Abstract
G2A is a stress-inducible G protein-coupled receptor that is expressed on several cell types within atherosclerotic lesions. We demonstrated previously that G2A deficiency in mice increased aortic monocyte recruitment and increased monocyte:endothelial interactions. To investigate the impact of G2A deficiency in macrophages, we isolated peritoneal macrophages from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice. G2A−/−ApoE−/− macrophages had significantly lower apoptosis than control macrophages. The pro-survival genes BCL-2, BCL-xL, and cFLIP were increased in G2A−/−ApoE−/− macrophages. Macrophages from G2A−/−ApoE−/− mice also had increased pro-inflammatory status that was indicative of a M1 macrophage phenotype. This was indicated by significantly increased nuclear translocation of NFκB, as well as production of IL-12p40, TNFα, and IL-6, and reduced expression of Arginase-1. Moreover, G2A−/−ApoE−/− macrophages had reduced ability to engulf apoptotic cells in vitro. We examined atherosclerosis in mice fed a Western diet for 10 weeks and found that G2A deficiency increased lesion size in the aortic root by 50%. Plasma lipid levels were not changed in G2A−/−ApoE−/− mice. However, we found that absence of G2A increased the number of aortic macrophages and attenuated apoptosis in this cell type. Moreover, bone marrow transplantation studies indicated that deficiency of G2A in marrow-derived cells significantly contributed to atherosclerosis development. In the absence of G2A, increased macrophage activation and decreased apoptosis is associated with accumulation of macrophages in the aorta and increased atherosclerosis.
Keywords: apoptosis, macrophages, vascular inflammation, atherosclerosis
Introduction
Macrophages and T-lymphocytes play critical roles in the initiation and development of atherosclerosis1. Activated endothelium recruits monocytes by secretion of chemoattractants, after which monocytes bind to and subsequently transmigrate though the endothelial layer 2–6. In addition to macrophages, CD4+ lymphocytes are detected in early atherogenesis and late-stage unstable atherosclerotic lesions, consistent with a role for acquired immunity in lesion development7–9. Lesional lymphocytes secrete the inflammatory cytokine IFN-γ which further activate macrophages and vascular cells 10–13.
The G protein-coupled receptor G2A is a stress-inducible receptor. Overexpression in fibroblasts causes cell cycle arrest at the G2 phase of mitosis, thus the name G2A for G2 accumulation 14. G2A expression attenuates Bcr-Abl oncogene-mediated cell proliferation while mice lacking G2A have an increased mortality rate in an oncogene-induced model of leukemia 15. The endogenous ligand for G2A is unknown, although putative ligands include LPC16;17, 9(S)HODE18, and possibly other free fatty acids18. G2A and other receptors within the OGR1 family (TDAG8, GPR4, and OGR1) respond to changes in extracellular pH 19. However G2A is less responsive to pH changes compared to other receptor family members20.
G2A is highly expressed on macrophages and lymphocytes, with lower expression found on macrovascular endothelium 21;22. G2A has been localized to atherosclerotic lesions in mice, consistent with a contributory role in the disease process 23. We have reported that G2A deficiency increases monocyte:endothelial interactions in vivo resulting in increased monocyte accumulation in aorta 22. In this prior study, we demonstrated a critical role of G2A in endothelium, however, the impact of G2A-deficiency on the macrophage was not investigated.
In the current study, we hypothesize that G2A deficiency in macrophages would result in a pro-inflammatory macrophage phenotype. Our data is consistent with this hypothesis, as macrophages from G2A−/−ApoE−/− show increased cytokine secretion, NFκB activation, and associated increases in survival gene expression. G2A−/−ApoE−/− mice fed a diet high in saturated fat for 10 weeks develop increased aortic root atherosclerosis compared to G2A+/+ApoE−/−. These data demonstrate that G2A-deficiency results in a pro-inflammatory macrophage M1 phenotype that is associated with increased atherosclerosis.
Materials and Methods
Detailed methods can be found online at www.circres.ahajournals.org. For the current studies, we fed G2A+/+ApoE−/− and G2A−/−ApoE−/− double knockout mice a Western (Harlan Teklad 88137) for ten weeks. We measured atherosclerosis using aortic root and en face techniques. In some studies, thioglycollate-elicited peritoneal macrophages were obtained from mice for measurements of apoptosis using flow cytometry and real-time PCR and macrophage inflammatory phenotype using ELISA and real-time PCR. We also performed atherosclerosis measurements in ApoE−/− and Ldlr−/− recipient mice fed a Western diet for ten weeks that had received ApoE−/−, G2A−/−ApoE−/−, Ldlr−/−, or G2A−/−Ldlr−/− bone marrow cells in a series of bone marrow transplantation studies.
Results
Activation of NFκB in G2A deficient macrophages
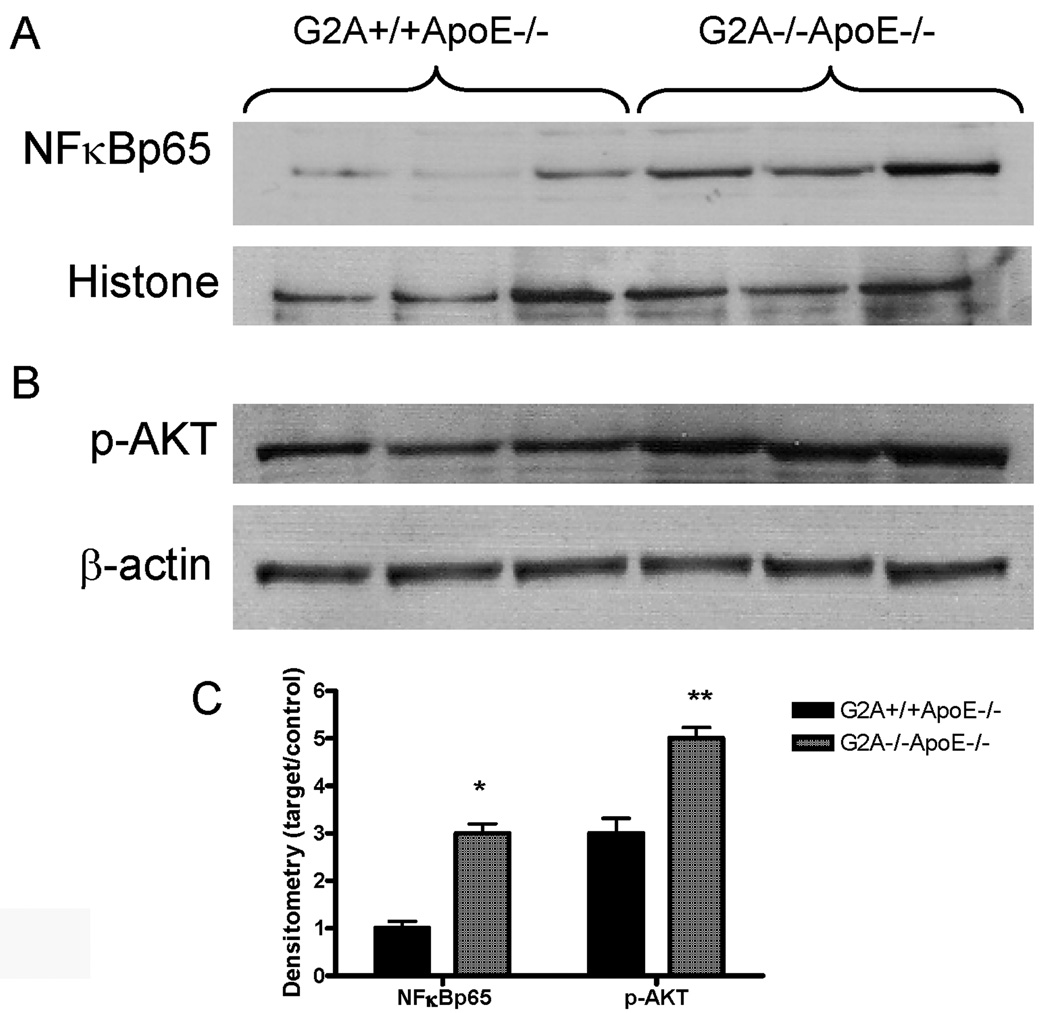
Upon activation, NFκB is translocated to the nucleus where it acts as a transcription factor. We found that NFκB p65 levels were significantly increased in macrophage nuclear extracts from G2A−/−ApoE−/− mice (Figure 1A). Phosphorylation of nuclear NFκB p65, another measure of NFκB activation, was also increased in G2A−/−ApoE−/− macrophages (data not shown). AKT is involved in NFκB signaling 37 and AKT phosphorylation activates the BCL family of anti-apoptotic factors. Peritoneal macrophages from G2A−/−ApoE−/− mice showed increased AKT phosphorylation compared to G2A+/+ApoE−/− control (Figure 1B). Figure 1C represents densitometry of 6 mice per group.
Figure 1. NFκB is activated in G2A−/− macrophages.
Cytosol and nuclear extracts were isolated as described in Methods from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice fed a Western diet for 10 weeks. Images shown are peritoneal macrophages isolated from three mice from each group. Panel A. 50µg of nuclear proteins were analyzed by SDS-PAGE for NFκBp65 or histone. Panel B. 50µg of cytosolic proteins were used to analyze levels of phosphorylated AKT and β-actin. Panel C. Graph represents densitometry of 6 mice per group. *Significantly greater than G2A+/+ApoE−/− control, P<0.002, **significantly greater than control, P<0.005.
G2A−/−ApoE−/− macrophages have reduced apoptosis
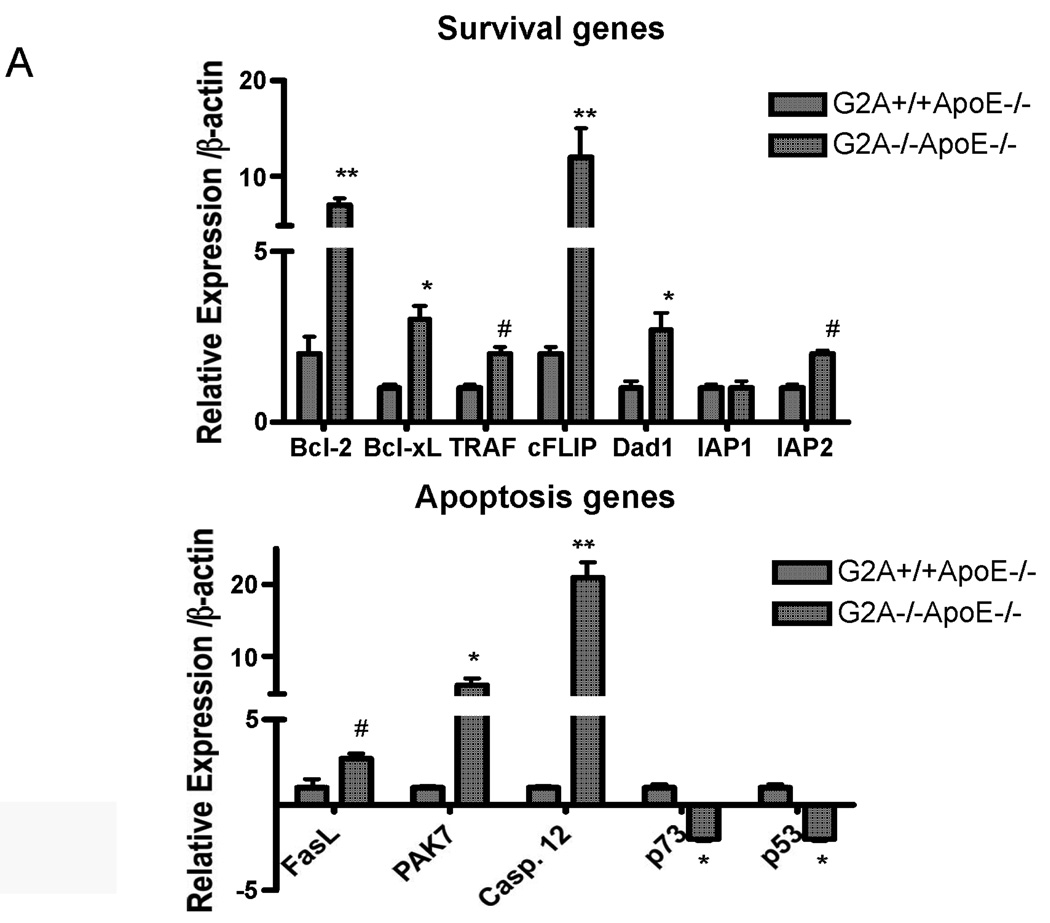
We examined expression of genes involved in inflammation and apoptosis. Several survival gene targets of NFκB were upregulated in G2A−/−ApoE−/− macrophages compared to control, including BCL-2, BCL-xL, and cFLIP (Figure 2A). In addition, IAP2 was increased 1.8-fold, and expression of p73 and p53, two pro-apoptotic genes, was reduced 2.7-fold (Figure 2A). Interestingly though, we observed significant upregulation of several other pro-apoptotic genes, including FasL, PAK7, and Caspase-12 (Figure 2A), suggesting that G2A expression in the macrophage must regulate apoptotic/survival pathways, and in the absence of G2A, these significantly pathways become dysregulated.
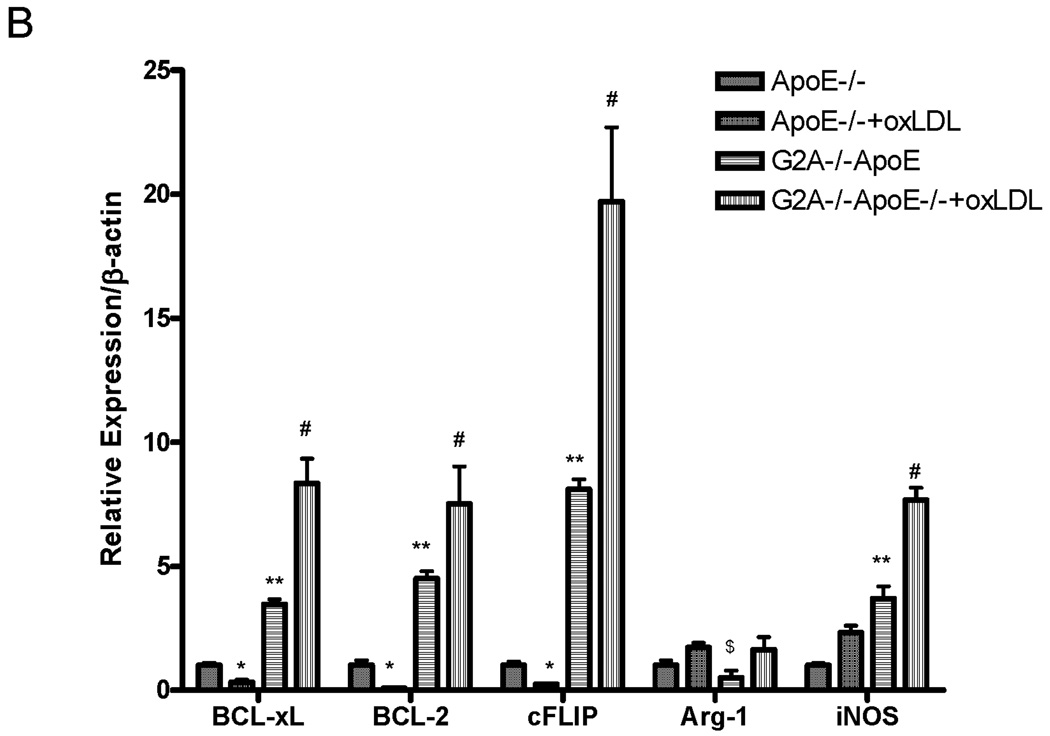
Figure 2. Increased survival gene expression and reduced apoptosis of macrophages in G2A−/− mice.
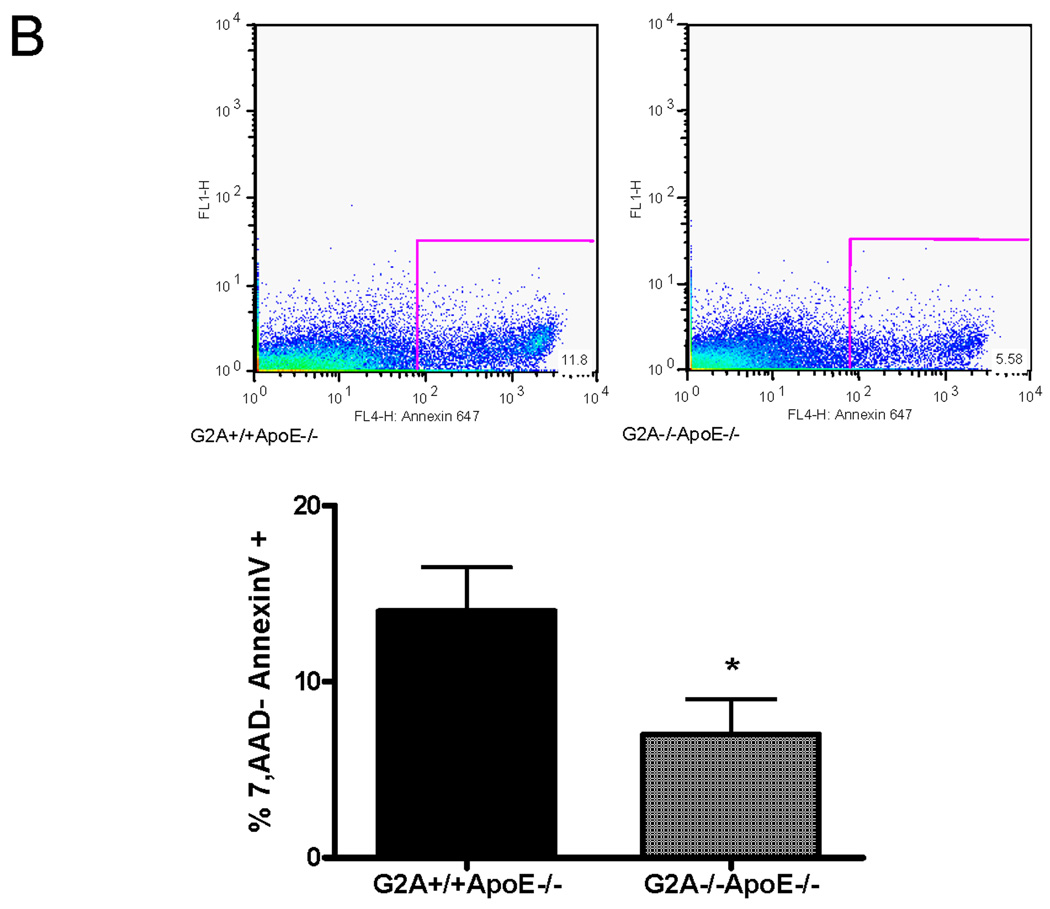
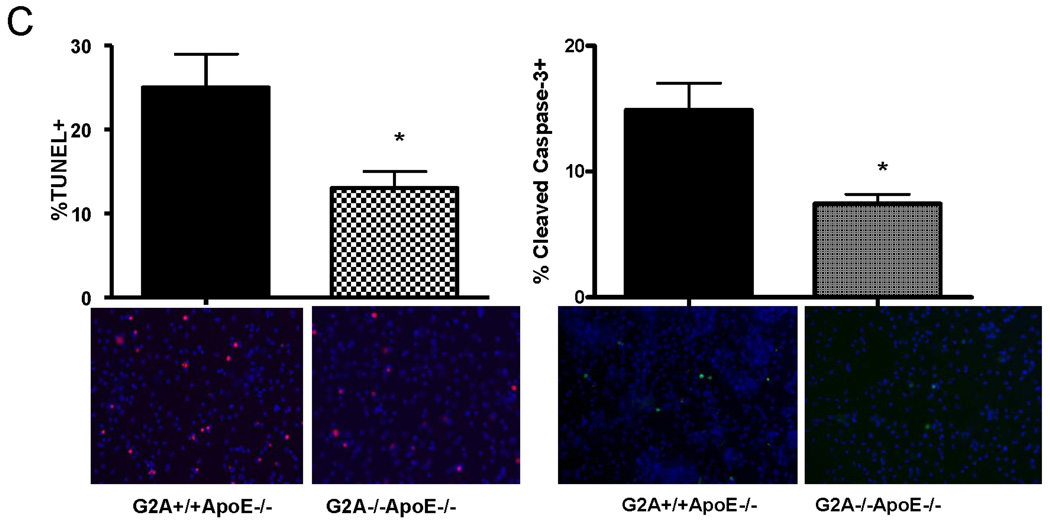
Panel A. Gene expression. Pro-survival and pro-apoptotic gene expression in peritoneal macrophages isolated from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice fed a Western diet for 10 weeks was analyzed by quantitative real-time RT-PCR. *p<0.005, **p<0.0001, #p<0.01 between G2A+/+ApoE−/− and G2A−/−ApoE−/−. Panel B. Measurements of macrophage apoptosis. Peritoneal macrophages were isolated as described in Methods from ten each of G2A+/+ApoE−/− and G2A−/−ApoE−/− mice fed a Western diet for 10 weeks. Macrophages were stained for AnnexinV-Alexa647 using the Vybrant Apoptosis assay kit (Molecular Probes) according to the manufacturer’s instructions. Data was analyzed using FlowJo software. Representative dot plots are shown for each group, and the mean percentage of 7,AAD-AnnexinV+ cells per group was plotted. *Significantly less than G2A+/+ApoE−/− control, P<0.0001. Panel C. TUNEL and caspase-3 staining. Peritoneal macrophages from each group were stained for TUNEL using the TMR red in situ TUNEL assay kit (Roche Applied Sciences) or stained for cleaved Caspase-3 as described in Methods. Nuclei were stained with DAPI (blue). *Significantly less than G2A+/+ApoE−/− control for TUNEL, p<0.0005; *Significantly less than G2A+/+ApoE−/− control for cleaved caspase-3, p<0.002.
To examine whether the macrophages exhibited a functionally pro-apoptotic or pro-survival phenotype, we performed several assays. First, we measured Annexin V staining on freshly isolated peritoneal macrophages from G2A−/−ApoE−/− and G2A+/+ApoE−/− mice using flow cytometry (Figure 2B). G2A−/−ApoE−/− macrophages showed significantly less Annexin V staining as measured by flow cytometry (Figure 2B). Alternatively, peritoneal macrophages were plated overnight on chamber slides and stained for TUNEL or cleaved Caspase-3. G2A−/−ApoE−/− macrophages showed significantly less TUNEL staining, (P<0.001; Figure 2C), and less cleaved Caspase-3 than G2A+/+ApoE−/− (Figure 2C). Taken together, these results indicate that G2A deficiency in macrophages activates pro-survival signaling pathways thereby preventing apoptosis, even though several pro-apoptotic pathways are activated in these macrophages.
G2A−/−ApoE−/− macrophages are pro-inflammatory and have impaired apoptotic cell engulfment
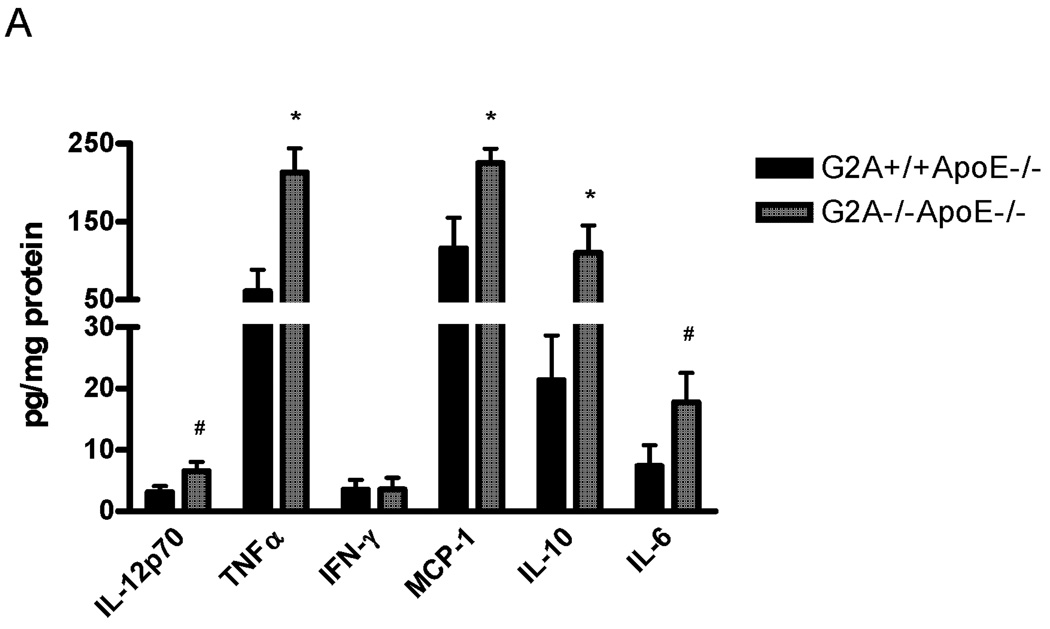
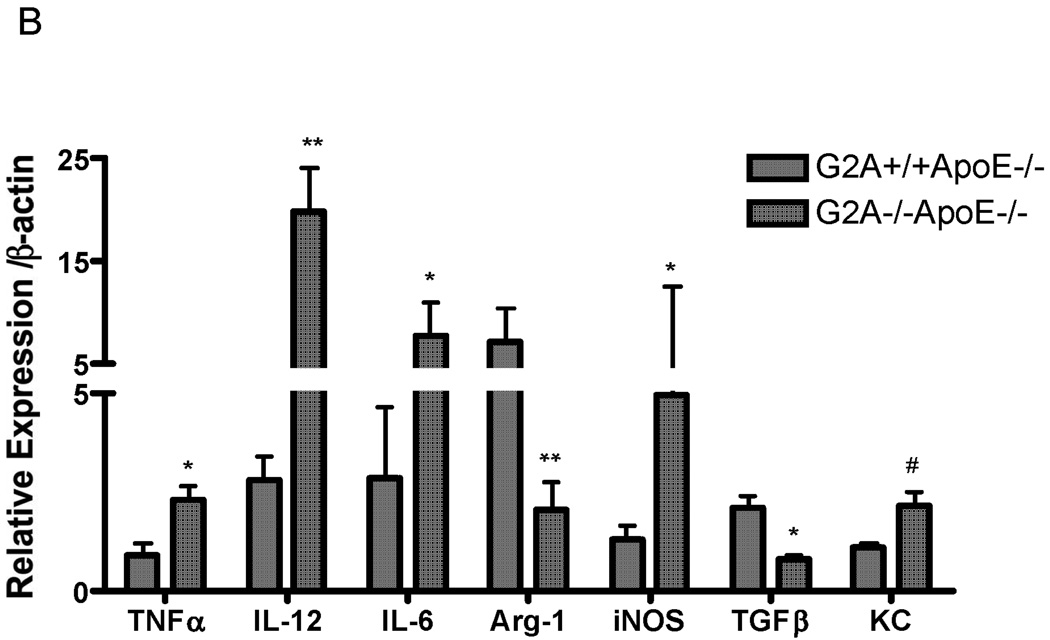
We next examined the inflammatory phenotype of G2A−/−ApoE−/− and G2A+/+ApoE−/− macrophages. IL-12p70, TNFα, MCP-1, IL-10, and IL-6 secretion into culture media were all significantly higher in G2A−/−ApoE−/− macrophages compared to control (Figure 3A). IFN-γ levels were unchanged. Additionally, quantitative real-time RT-PCR was performed on RNA isolated from peritoneal macrophages from G2A−/−ApoE−/− and G2A+/+ApoE−/− mice, and we observed similar inductions of cytokine mRNA expression (Figure 3B). In addition, we found significant reductions in Arg-1 and TGFβ expression in G2A−/−ApoE−/− macrophages (Figure 3B), both of which have been linked to a M2 alternative, anti-inflammatory macrophage phenotype38. We also found similar changes in gene expression in macrophages isolated from whole aorta (data not shown). Taken together, these data suggest that G2A deficiency promotes an inflammatory M1-like macrophage phenotype.
Figure 3. Inflammatory cytokine production by G2A−/− macrophages.
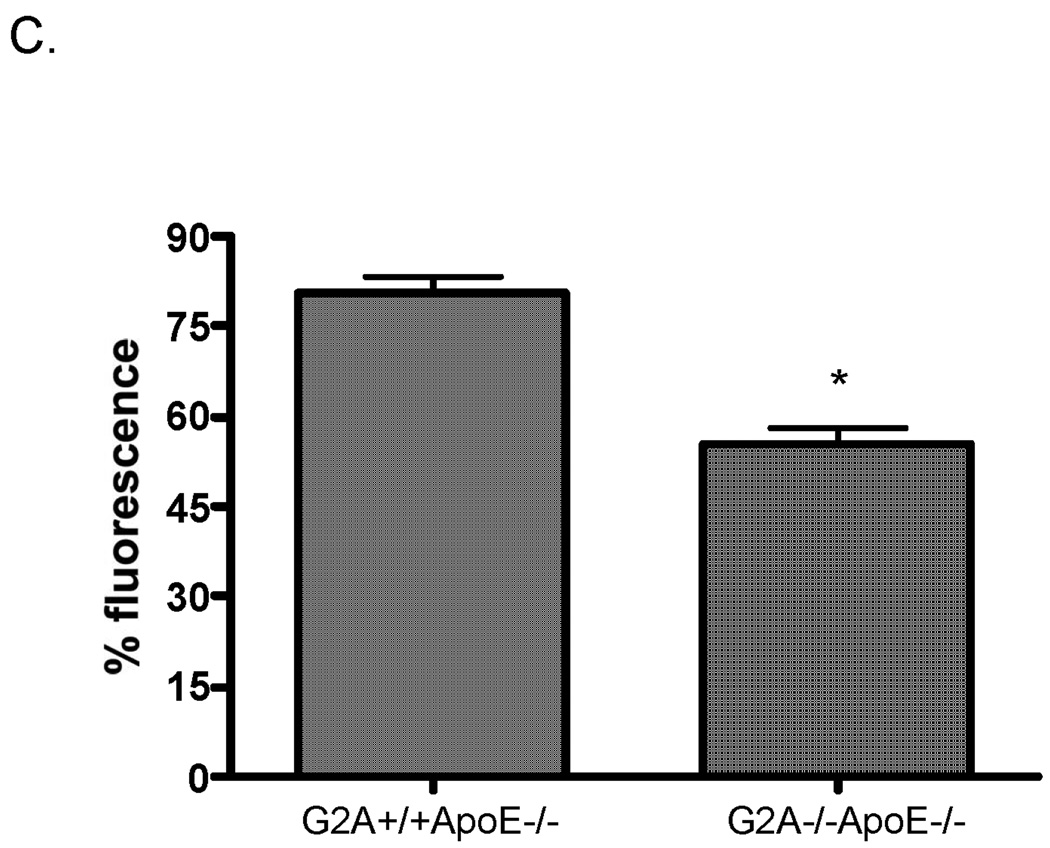
Panel A. Cytometric bead arrays were used to analyze inflammatory cytokine secretion from G2A+/+ApoE−/− and G2A−/−ApoE−/− peritoneal macrophages fed a Western diet for 10 weeks. Assays were conducted according to the manufacturer’s instructions. #Significantly greater than G2A+/+ApoE−/− control, P<0.02, *significantly greater than G2A+/+ApoE−/− control, P<0.005. Panel B. Quantitative real-time RT-PCR of macrophage inflammatory markers represented as relative expression and normalized to β-actin. *p<0.001, **p<0.0001, #p<0.008. Panel C. Peritoneal macrophages from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice fed a Western diet for 10 weeks were incubated with fluorescently-labeled apoptotic Jurkat cells for 15 minutes for an engulfment assay. The percentage of engulfed cells was determined as a measure of fluorescence by flow cytometry. *p<0.03, Data represent an ‘n’ of 4 mice per group.
Next, we measured the ability of the G2A−/−ApoE−/− macrophages to engulf apoptotic cells. Efficient clearance of apoptotic cells by phagocytic macrophages is associated with promotion of an anti-inflammatory, M2-like phenotype. As shown in Figure 3C, G2A−/−ApoE−/− macrophages showed reduced ability to engulf apoptotic cells in vitro. Since defective apoptotic cell clearance from the vessel wall is associated with increased atherosclerosis39;40, these data would suggest that G2A deficiency in macrophages would contribute to atherosclerosis development.
Apoptosis and anti-apoptotic gene expression after treatment with oxidized LDL
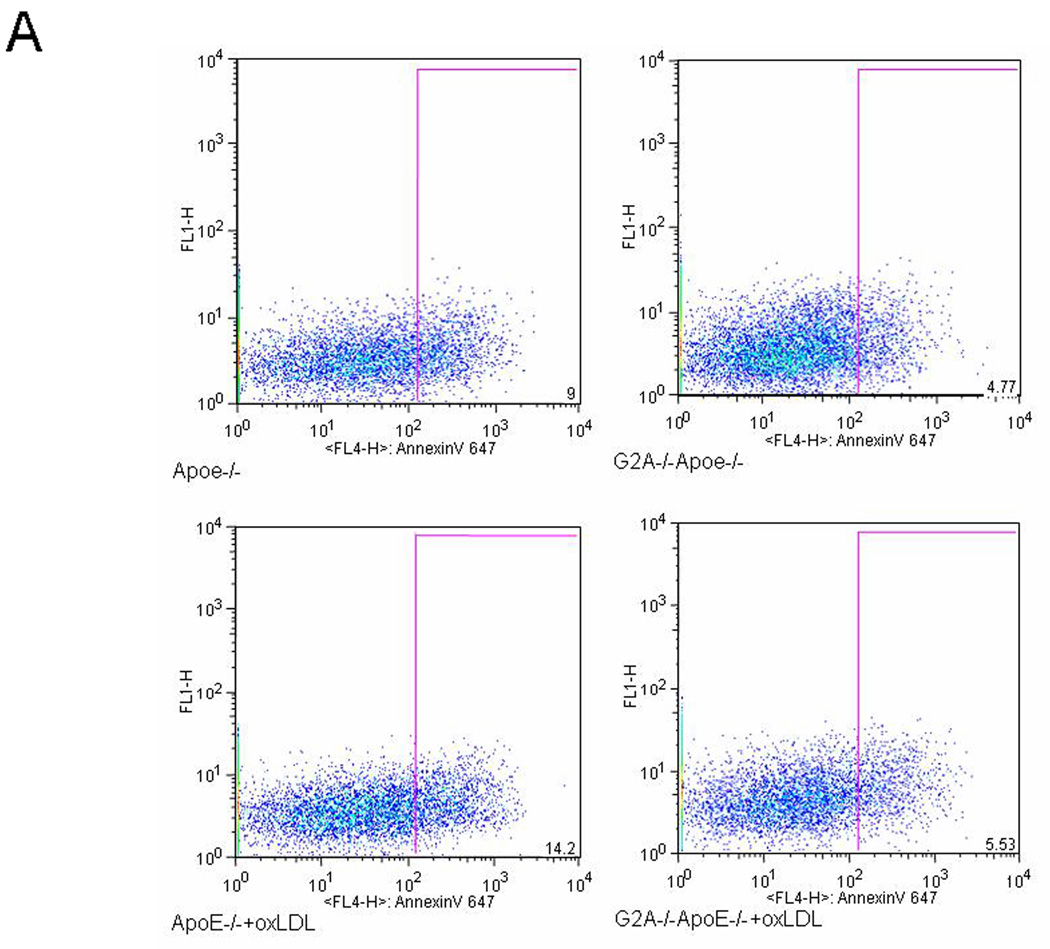
Peritoneal macrophages from 6 each of G2A+/+ApoE−/− and G2A−/−ApoE−/− mice were isolated and stimulated with the treatment of with oxidized LDL (50µg/mL) for 18 hours. After treatment, cells were collected for measurement of apoptosis and survival gene expression. Apoptosis, as measured by annexinV staining, was significantly reduced in G2A−/−ApoE−/− macrophages compared to G2A+/+ApoE−/− control (P<0.002). Treatment with oxLDL increased apoptosis in G2A+/+ApoE−/− by 50% (P<0.01), but did not significantly increase apoptosis in G2A−/−ApoE−/− macrophages. Figure 4A shows representative dot plots from each group. The survival genes BCL-2, BCL-xL, and cFLIP as well as iNOS expression were all significantly increased in G2A−/−ApoE−/− macrophages compared to control (P<0.001), while treatment with oxLDL further increased survival gene and iNOS expression in G2A−/−ApoE−/− macrophages (P<0.002) (Figure 4B). Treatment of G2A+/+ApoE−/− macrophages with oxLDL reduced expression of the survival genes BCL-2, BCL-xL, and cFLIP (P<0.005). Arginase I expression was significantly reduced in untreated G2A−/−ApoE−/− compared to G2A+/+ApoE−/− (P<0.02), but was restored to control levels upon stimulation with oxLDL (Figure 4B).
Figure 4. Oxidized LDL treatment of peritoneal macrophages.
Panel A. Peritoneal macrophages were isolated from 6 mice per group fed chow, treated +/− oxLDL (50µg/mL) for 18 hours and stained for AnnexinV-Alexa647 using the Vybrant Apoptosis assay kit (Molecular Probes) according to the manufacturer’s instructions. Data was analyzed using FlowJo software. Representative dot plots are shown for each group. Panel B. Quantitative real-time RT-PCR of anti-apoptosis genes and macrophage inflammatory markers represented as relative expression and normalized to β-actin. * Significantly lower than G2A+/+ApoE−/− control, P<0.005, **greater than G2A+/+ApoE−/− control, P<0.001, # greater than G2A−/−ApoE−/−, P<0.002, $ less than G2A+/+ApoE−/− control, P<0.02.
Atherosclerosis lesion analysis and characterization
We next examined atherosclerosis development in G2A-deficient mice after ten weeks of Western diet feeding. We found that fasting total cholesterol concentrations were elevated in both groups. There were no significant differences between G2A+/+ApoE−/− and G2A−/−ApoE−/− mice in blood glucose levels (306mg/dL+/−36.2 vs 282.7mg/dL +/−48.1), total cholesterol (1084mg/dL +/−107.4 vs 972.5mg/dL +/−161.5), HDL (16.1mg/dL +/−2.1 vs 16.4mg/dL +/−3.1), LDL (1052mg/dL +/−124.9 vs 943.3mg/dL +/−157), or triglycerides (128.7mg/dL +/−28.6 vs 132.4mg/dL +/−28.9) respectively. Body weight at the end of the experiment also did not differ between groups (G2A+/+ApoE−/−, 22.2g+/−2.8 vs G2A−/−ApoE−/−, 21.6g+/−3.6).
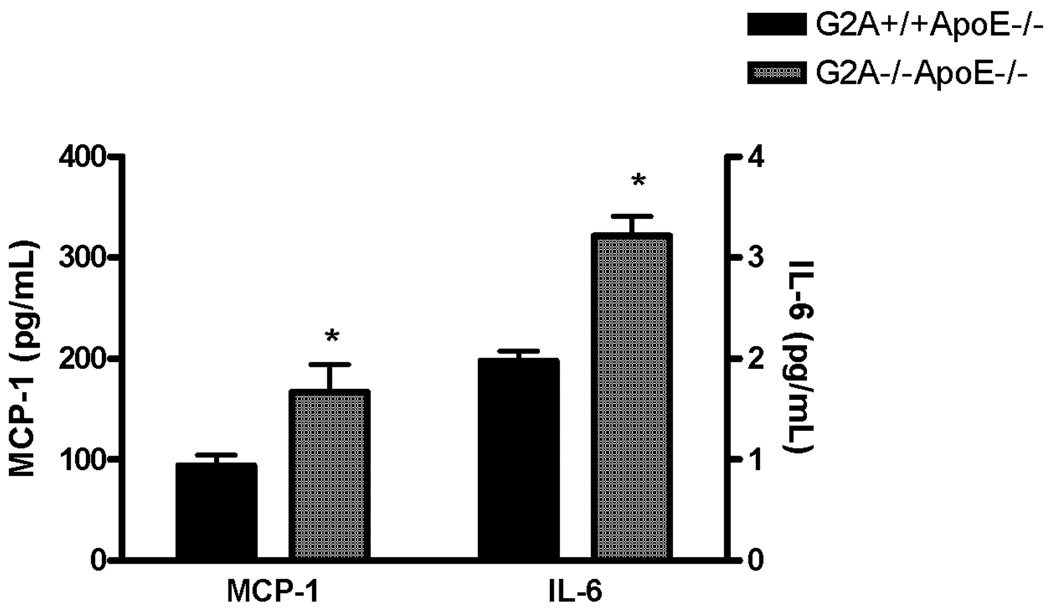
G2A deficiency did lead to significantly increased concentrations of MCP-1 and IL-6 in plasma, however (P<0.005; Figure 5). Plasma concentrations of IFN-γ, IL-12p70, IL-10, and TNFα were below the detection sensitivity of the assay in all groups (data not shown).
Figure 5. Plasma cytokine concentrations.
Blood was collected from 15 each of G2A+/+ApoE−/− and G2A−/−ApoE−/− mice after 10 weeks on Western diet via cardiac puncture. Plasma was analyzed for cytokine production using a cytometric bead array and flow cytometry. *Significantly higher than G2A+/+ApoE−/− control, P<0.002.
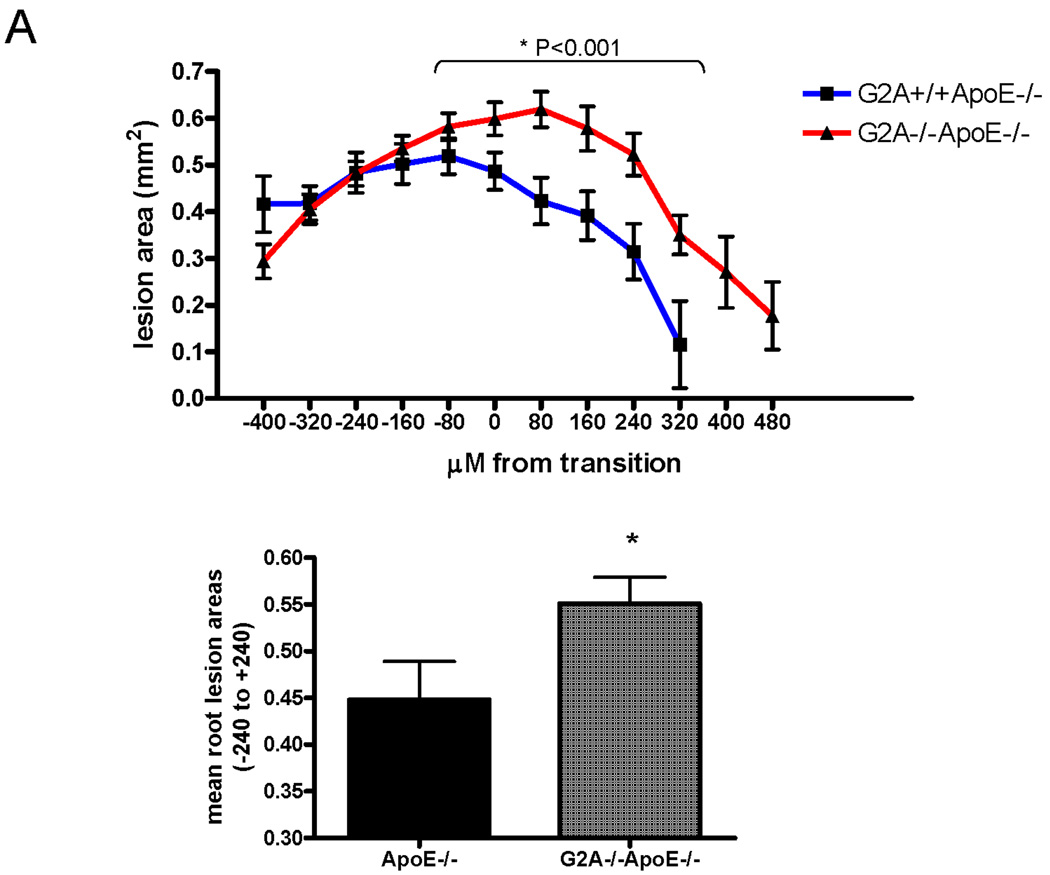
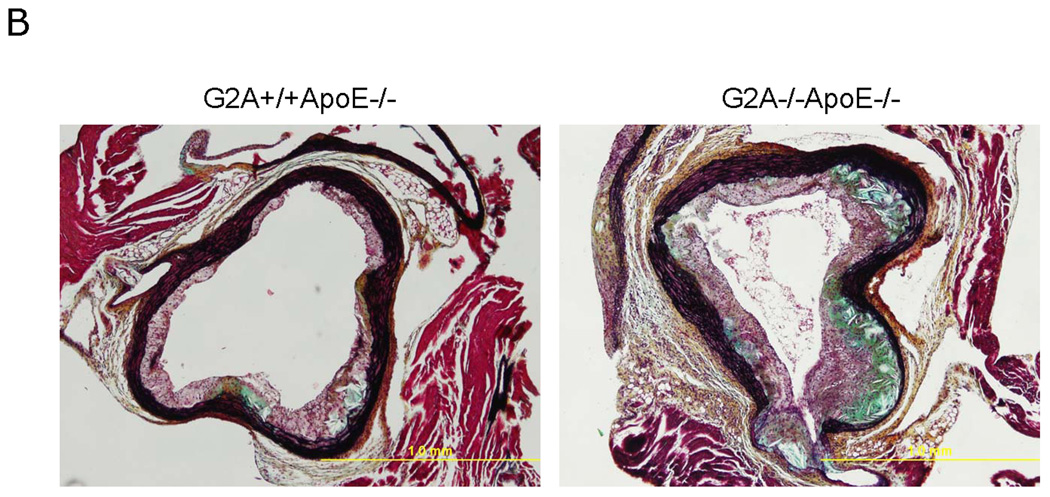
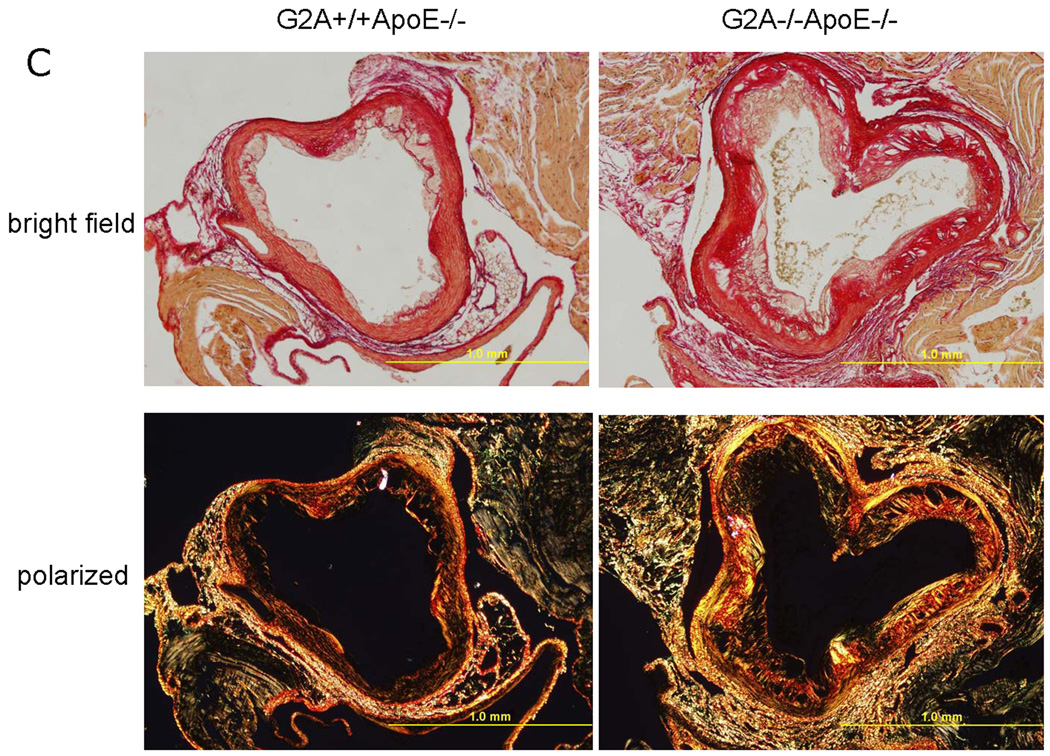
Aortic root sections from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice were collected and lesion area was measured in both the sinus and the ascending aortas as indicated in Figure 6A, with the “0” point indicating the transition between these areas. After 10 weeks Western diet feeding, G2A−/−ApoE−/− mice showed increased atherosclerosis development within −240µM to +240µM from the transition (P<0.001; Figure 6A). Movats pentachrome and picrosirius red staining of 5µM sections from aortic roots (taken at +160µM from the transition) demonstrated that G2A deficiency led to greater collagen content in the aortic root suggesting formation of complex atherosclerotic plaques (Figure 6B). Using Movats stain, collagen content is apparent by dark purple to black coloration 35. Using picrosirius red staining, collagen content of the lesion is visible by red staining under normal light. Under polarized light, red, orange, yellow, and green colors are apparent (the colors of collagen fibers in order of decreasing thickness) (Figure 6C) 36.
Figure 6. G2A−/−ApoE−/− mice have increased atherosclerosis after 10 weeks of diet feeding.
Panel A. Atherosclerotic lesion analysis. Aortic roots from G2A+/+ApoE−/− and G2A−/−ApoE−/− mice were embedded in paraffin. 5µM sequential sections from the aortic sinus and the descending aorta (−400µM to +480µM from the transition) were sectioned and stained using oil red O, and lesion area was quantified using Image Pro software analysis. *Significantly greater lesion area than G2A+/+ApoE−/− control (from −240µM to +240µM, P<0.001). Panel B. Movat staining of aortic root sections. 5µM sections from the aortic sinus and the descending aorta at +160µM were stained using pentachrome MOVAT stain. Collagen and reticulum fibers - yellow to greenish yellow. Panel C. Picrosirius red staining of aortic root sections. 5µM sections at +160µM from the transition were stained using picrosirius red stain and visualized under bright field and polarized light. Under normal light, collagen content of the lesion is visible by red staining. Under polarized light, red, orange, yellow, and green colors are apparent (the colors of collagen fibers in order of decreasing thickness) 36.
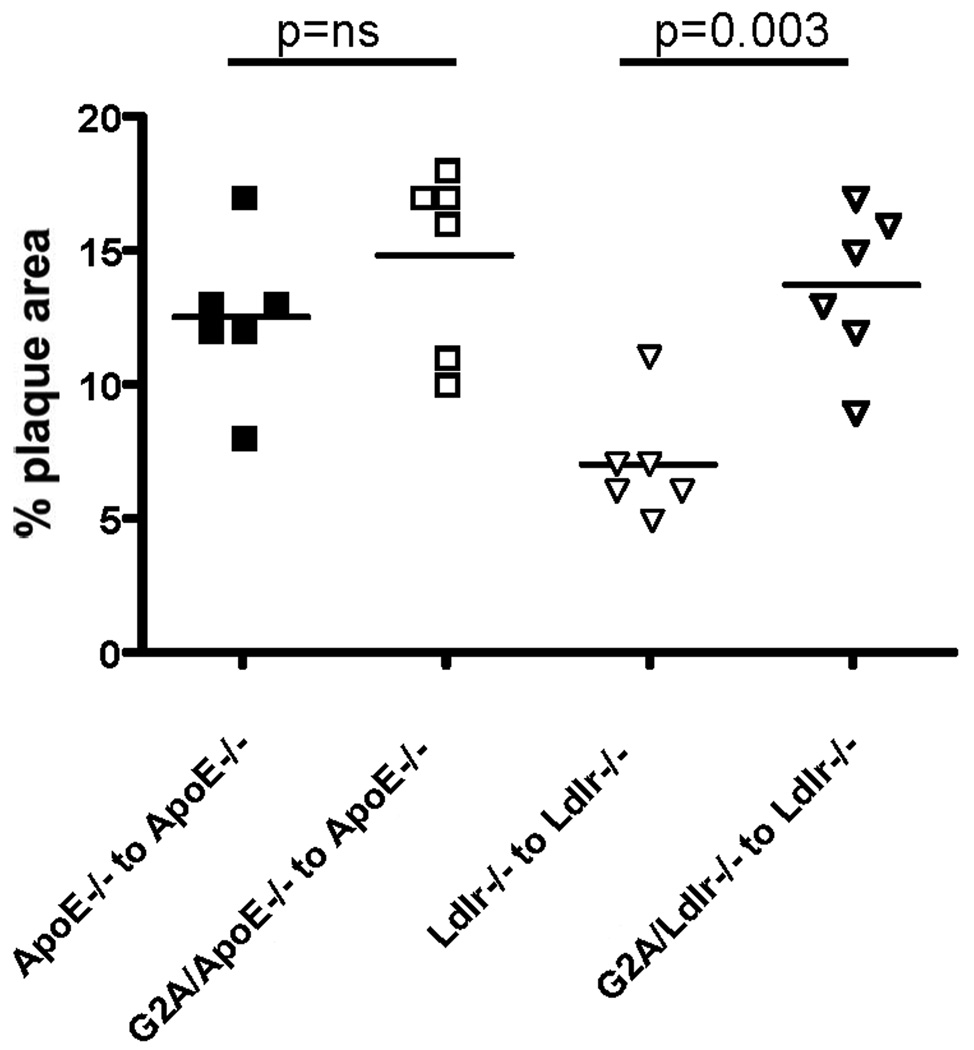
Absence of G2A in marrow-derived cells contributes to early atherosclerosis development
Bone marrow transplantation studies were performed in ApoE−/− recipients using ApoE−/− and G2A−/−ApoE−/− bone marrow and in Ldlr−/− recipients using Ldlr−/− and G2A−/−Ldlr−/− bone marrow. After 6 weeks of reconstitution, mice were placed on a Western diet for 10 weeks. Atherosclerosis was measured using en face analysis. As shown in Figure 7, the percent plaque area was greater in ApoE−/− recipients receiving apoE−/− marrow compared to Ldlr−/− recipients receiving Ldlr−/− marrow. In the apoE−/− recipients, we observed a trend towards increased plaque area in the mice that received G2A−/−ApoE−/− bone marrow, but the data did not reach statistical significance. However, in the Ldlr−/− recipients, absence of G2A in marrow-derived cells significantly increased aortic plaque area, p<0.003 (Figure 7). Taken together, these data suggest that G2A expression in macrophages contributes significantly to atherosclerosis development, yet does not rule out some contribution of non-marrow derived cells.
Figure 7. Absence of G2A in marrow-derived cells contributes to atherosclerosis.
Female LDLR−/− or apoE−/− mice were irradiated and transplanted with donor bone marrow (Ldlr−/−, G2A−/−Ldlr−/−, apoE−/−, G2A−/−ApoE−/−) as indicated in the figure. Mice were reconstituted for 6 weeks, after which they were fed a Western diet for 10 weeks. Atherosclerosis was assessed by measuring en face aortic lesion area.
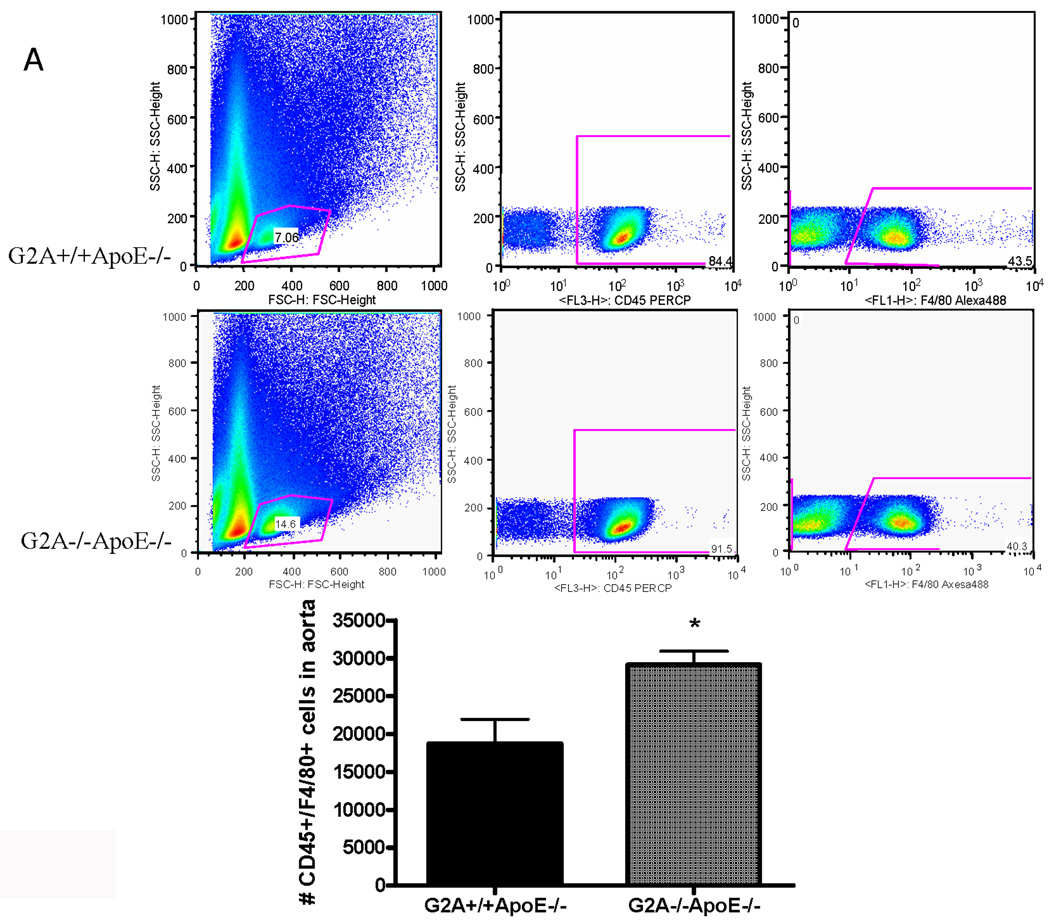
Higher macrophage content in aortic wall of G2A−/− mice
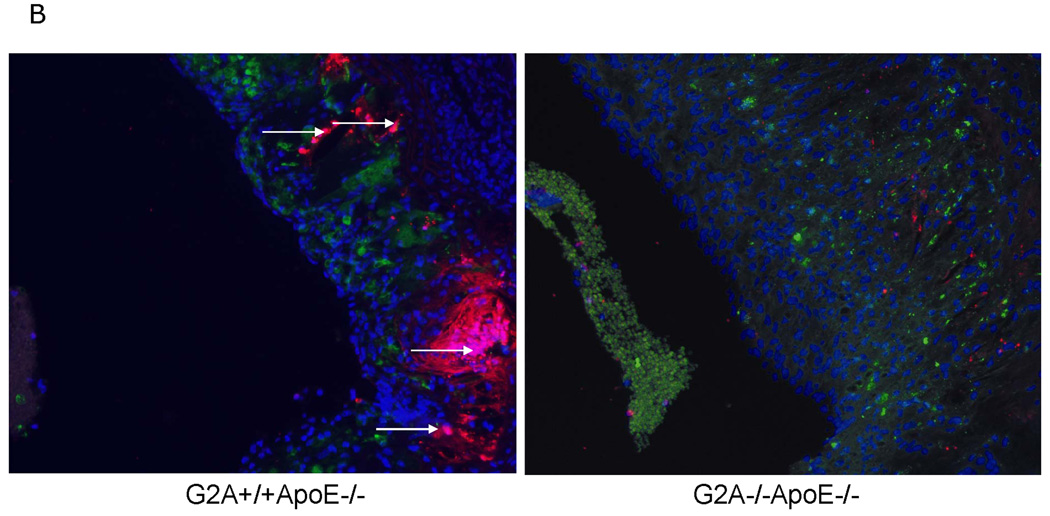
Finally, we examined macrophage numbers in the aortic wall using a novel flow cytometry method developed by Ley and colleagues31. Macrophage content in vivo in the aortic wall of G2A−/−ApoE−/− mice was significantly higher than that of ApoE−/− control (P<0.002) as measured by dual staining for CD45 and F4/80 (Figure 8A). However, total monocyte and lymphocyte counts in blood were similar between the two experimental groups (data not shown). Thus, the increase in aortic wall macrophage accumulation does not occur as a result of increased leukocyte numbers in blood of the G2A−/−ApoE−/− mice. We anticipate that the increased aortic wall content is due to increased monocyte:endothelial cell interactions in the arterial wall in the absence of G2A, which we have previously reported22. Moreover, G2A−/−ApoE aortic root cross-sections, while having a greater plaque area and macrophage content, had significantly less TUNEL+/DAPI+ co-staining (Figure 8B). The regions of positive TUNEL staining in G2A−/−ApoE−/− lesions showed very little co-staining for either DAPI nuclei or MAC-2 macrophage indicating these areas are necrotic. We found approximately a 5% increase in %necrotic core/total plaque area ratio in the aortic roots of G2A−/−ApoE−/− mice compared to G2A+/+ApoE−/− mice. Aortic root sections from ApoE−/− mice had more macrophage staining as measured by MAC-2+ staining as well as greater TUNEL+/DAPI+ staining. These data combined with our in vitro data on macrophage function suggest that the reduced macrophage apoptosis observed in G2A−/−ApoE−/− mice contributes to aortic macrophage accumulation and atherosclerosis.
Figure 8. Increased numbers of macrophages in the aortic wall of G2A−/−ApoE−/− mice.
Panel A. Whole aorta flow cytometry for macrophage content. Aortas were isolated from ten each of G2A+/+ApoE−/− and G2A−/−ApoE−/− mice after 10 weeks on Western diet. Aortas were digested and analyzed for leukocyte content by flow cytometry as described in Methods. Data was analyzed using FlowJo software and the total number of CD45+F4/80+ cells/aorta was plotted. *Significantly greater than G2A+/+ApoE−/− control, P<0.005. Panel B. Fluorescent imaging of lesional macrophage apoptosis. 5µM aortic root sections at +160µM from the transition were stained for resident macrophages and apoptosis. Green: MAC-2; Red: TUNEL; Blue: DAPI nuclear stain. G2A−/−ApoE−/− aortic root sections showed increased individual macrophage staining and decreased TUNEL. G2A+/+ApoE−/− sections showed increased MAC-2/TUNEL colocalization to nuclear intact cells as indicated by the arrows (pink color).
Discussion
G2A is a G protein-coupled receptor that is highly expressed in macrophages and lymphocytes41. The endogenous ligand for G2A remains unclear 18;42–44, however there is some evidence that G2A is involved in atherosclerotic lesion development in animal models 21;23. We have recently found that absence of G2A in mice contributes to cholesterol gallstone disease45, although G2A has not, to date, been defined as a Lith gene. The gene in humans that corresponds to G2A is GPR132. Currently, there are no known reported associations of polymorphisms in GPR132 with clinical disease in humans. Recently, however, we have discovered single-nucleotide polymorphisms (SNPs) within GPR132 that are associated with internal intimal media thickness (IMT) of the carotid artery in patients (data not shown). Thus, GPR132 (G2A) may indeed represent a clinically relevant gene for lipid metabolism and atherosclerosis.
In the current study, we investigated the impact of G2A-deficiency on macrophage function and atherosclerosis in ApoE−/− mice. G2A−/−ApoE−/− mice fed a fat-enriched diet for 10 weeks had significantly greater aortic sinus lesion area compared to G2A+/+ApoE−/− mice. G2A−/−ApoE−/− mice additionally had significantly higher numbers of macrophages present in the aortic wall compared to G2A+/+ApoE−/−. While there was no difference in circulating lipid levels, G2A−/−ApoE−/− mice had significantly greater plasma IL-6 and MCP-1 levels. Moreover, macrophages isolated from these mice had increased production of pro-inflammatory cytokines, and reduced expression of anti-inflammatory genes. These data suggest that G2A deficiency promotes a pro-inflammatory M1 macrophage phenotype that contributes to atherosclerotic lesion development.
In related studies of G2A function, Parks et al. examined G2A-deficient mice on a LDLR−/− background and noted increased macrophage content and decreased macrophage apoptosis with no effect on atherosclerotic lesion size after e0069ther 6 or 12 weeks of Western diet feeding 21. A second study from the same group demonstrated that G2A-deficiency decreased atherosclerosis in G2A−/−LDLr−/− mice at later times points of diet feeding 46. In the second study, these investigators reported significant elevations in plasma HDL levels in the G2A−/−LDLr−/− mice when fed a Western diet for both 9 and 20 weeks. They observed a significant decrease in atherosclerosis in G2A−/−LDLr−/− mice at both time points of feeding that could possibly be attributed to increased plasma HDL concentrations. Our studies were performed in ApoE−/− mice for ten weeks and we did not observe changes in HDL. Moreover, we performed bone marrow transplantation studies in both genetic backgrounds and found that deficiency of G2A in bone marrow-derived cells significantly contributed to atherosclerosis (Figure 7). In the apoE−/− background, this trend did not reach statistical significance, most likely due to the small number of animals available for study. However, the effect was quite dramatic in the Ldlr−/− mice, and our findings are opposite to those of Parks et al 21;46. HDL levels were similar among groups in our BMT studies (data not shown), which is different from the studies by Parks. However, taken together, the collective results of our studies and those of Parks et al. suggest that G2A expression in multiple cell types influences atherosclerosis. Indeed, absence of G2A in hepatocytes, lymphocytes and in endothelium clearly influences inflammatory and immune processes related to atherosclerosis22;41;45. Development of floxed mice for cell-specific studies of G2A deficiency is needed to dissect the important contributions of G2A in each cell type on atherosclerosis.
G2A expression has been shown to influence apoptosis in leukocytes 16;21;47. Recent studies suggest an important role of apoptosis in atherosclerotic plaque formation 48;49. Our previous study demonstrated that G2A-deficiency resulted in increased NFκB activation in murine aortic endothelial cells 22. Since NFκB activation targets survival gene expression resulting in decreased apoptosis 50, we investigated whether this was relevant in macrophages in the current study. Indeed, G2A−/−ApoE−/− macrophages showed significantly higher levels of NFκB p65 expression in the nucleus that corresponded with increased survival gene expression. Expression of NFκB-controlled genes that are important for cell survival, including bcl-2, bcl-xL, TRAF, and cFLIP was elevated in macrophages from G2A−/− mice. These G2A-deficient macrophages also showed significantly lower TUNEL, cleaved Caspase-3, and Annexin V staining. Furthermore, we observed downregulation of the pro-apoptotic genes, p53 and p73, which have been shown to be downregulated by NFκB 51. Concomitantly with the increase in pro-survival genes, we observed increased numbers of macrophages in the aortic wall of G2A−/− mice in vivo, suggesting that G2A deficiency causes macrophage accumulation in the aortic wall through promoting macrophage survival. We speculate from our data that NFκB activation is the primary regulator of macrophage survival in the absence of G2A. Since we have observed activation of NFκB in endothelium as well, we anticipate that G2A expression somehow serves to inhibit NFκB. The mechanisms for this are unknown, but are currently being studied in the laboratory. In preliminary studies, we have not observed significant changes in IκB expression (data not shown), but we have observed increased AKT activity in the G2A−/−ApoE−/− macrophages, which has been shown to increase NFκB 52. However, we cannot rule out contributions of other survival pathway genes in regulating apoptosis in the G2A−/− macrophages. Moreover, we found upregulation of a few pro-apoptotic genes, including FasL, and caspase-12 in G2A−/− macrophages, which suggest that the macrophages in G2A−/− mice have become dysregulated, most likely impacting their inflammatory phenotype as well. Indeed, caspase-12 is induced by ER stress, which often occurs as a result of free cholesterol loading in macrophages 53. ER stress can contribute to a pro-inflammatory macrophage phenotype 49.
Under normal conditions, macrophages act to maintain homeostasis in the aortic wall. Classically-activated (M1-type) macrophages exhibit strong microbicidal properties, thereby promoting IL-12 and TNFα-mediated Th1 responses 54. In contrast, alternatively activated (M2-type) macrophages secrete anti-inflammatory cytokines such as TGFβ, ingest and clear cell debris, and are rapidly cleared from the wall, thereby contributing to the resolution of inflammation. In normal tissue and during early atherosclerosis, M2-type macrophages help to stabilize the environment of the vessel wall by promoting effective efferocytosis of dying cells 55. Chronic activation of M1-like macrophages promotes an unstable vessel environment, reducing efferocytosis and triggering secondary necrosis of vascular wall cells, thereby contributing to the advanced atherosclerotic plaque formation 49. G2A has recently been identified as a phagocyte receptor on macrophages, in which G2A recognizes ‘find me’ signals such as lysophosphatidylcholine metabolites that are secreted by dying cells. The increased collagen content observed in aortic roots of G2A-deficient mice (Figure 6) suggests that there is increased secondary necrosis of vascular wall cells that contribute to plaque complexity. This finding is consistent with the notion of Peter, et al. that G2A may serve as a phagocytic receptor 56. Thus, the absence of G2A on macrophages may contribute to atherosclerosis through impairing efferocytosis pathways, in which various ‘find me’ signals secreted by dying cells are no longer recognized by the macrophage in the absence of G2A. We did find reductions in apoptotic cell engulfment in G2A−/− macrophages in vitro, supporting this hypothesis (Figure 3). Moreover, defective apoptotic cell clearance has been linked to atherosclerosis in mice 39. Future studies to determine the specific roles of the G2A receptor in apoptotic cell clearance and its subsequent impact on atherosclerosis will be needed to fully address this hypothesis.
In conclusion, we demonstrate that G2A deficiency caused increased atherosclerosis in the aortic sinus of ApoE−/− mice fed a Western diet. We propose this is due to increased numbers of lesional macrophages and decreased macrophage apoptosis in the aortic wall. These findings demonstrate that G2A-deficiency triggers an abnormal inflammatory macrophage phenotype that contributes to atherosclerosis development, suggesting a critical role of G2A in macrophage homeostasis.
Acknowledgment
The authors would like to thank Nathan Linden for assistance with real-time RT-PCR, and Angela Whetzel for helpful technical assistance. We thank Dr. Owen Witte (UCLA) for the gift of the G2A−/− mice.
Sources of Funding
These studies were funded by NIH R01 HL071141 (C.C.H.).
Footnotes
Disclosures
None.
Reference List
- 1.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 3.Cybulsky MI, Lichtman AH, Hajra L, Iiyama K. Leukocyte adhesion molecules in atherogenesis. Clin Chim Acta. 1999;286:207–218. doi: 10.1016/s0009-8981(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 4.Berliner JA, Vora DK, Shih PT. Control of leukocyte adhesion and activation in atherogenesis. In: Pearson J, editor. Vascular Adhesion and Inflammation. Basel, Switzerland: Birkhauser Verlag; 2001. [Google Scholar]
- 5.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 6.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 8.Lichtman AH, Cybulsky M, Luscinskas FW. Immunology of atherosclerosis: the promise of mouse models. Am J Pathol. 1996;149:351–357. [PMC free article] [PubMed] [Google Scholar]
- 9.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 11.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 12.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 13.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng Z, Fluckiger AC, Nisitani S, Wahl MI, Le LQ, Hunter CA, Fernal AA, Le Beau MM, Witte ON. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc Natl Acad Sci U S A. 1998;95:12334–12339. doi: 10.1073/pnas.95.21.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le LQ, Kabarowski JH, Wong S, Nguyen K, Gambhir SS, Witte ON. Positron emission tomography imaging analysis of G2A as a negative modifier of lymphoid leukemogenesis initiated by the BCR-ABL oncogene. Cancer Cell. 2002;1:381–391. doi: 10.1016/s1535-6108(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 16.Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 17.Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- 18.Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 19.Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005;17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Radu CG, Nijagal A, McLaughlin J, Wang L, Witte ON. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci U S A. 2005;102:1632–1637. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parks BW, Gambill GP, Lusis AJ, Kabarowski JH. Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J Lipid Res. 2005;46:1405–1415. doi: 10.1194/jlr.M500085-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Bolick DT, Whetzel AM, Skaflen M, Deem TL, Lee J, Hedrick CC. Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ Res. 2007;100:572–580. doi: 10.1161/01.RES.0000258877.57836.d2. [DOI] [PubMed] [Google Scholar]
- 23.Rikitake Y, Hirata K, Yamashita T, Iwai K, Kobayashi S, Itoh H, Ozaki M, Ejiri J, Shiomi M, Inoue N, Kawashima S, Yokoyama M. Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:2049–2053. doi: 10.1161/01.atv.0000040598.18570.54. [DOI] [PubMed] [Google Scholar]
- 24.Le LQ, Kabarowski JH, Weng Z, Satterthwaite AB, Harvill ET, Jensen ER, Miller JF, Witte ON. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity. 2001;14:561–571. doi: 10.1016/s1074-7613(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 25.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 26.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–138. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-Phosphate Induces an Antiinflammatory Phenotype in Macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolick DT, Srinivasan S, Whetzel A, Fuller LC, Hedrick CC. 12/15 Lipoxygenase Mediates Monocyte Adhesion to Aortic Endothelium in Apolipoprotein E-Deficient Mice Through Activation of RhoA and NF-{kappa}B. Arterioscler Thromb Vasc Biol. 2006;26:1260–1266. doi: 10.1161/01.ATV.0000217909.09198.d6. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. Increased Production of 12/15 Lipoxygenase Eicosanoids Accelerates Monocyte/Endothelial Interactions in Diabetic db/db Mice. J Biol Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 31.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 33.Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem. 2002;277:20453–20460. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- 34.Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, Hedrick CC. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:2301–2307. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- 35.Movat HZ. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch Pathol. 1955;60:289–295. [PubMed] [Google Scholar]
- 36.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 37.Adhikari N, Charles N, Lehmann U, Hall JL. Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr Atheroscler Rep. 2006;8:252–260. doi: 10.1007/s11883-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 38.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–C640. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- 39.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 40.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 41.Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci U S A. 2004;101:245–250. doi: 10.1073/pnas.2536801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell. 2005;16:2234–2247. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witte ON, Kabarowski JH, Xu Y, Le LQ, Zhu K. Retraction. Science. 2005;307:206. doi: 10.1126/science.307.5707.206b. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LE, Elias MS, Bolick DT, Skaflen MD, Green RM, Hedrick CC. The G protein-coupled receptor G2A: involvement in hepatic lipid metabolism and gallstone formation in mice. Hepatology. 2008;48:1138–1148. doi: 10.1002/hep.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks BW, Lusis AJ, Kabarowski JH. Loss of the lysophosphatidylcholine effector, G2A, ameliorates aortic atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2703–2709. doi: 10.1161/01.ATV.0000246774.02426.71. [DOI] [PubMed] [Google Scholar]
- 47.Lin P, Ye RD. The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J Biol Chem. 2003;278:14379–14386. doi: 10.1074/jbc.M209101200. [DOI] [PubMed] [Google Scholar]
- 48.Kutuk O, Basaga H. Bcl-2 protein family: implications in vascular apoptosis and atherosclerosis. Apoptosis. 2006;11:1661–1675. doi: 10.1007/s10495-006-9402-7. [DOI] [PubMed] [Google Scholar]
- 49.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–2959. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 51.Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: role of p53 and NFkB. Mol Pathol. 1998;51:55–61. doi: 10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 53.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 54.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 55.Tabas I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr Drug Targets. 2007;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 56.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic "find-me" signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]