h.
Expression of the active form of caspase-3 and –6 and cleavage of caspase-3 substrate acinus
We further evaluated the activation of caspase-3 and -6 during the development of epidermal blisters in neonatal mice injected with PF1 IgG. As shown in the top panel of Figure 3B, a monoclonal antibody specific for the cleaved caspase-3 detected the processed active form of caspase-3 (p17) in the mouse skin extracts at time points 6, 8 and 20 h post PF IgG injection. At 20 h, two bands (p17 and p19) with reduced intensity were detected, indicating that caspase-3 was not processed efficiently at this late time point. When the blot was reprobed with a polyclonal antibody that recognizes both the unprocessed (inactive) and processed (active) caspase-6, the unprocessed caspase-6 (35 kDa) was detected throughout the time points, but the intensity level was dramatically reduced at 6 and 8 h and moderately reduced at 20 h (Figure 3B, the second panel from the top). The fully processed caspase-6 (p15) was detected at 6 and 8 h (Figure 3B, the third panel from the top). Additional bands (p18 and p20) were observed at 6 and 8, and 20 h, which are likely the products of partially processed caspase-6. In addition, we also detected the cleaved acinus (23 kDa), a caspase-3 substrate, at 8 h post PF1 IgG injection (Figure 3C).
Administration of caspase inhibitors protects mice from developing PF
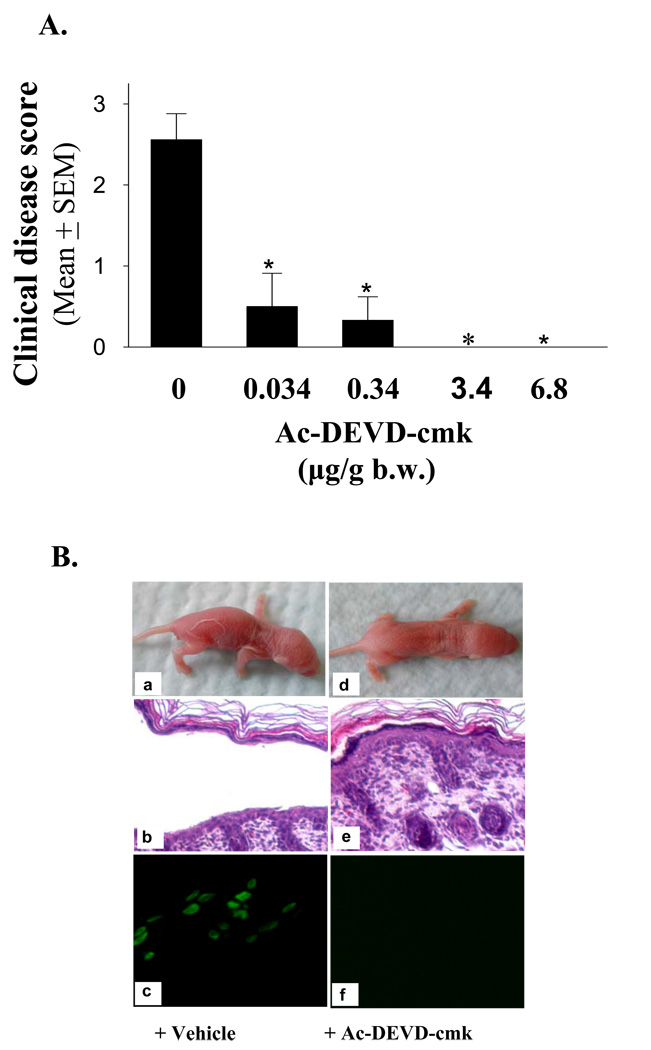
To test the role of caspase-3 in PF IgG pathogenicity, we further evaluated whether caspase inhibitors would have beneficial effect on PF model mice. We first assessed the effect of various doses of Ac-DEVD-cmk, a cell-permeable and irreversible inhibitor for caspase-3/7, on PF1 IgG-induced disease. Control mice that received the vehicle developed skin lesions 20 h post PF1 IgG injection (Figure 4A, and 4B panel a). In sharp contrast, mice that received the inhibitor were protected from disease in a dose-dependent manner, in a range of 0.034 to 3.4 µg per gram body weight (Figure 4A). When the dose was equal or larger than 3.4 µg/g body weight, Ac-DEVD-cmk blocked disease completely (Figure 4A, also see Table I). Figure 4B is the representative results derived from mice treated with the vehicle alone (left panel) or Ac-DEVD-cmk (6.8 ug/g body weight) (right panel). Twenty hours post PF1 IgG injection, mice treated with the vehicle revealed skin lesions and intraepidermal cleft at the upper layer (Figure 4B, panels a and b), mimicking the human disease. In contrast, the same dose of PF1 IgG failed to induce clinical or histological blisters in mice that received Ac-DEVD-cmk (Figure 4B, panels d and e). TUNEL assay revealed a positive staining on the epidermis from mice treated with the vehicle (Figure 2B, panel c), but a negative staining from mice treated with Ac-DEVDcmk (Figure 4B, panel f).
Figure 4. The caspase-3/7 inhibitor Ac-DEVD-cmk protects mice against PF.
(A) Dose-dependent protective effects. Various doses of caspase-3/7 inhibitor Ac-DEVD-cmk were administrated into neonatal mice by two subcutaneous injections. Half of the dose was given 1.5 h before IgG injection, and the second half dose was injected concomitantly with PF1 IgG. Control mice were injected with vehicle. The same dose of pathogenic PF1 IgG was used for disease induction in each experiment. The extent of disease was examined and scored 20 h post IgG injection. The protective effect is highly significant (n=8 for the control group, n=3 for each treatment group, *p<0.001, Student t-test). (B) Suppression of TUNEL-staining and blockade of intraepidermal blisters and clinical disease. Neonatal mice were injected with pathogenic PF1 IgG with (d–f) or without (a–c) Ac-DEVD-cmk. Control mice treated with vehicle developed clinical and histological blisters (a and c) 20 hours post IgG injection. In contrast, mice treated with the caspase-3 inhibitor did not develop clinical and histological lesions (d and f). TUNEL assay showed a positive staining in control mice but not in mice treated with the inhibitor (e).
Table I.
Blockade of experimental PF by the caspase inhibitors*
| IgG injected | Treatment | # of mice | Disease Score (Mean ± SD) |
|---|---|---|---|
| PF1 | Vehicle | 8 | 2.5 ± 0.3 |
| Ac-DEVD-cmk | 6 | 0 | |
| Boc-D-fmk | 3 | 0 | |
| PF2 | Vehicle | 6 | 2.3 ± 0.3 |
| Ac-DEVD-cmk | 3 | 0 | |
| Boc-D-fmk | 3 | 0 |
Neonatal mice were preinjected (s.c.) with the caspase inhibitor or control vehicle. One and a half hour later, mice were injected (s.c.) with the pathogenic IgG prepared from two PF patients (PF1 and PF2) along with the inhibitor or vehicl. The total doses for Ac-DEVD-cmk were 3.4 or 6.8 µg/g b.w. and for Boc-D-fmk was 6.8 µg/g b.w. Injected animals were examined clinically 20 h after IgG injection, and clinical disease was scored. There is a significant difference in clinical disease scores between mice treated with vehicle and mice treated with Ac-DEVD-cmk or Boc-D-fmk (p < 0.0001, Student’s t test).
The effect of Ac-DEVD-cmk on skin blistering was also tested on mice induced by IgG from PF2 serum. The same beneficial effect was observed (Table I). In addition to Ac-DEVD-cmk, we also tested the effect of Boc-D-fmk, a broad-spectrum caspase inhibitor on experimental PF. Boc-D-fmk (6.8 ug/g body weight) exhibited a similar protective effect against PF IgG-induced histological blister and clinical disease (Table I).
DISCUSSION
The aim of this study was to investigate the relevance of the apoptotic mechanism in cell/tissue injury in PF, an organ-specific autoantibody-mediated autoimmune blistering disease. Our study provides five lines of evidence that pathogenic PF IgG is able to provoke the biochemical response of apoptosis in epidermis of the mouse model. As a consequence, caspases are activated, which contribute to the development of acantholytic blisters and disease pathogenesis.
First, we demonstrated that administration of PF IgG to neonatal mice triggered DNA fragmentation of lesional epidermal cells as demonstrated by two techniques, the TUNEL assay and the nucleosome release assay. The former labels the free 3’-OH ends of the DNA strands resulting from DNA breaks, and the latter measures the amount of oligonucleosomes released in the cytosolic fraction of the epidermal cells. TUNEL-positive epidermal cells were detected on the roof and floor of the intraepidermal blisters of the PF mice (Figure 1A). Consistent with the TUNEL result, the nucleosome release assay also reveals increased epidermal DNA fragmentation in lysates of the lesional skin of animals injected with PF IgG (Figure 1B). These results are not only in line with previous findings in lesional epidermis of PF and PV patients (33–35, 38), but also demonstrate a cause–effect relationship between the injected PF autoantibodies and DNA fragmentation. Together, the data generated in the mouse model and the previous observations in patients strongly suggest that apoptotic DNA fragmentation is associated with the ongoing acantholysis induced by PF IgG.
Second, we observed that the appearance of TUNEL-positive epidermal cells precedes the onset of intraepidermal blisters in neonatal mice injected with PF IgG (Figures 2). The time course study of the model mice post PF IgG injections revealed that the TUNEL-positive epidermal cells first appeared at 8 h, whereas the initial histological blisters occurred at 12 h. This observation argues against the possibility that the apoptotic response to PF autoantibodies is the result of the intraepidermal blistering. It has been previously reported that TUNEL-positive epidermal cells are present not only in lesional epidermis but also in perilesional or apparently normal epidermis of patients with PF and PV, suggesting that apoptosis may precede the onset of acantholysis (33–35, 38). The current time course study provides direct evidence that the occurrence of apoptotic DNA fragmentation of epidermal cells precedes intraepidermal blister formation and clinical disease.
Third, we detected up- and down- expression of Bax and Bcl-xl, two Bcl-2 family members exhibiting opposite effects in regulating apoptotic cell death via the mitochondrial pathway (39). Western blot analysis of skin lysates of PF IgG-injected animals revealed an increased amount of the pro-apoptotic factor Bax at earlier time points (2 and 4 h) and a decreased level of the anti-apoptotic factor Bcl-xl at later time periods (6, 8 and 20 h) (Figure 3A). These results indicate that the mitochondrial pathway of apoptosis might be evoked in the epidermal cells by PF IgG.
Fourth, we detected the activated form of two effector caspases, caspase-3 and caspase-6, by Western blotting of skin lysates from mice injected with PF IgG. Both caspase-3 and -6 were activated at later time points (6, 8, and 20 h) post PF IgG injection (Figure 3B). The experiment was repeated using a second IgG from PF2, and similar time-dependent expression of the cleaved caspase 3 was also observed (data not shown). Further, cleaved caspase 3 was not detected in the skin lysate of the mice that were injected with normal human IgG (data not shown). To further verify the activation of caspase-3, we examined the cleavage of the protein acinus, which is a substrate for caspase-3 and functions in inducing chromatin condensation (40). We found that the activated form of acinus (23-kDa) was transiently expressed in mouse skin following the injection of PF IgG (Figure 3C). However, we did not detect the 85-kDa cleaved fragment of poly-(ADP-ribose) polymerase (PARP), another well-known substrate for caspase-3 (data not shown). Failure to detect the cleaved PARP fragment may indicate that the level of caspase-3 activity in the epidermis of mice injected with PF IgG is low or limited. Caspase-3 activity has been detected in cultured keratinocytes exposed to PV IgG (31, 35, 41). Interestingly, Frusic-Zlotkin et al. (41) found that the induced caspase-3 activity level roughly correlated with the IF titers of the PV sera utilized. The result of the present study demonstrates for the first time that caspase-3, as well as caspase-6, is activated in vivo before the onset of intraepidermal blistering.
Finally, another novel observation reported in this study demonstrates that two caspase inhibitors were able to block the PF blistering in mice. Administration of the caspase-3/7 selective inhibitor Ac-DEVD-cmk abrogated DNA fragmentation and protected mice from developing intraepidermal blisters and clinical phenotype (Figure 4B). The inhibitor was effective in a dose-dependent manner (Figure 4A). Moreover, the pan-caspase inhibitor Boc-D-fmk was also effective in preventing skin disease in neonatal mice induced by PF IgG (Table 1). The protective effect of these inhibitors against PF IgG-induced skin blistering is more likely a local effect since the inhibitors were delivered into the animals by local (s.c.) injections and the skin blistering was examined only in the dorsal area where IgG was injected. Although it is also possible that a small fraction of the injected inhibitors may diffuse into the intravascular compartment, enter into the general circulation, and thus have some systemic effect.
It has been reported that many peptide-based caspase inhibitors, such as z-VAD-fmk, z-DEVD-fmk, and z-YVAD-fmk, also inhibit papain-like cathepsins at a higher concentration (100 µM) commonly used for cell-based studies (42, 43). However, the non-specific effects are only observed in the fluromethylketone (-fmk) modified inhibitors, but not in the chloromethylketon (-cmk) or aldehyde (-cho) modified inhibitors (42, 43). Indeed, z-DEVD-cmk and z-DEVD-cho do not exhibit any inhibitory effect on cathepsin activity at the high concentration of 100 µM when tested in cells (43). The finding in this study that local injection of Ac-DEVD-cmk blocked experimental PF at the low-micro molar concentration range (5 µg/50 µl, < 0.2 µM) indicates that the possible non-specific effect of this inhibitor on papain-like cathepsins is negligible, if exists. Taken together, the protective effect of the caspase inhibitors on PF strongly suggest that caspase activation is critically involved in the formation of acantholytic blisters and subsequent clinical disease of PF.
Collectively, the results of this study suggest that PF IgG induces a pro-apoptotic response in epidermal cells of the neonatal mice. As a consequence, executioner caspases are activated and contributes to intraepidermal blistering. The observation that the cleaved caspase-3 and -6 are detected at late time points (6 h and afterward) indicates that activation of these executioner caspases is a downstream event proximal to the onset of histological blistering of PF. Possible upstream apoptotic pathways may include the mitochondrial pathway as well as the death receptor pathway. The former possibility is supported by the observation that Bax is upregulated at 2 h post PF IgG injection. Activation of the death receptor pathway is also possible as increased expression of Fas, Fas ligand and activated initiator caspase-8 in this pathway have been detected in lesional skin biopsy of PV patients and in cultured keratinocytes exposed to PV IgG (34, 35, 41). In addition, it has been found that two signaling events, the p38 MAPK activation and the RhoA inactivation, are involved in acantholysis induced by PF IgG as well as PV IgG (26, 28). The pathogenic role of these two signaling pathways in PF and PV are demonstrated by blocking skin blistering using pharmacologic inhibitors of p38 MAPK (22) or activator of RhoA (26). While the relationship of the RhoA inactivation to the apoptotic pathway is not clear, p38 MAPK activation is known to be an upstream event of keratinocyte apoptosis (44). Indeed, s.c. injection of a p38 MAPK inhibitor prevents PF IgG-induced keratinocyte apoptosis in mice (David Rubenstein, personal communication), indicating that apoptosis is one of the downstream events following p38 MAPK activation in PF mouse model.
An intriguing observation in this study is that although we detected the key biochemical hallmarks of apoptosis (caspase-3 activation and DNA fragmentation), the morphological characteristics of apoptosis are not obvious by H&E examination of the epidermis of mice injected with pathogenic PF IgG. This observation may suggest that activation of caspases leads to epidermal cell injury and dysfunction, which in turn is manifested as epidermal cell detachment and blister formation. Acantholytic blisters may occur before or without the endpoint of epidermal cell death. Caspase-3 activation without cell death has been reported in several physiological processes such as terminal differentiation of various cell types, proliferation of resting peripheral T lymphocytes, and inhibition of the cell cycle in peripheral B cells (45). It is unclear how cells are able to survive with an activated “killer” caspase. It has been suggested that compartmentalized or low magnitude activation of caspase-3, or activation of pro-survival factors may account for this enigma. Regardless of the possible explanation, we hypothesize that the contribution of activated executioner caspases to the process of epidermal cell detachment is through proteolytic cleavage of structural proteins involved in epidermal cell-cell adhesion. It should be noted that acantholysis is a process of the loss of cell-cell cohesion, and the formation of intraepidermal cleft is a result of this process. Therefore, although our time course study clearly shows that the activation of caspase-3 and -6 is prior and close to the onset of visible intraepidermal cleavage, it does not exclude the possibility that the acantholytic process has not started before executioner caspases are activated. It is probable that weakened intercellular adhesion and some extent of acantholysis already occurred at early course. Activation of executioner caspases may be one of the later events that aggravate this process by proteolytic cleavage of structural proteins involving the epidermal cell-cell junctions. Possible candidates may include components of the desmosomes, adherens junctions, and cytoskeletons. Many cell adhesion molecules are caspase substrates (46) or are degraded during apoptosis, such as Dsg1 (47, 48), Dsg3, plakoglobin, plakophillin, plakin proteins (49–51), E-cadherin, P-cadherin and β-catenin (46). Interestingly, it has been shown that shedding of the ectodomain of Dsg1 or Dsg3 during the process of apoptosis is inhibited by caspase inhibitors (47, 51).
Apoptotic epidermal keratinocytes have been found in cutaneous diseases such as lichen planus, atopic dermatitis, and toxic epidermal necrolysis. Excessive apoptosis of keratinocytes at basal or suprabasal layers of the epidermis is thought to account for the characteristic histological features found in lichennoid tissue reaction, which is characterized by the presence of colloid bodies and basal cell damage (52), and in atopic dermatitis characterized by suprabasal intercellular edema (spongiosis) and vesicle formation (53). Massive apoptotic cell death is recognized as a key mechanism involved in the pathogenesis of toxic epidermal necrolysis (54, 55), in which the epidermis is detached from the dermis due to the loss of adhesion between the basal cell and its underlying extracellular matrix. Apparently, the apoptotic mechanism is only part of the complex pathogenic mechanisms underlying these diseases, and apoptosis alone may not explain the distinct histological presentations of these diseases. Other factors may also account for the different forms of epidermal injury caused by keratinocyte apoptosis. These may include the trigger of apoptosis, the magnitude of apoptotic response, the other pathways that are activated concurrently, and the unique feature of the target layer of the epidermis (basal, spinous, or granular keratinocytes).
In summary, the finding of this mouse model study suggests that the biochemical pathway of apoptosis is involved in the epidermal cell/tissue injury of PF, an organ-specific autoimmune disease. Activated caspases may be one of the final events that contribute to acantholytic blistering. Further studies are required to identify the potential targets for caspases that may be cleaved during PF blistering.
Acknowledgments
Grant support: This work was supported in part by US Public Health Service National Institutes of Health (NIH) Grants AR053313, AR052109 awarded to N. Li., AI40768 and AI61430 to Z Liu, and AR30281, AR32599 awarded to L.A. Diaz
Abbreviations
- Dsg
desmoglein
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
- IF
immunofluorescence
- s.c.
subcutaneous
Footnotes
Part of the data has been presented at the 67th annual meeting of Society for Investigative Dermatology held in Philadelphia, PA (May 3–6, 2006). Li N, Zhao M, Liu Z, Diaz LA (2006). Pathogenic role of apoptosis in experimental pemphigus. J Invest Dermatol 126: 29 (Abstract 173)
Disclosures: The authors state no conflict of interest.
Publisher's Disclaimer: DISCLAIMER
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
REFERENCES
- 1.Anhalt GJ, Diaz LA. Prospects for autoimmune disease: Research advances in pemphigus. JAMA. 2001;285:652–654. doi: 10.1001/jama.285.5.652. [DOI] [PubMed] [Google Scholar]
- 2.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 3.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 4.Stanley JR, Koulu L, Thivolet C. Distinction between epidermal antigens binding pemphigus vulgaris and pemphigus foliaceus autoantibodies. J Clin Invest. 1984;74:313–320. doi: 10.1172/JCI111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 6.Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, Patel H, Anhalt GJ. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985;85:538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- 7.Amagai M, Hashimoto T, Green KJ, Shimizu N, Nishikawa T. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895–901. doi: 10.1111/1523-1747.ep12606168. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen V, Lee TX, Ndoye A, Shultz LD, Pittelkow MR, Dahl MV, Lynch PJ, Grando SA. The pathophysiological significance of nondesmoglein targets of pemphigus autoimmunity. Development of antibodies against keratinocyte cholinergic receptors in patients with pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol. 1998;134:971–980. doi: 10.1001/archderm.134.8.971. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista F, Dasher DA, Diaz LA, Prisayanh PS, Li N. E-cadherin is an additional immunological target for pemphigus autoantibodies. J Invest Dermatol. 2008;128:1710–1718. doi: 10.1038/sj.jid.5701260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz LA, Marcelo CL. Pemphigoid and pemphigus antigens in cultured epidermal cells. Br J Dermatol. 1978;98:631–637. doi: 10.1111/j.1365-2133.1978.tb03581.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci. 2007;48:1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heupel WM, Zillikens D, Drenckhahn D, Waschke J. Pemphigus vulgaris IgG directly inhibit desmoglein 3-mediated transinteraction. J Immunol. 2008;181:1825–1834. doi: 10.4049/jimmunol.181.3.1825. [DOI] [PubMed] [Google Scholar]
- 15.Patel HP, Diaz LA, Anhalt GJ, Labib RS, Takahashi Y. Demonstration of pemphigus antibodies on the cell surface of murine epidermal cell monolayers and their internalization. J Invest Dermatol. 1984;83:409–415. doi: 10.1111/1523-1747.ep12273480. [DOI] [PubMed] [Google Scholar]
- 16.Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama Y, Owada MK, Kitajima Y. A pathogenic autoantibody, pemphigus vulgaris-IgG, induces phosphorylation of desmoglein 3, and its dissociation from plakoglobin in cultured keratinocytes. Eur J Immunol. 1999;29:2233–2240. doi: 10.1002/(SICI)1521-4141(199907)29:07<2233::AID-IMMU2233>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Cirillo N, Femiano F, Gombos F, Lanza A. Serum from pemphigus vulgaris reduces desmoglein 3 half-life and perturbs its de novo assembly to desmosomal sites in cultured keratinocytes. FEBS Lett. 2006;580:3276–3281. doi: 10.1016/j.febslet.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 19.Cirillo N, Gombos F, Lanza A. Changes in desmoglein 1 expression and subcellular localization in cultured keratinocytes subjected to anti-desmoglein 1 pemphigus autoimmunity. J Cell Physiol. 2007;210:411–416. doi: 10.1002/jcp.20856. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Aoyama Y, Shu E, Tsunoda K, Amagai M, Kitajima Y. Anti-desmoglein 3 (Dsg3) monoclonal antibodies deplete desmosomes of Dsg3 and differ in their Dsg3-depleting activities related to pathogenicity. J Biol Chem. 2007;282:17866–17876. doi: 10.1074/jbc.M607963200. [DOI] [PubMed] [Google Scholar]
- 21.Delva E, Jennings JM, Calkins CC, Kottke MD, Faundez V, Kowalczyk AP. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J Biol Chem. 2008;283:18303–18313. doi: 10.1074/jbc.M710046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci U S A. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanza A, Cirillo N, Rossiello R, Rienzo M, Cutillo L, Casamassimi A, de Nigris F, Schiano C, Rossiello L, Femiano F, Gombos F, Napoli C. Evidence of key role of Cdk2 overexpression in pemphigus vulgaris. J Biol Chem. 2008;283:8736–8745. doi: 10.1074/jbc.M702186200. [DOI] [PubMed] [Google Scholar]
- 24.Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem. 2007;282:13804–13812. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- 25.Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, Bolli R, Hunziker T, Suter MM, Muller EJ. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frusic-Zlotkin M, Raichenberg D, Wang X, David M, Michel B, Milner Y. Apoptotic mechanism in pemphigus autoimmunoglobulins-induced acantholysis--possible involvement of the EGF receptor. Autoimmunity. 2006;39:563–575. doi: 10.1080/08916930600971836. [DOI] [PubMed] [Google Scholar]
- 28.Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 29.Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, Muller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol. 2001;153:823–834. doi: 10.1083/jcb.153.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seishima M, Iwasaki-Bessho Y, Itoh Y, Nozawa Y, Amagai M, Kitajima Y. Phosphatidylcholine-specific phospholipase C, but not phospholipase D, is involved in pemphigus IgG-induced signal transduction. Arch Dermatol Res. 1999;291:606–613. doi: 10.1007/s004030050462. [DOI] [PubMed] [Google Scholar]
- 31.Arredondo J, Chernyavsky AI, Karaouni A, Grando SA. Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in Pemphigus. Am J Pathol. 2005;167:1531–1544. doi: 10.1016/S0002-9440(10)61239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baroni A, Buommino E, Paoletti I, Orlando M, Ruocco E, Ruocco V. Pemphigus serum and captopril induce heat shock protein 70 and inducible nitric oxide synthase overexpression, triggering apoptosis in human keratinocytes. Br J Dermatol. 2004;150:1070–1080. doi: 10.1111/j.1365-2133.2004.05919.x. [DOI] [PubMed] [Google Scholar]
- 33.Gniadecki R, Jemec GB, Thomsen BM, Hansen M. Relationship between keratinocyte adhesion and death: anoikis in acantholytic diseases. Arch Dermatol Res. 1998;290:528–532. doi: 10.1007/s004030050347. [DOI] [PubMed] [Google Scholar]
- 34.Puviani M, Marconi A, Cozzani E, Pincelli C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J Invest Dermatol. 2003;120:164–167. doi: 10.1046/j.1523-1747.2003.12014.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Bregegere F, Frusic-Zlotkin M, Feinmesser M, Michel B, Milner Y. Possible apoptotic mechanism in epidermal cell acantholysis induced by pemphigus vulgaris autoimmunoglobulins. Apoptosis. 2004;9:131–143. doi: 10.1023/B:APPT.0000018795.05766.1f. [DOI] [PubMed] [Google Scholar]
- 36.Pelacho B, Natal C, Espana A, Sanchez-Carpintero I, Iraburu MJ, Lopez-Zabalza MJ. Pemphigus vulgaris autoantibodies induce apoptosis in HaCaT keratinocytes. FEBS Lett. 2004;566:6–10. doi: 10.1016/j.febslet.2004.03.107. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, Roopenian DC, Liu Z. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuccolotto I, Roselino AM, Ramalho LN, Zucoloto S. Apoptosis and p63 expression in the pathogenesis of bullous lesions of endemic pemphigus foliaceus. Arch Dermatol Res. 2003;295:284–286. doi: 10.1007/s00403-003-0434-3. [DOI] [PubMed] [Google Scholar]
- 39.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 40.Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401:168–173. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- 41.Frusic-Zlotkin M, Pergamentz R, Michel B, David M, Mimouni D, Bregegere F, Milner Y. The interaction of pemphigus autoimmunoglobulins with epidermal cells: activation of the fas apoptotic pathway and the use of caspase activity for pathogenicity tests of pemphigus patients. Ann N Y Acad Sci. 2005;1050:371–379. doi: 10.1196/annals.1313.040. [DOI] [PubMed] [Google Scholar]
- 42.Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- 43.Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, Turk D, Vasiljeva O, Stefe I, Vandenabeele P, Bromme D, Puizdar V, Fonovic M, Trstenjak-Prebanda M, Dolenc I, Turk V, Turk B. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 2003;10:881–888. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu H, Banno Y, Sumi N, Naganawa T, Kitajima Y, Nozawa Y. Activation of p38 mitogen-activated protein kinase and caspases in UVB-induced apoptosis of human keratinocyte HaCaT cells. J Invest Dermatol. 1999;112:769–774. doi: 10.1046/j.1523-1747.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwerk C, Schulze-Osthoff K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem Pharmacol. 2003;66:1453–1458. doi: 10.1016/s0006-2952(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 46.Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dusek RL, Getsios S, Chen F, Park JK, Amargo EV, Cryns VL, Green KJ. The differentiation-dependent desmosomal cadherin desmoglein 1 is a novel caspase-3 target that regulates apoptosis in keratinocytes. J Biol Chem. 2006;281:3614–3624. doi: 10.1074/jbc.M508258200. [DOI] [PubMed] [Google Scholar]
- 48.Lanza A, Cirillo N. Caspase-dependent cleavage of desmoglein 1 depends on the apoptotic stimulus. Br J Dermatol. 2007;156:400–402. doi: 10.1111/j.1365-2133.2006.07654.x. [DOI] [PubMed] [Google Scholar]
- 49.Aho S. Plakin proteins are coordinately cleaved during apoptosis but preferentially through the action of different caspases. Exp Dermatol. 2004;13:700–707. doi: 10.1111/j.0906-6705.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 50.Kalinin AE, Aho M, Uitto J, Aho S. Breaking the connection: caspase 6 disconnects intermediate filament-binding domain of periplakin from its actin-binding N-terminal region. J Invest Dermatol. 2005;124:46–55. doi: 10.1111/j.0022-202X.2004.23507.x. [DOI] [PubMed] [Google Scholar]
- 51.Weiske J, Schoneberg T, Schroder W, Hatzfeld M, Tauber R, Huber O. The fate of desmosomal proteins in apoptotic cells. J Biol Chem. 2001;276:41175–41181. doi: 10.1074/jbc.M105769200. [DOI] [PubMed] [Google Scholar]
- 52.Weedon D, Searle J, Kerr JF. Apoptosis. Its nature and implications for dermatopathology. Am J Dermatopathol. 1979;1:133–144. [PubMed] [Google Scholar]
- 53.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 55.Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, Roujeau JC. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996;134:710–714. doi: 10.1111/j.1365-2133.1996.tb06976.x. [DOI] [PubMed] [Google Scholar]