Abstract
Purpose
This randomized, double-blind, placebo-controlled study was undertaken to evaluate the efficacy and safety of entecavir in Chinese patients with lamivudine-refractory chronic hepatitis B.
Methods
One hundred forty-five lamivudine-refractory patients with chronic hepatitis B were randomized to double-blind treatment with oral entecavir 1 mg (n = 116) or placebo (n = 29) daily for 12 weeks, followed by 36 weeks of open-label entecavir treatment. The primary efficacy endpoint was the mean change from baseline in serum hepatitis B virus (HBV) DNA by polymerase chain reaction (PCR) assay at week 12.
Results
At week 12, the mean change from baseline in serum HBV DNA by PCR assay was –4.30 log10 copies/ml for patients on entecavir compared to –0.15 log10 copies/ml for patients on placebo (P < .0001). Among patients with baseline serum alanine aminotransferase (ALT) >1 × upper limit of normal (ULN), a higher proportion of entecavir than placebo patients (68% vs. 6%, respectively) achieved ALT normalization by week 12 (P < .0001). After 48 weeks of entecavir treatment, the mean change in HBV DNA by PCR assay was –5.08 log10 copies/ml, and 85% of patients with baseline ALT >1 × ULN had achieved ALT normalization. The safety profile of entecavir was similar to that of placebo during the first 12 weeks of blinded dosing. Entecavir was also well tolerated during 36 weeks of open-label treatment.
Conclusions
Lamivudine-refractory chronic hepatitis B patients treated with entecavir demonstrated marked HBV DNA reduction and normalization of ALT in most cases. Entecavir treatment for 48 weeks was well tolerated.
Keywords: Chronic hepatitis B, Entecavir, Lamivudine-refractory, Chinese
Introduction
Approximately out of the 400 million people who are chronically infected with hepatitis B virus (HBV), 275 million live in the Asia-Pacific region [1, 2]. In China, approximately 170 million people are chronically infected with HBV [3], and between 250,000 and 280,000 deaths per year are attributed to HBV infection [4]. The primary treatment goal for chronic hepatitis B is suppression of viral replication, thereby reducing the risk of necroinflammatory activity and liver disease progression [5].
Lamivudine was the first nucleoside analogue to be approved for treatment of chronic hepatitis B. Although efficacious and well tolerated, lamivudine therapy is associated with a high rate of viral resistance [6–9] that may reach up to 70% after 4 years of treatment [6, 10, 11]. While some studies have suggested that continuing lamivudine in the presence of resistance may promote HBeAg seroconversion and maintain lower alanine aminotransferase (ALT) and HBV DNA levels than were present at baseline [11–13], accumulated evidence now shows that there is no benefit to continuing lamivudine in this population. Patients with lamivudine-resistant HBV who continue to receive lamivudine have been shown to experience increases in viral load [6, 10, 14–16], hepatic flares that may lead to decompensation [15, 17–19], and reduction or reversal of histologic improvement [20]. These observations highlight the need for a treatment alternative for chronic hepatitis B patients who have developed lamivudine resistance.
Entecavir is a guanosine analogue and a potent and selective inhibitor of HBV polymerase [21]. In vitro studies have shown that entecavir effectively suppresses the replication of lamivudine-resistant HBV with an EC50 of 0.026 μM [22]. The selection of the 1 mg dose of entecavir for evaluation in the current study in lamivudine-refractory patients resulted from a multinational phase II dose-ranging study in which entecavir 1 mg demonstrated superior efficacy to continued lamivudine 100 mg, and yielded the greatest viral load reduction compared with the other doses of entecavir studied (0.5 and 0.1 mg) [23]. The results of a large, multinational phase III study conducted outside China have recently been reported and demonstrate that switching lamivudine-refractory patients to entecavir results in significantly improved rates of histologic, virologic, and biochemical response [24].
The current study was designed to assess the efficacy and safety of entecavir 1 mg daily compared with placebo in Chinese patients with lamivudine-refractory chronic hepatitis B.
Patients and methods
Study population
Patients were eligible for the study if they met the following inclusion criteria: males and females of at least 16 years of age; documented history of chronic hepatitis B (hepatitis B surface antigen [HBsAg](+) for ≥6 months); HBV DNA levels ≥105 copies/ml by polymerase chain reaction (PCR) assay at screening; ALT levels in the range of normal to ≤10 × ULN; and history of prior lamivudine therapy. Evidence of lamivudine-refractory status was defined as follows: persistent HBV viremia (HBV DNA level ≥0.7 MEq/ml by branched-chain DNA (bDNA) assay or ≥105 copies/ml by PCR assay) after at least 36 weeks of lamivudine; or breakthrough viremia after achieving undetectable HBV DNA (by bDNA assay) following at least 24 weeks of lamivudine; or recurrence of viremia after discontinuing lamivudine in patients who had achieved undetectable HBV DNA (by bDNA assay) and were hepatitis B e antigen negative (HBeAg(−)) following at least 36 weeks of lamivudine; or documented YMDD mutation and HBV viremia, regardless of duration of lamivudine therapy. Patients must have discontinued lamivudine at least 12 weeks prior to enrollment. Patients were also required to have compensated liver function, with prothrombin international normalized ratio ≤1.5, serum albumin ≥3.5 g/dl, and total serum bilirubin ≤2.5 mg/dl. Patients who were either HBeAg(+) or HBeAg(–), or had hepatitis B e antibody (HBeAb(+)) disease, were eligible.
Exclusion criteria included the following: coinfection with human immunodeficiency virus, hepatitis C virus, or hepatitis D virus; other forms of liver disease; 12 or more weeks of therapy with a nucleoside or nucleotide analogue other than lamivudine; and therapy with an immunomodulator or nucleoside or nucleotide analogue (other than lamivudine) with activity against HBV within 24 weeks of randomization.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in a manner consistent with Good Clinical Practice and applicable regulatory requirements. Written informed consent was obtained from all patients.
Study design
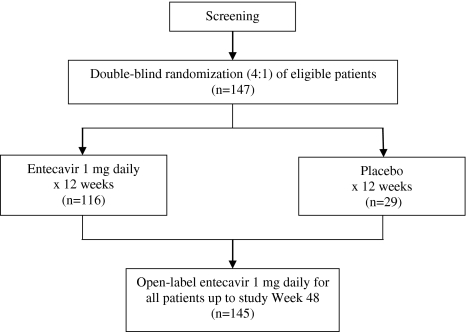
This randomized (4:1), double-blind, placebo-controlled study was conducted at five investigative sites in China. Patients were randomized (4:1) to receive oral entecavir 1 mg daily or placebo for 12 weeks (Fig. 1). All patients completing 12 weeks of blinded treatment, including those initially randomized to placebo, received open-label entecavir 1 mg daily for the next 36 weeks. HBV DNA levels by PCR assay were determined at baseline and at weeks 2, 4, 8, 12, 24, 36, and 48. HBV DNA levels by bDNA assay and HBV serologies (HBsAg, HBsAb, HBeAg, and HBeAb) were evaluated at baseline and at weeks 12 and 48. Serum ALT was assessed at every clinic visit: baseline, weeks 2 and 4, and every 4 weeks thereafter through the end of the study. Patients were regularly monitored for laboratory safety evaluations and adverse events during blinded and open-label therapy. Patients who completed 48 weeks of treatment were given the option of enrolling in an open-label rollover study of entecavir. Patients could continue on entecavir in the current study until the rollover protocol was available at the investigative site.
Fig. 1.
Study design
Study objectives
The primary efficacy endpoint was the mean change from baseline in serum HBV DNA by PCR assay at week 12. Secondary endpoints included the following: mean change in HBV DNA by PCR assay at week 48; proportions of patients achieving HBV DNA <300 copies/ml by PCR assay at weeks 12 and 48; proportions achieving HBV DNA <0.7 MEq/ml by bDNA assay at weeks 12 and 48; ALT normalization (≤1 × ULN) at weeks 12 and 48; and HBeAg seroconversion (HBeAg loss and appearance of HBeAb) at week 48. The primary safety endpoint was the proportion of patients discontinuing study drug owing to adverse events. Secondary safety endpoints included proportions of patients experiencing adverse events, serious adverse events, and laboratory abnormalities. An ALT flare was defined as an ALT measurement >2 × baseline and >10 × ULN.
Resistance analysis
All HBV DNA samples from treated patients were evaluated at baseline for substitutions in the HBV DNA polymerase associated with lamivudine resistance (rtM204 and rtL180). For patients displaying virologic breakthrough, genotypic analysis was performed on samples obtained at baseline and at week 48 to assess the emergence of substitutions conferring resistance to entecavir. Virologic breakthrough was defined as an increase of ≥1 log10 copies/ml above the patient’s nadir on entecavir therapy as determined by two sequential HBV DNA measurements by PCR assay at least 2 weeks apart or last on-treatment measurement. HBV DNA was extracted from patient samples, PCR amplified, and amino acids 1–344 of the reverse transcriptase sequenced as described elsewhere [25].
Assay methodology
Serum HBV DNA was quantified using the Roche Cobas Amplicor® Monitor PCR assay (Roche, IN, USA; limit of quantification, 300 copies/ml) and the Quantiplex® bDNA assay (Bayer-Versant Diagnostics, formerly Chiron Diagnostics, Emeryville, CA, USA; limit of quantification, 0.7 MEq/ml). HBV serologies (HBsAg, HBsAb, HBeAg, HBeAb) were measured using the Abbott AxSYM microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, IL, USA). Serum ALT was quantified by ultraviolet enzymatic assay. Detection of HBV genotypes was performed using the Inno-LiPA HBV genotyping assay (Innogenetics NV, Ghent, Belgium). Lamivudine-resistance mutations in the HBV DNA polymerase sequence were detected using the Inno-LiPA HBV DR assay (Innogenetics NV, Ghent, Belgium).
Data analysis
The planned sample size of 125 patients, 100 patients randomized to receive entecavir and 25 patients randomized to receive placebo, provided >90% power to demonstrate superiority of entecavir over placebo for the primary efficacy endpoint. Treatment comparisons for the primary endpoint focused on evaluable patients and were based on a linear regression model for HBV DNA by PCR assay at week 12 with covariates for baseline HBV DNA and treatment. The test for superiority of entecavir over placebo was based on a two-sided t-test for the treatment difference estimated from the linear regression model. A P value of <.05 was used to demonstrate superiority. For the analyses of binary outcomes, patients with a missing measurement for an endpoint were considered as having nonresponse for that endpoint.
Results
Study population
Of the 147 randomized patients, 145 received at least one dose of blinded study medication: 116 in the entecavir arm and 29 in the placebo arm. Of the 145 patients who started study therapy, 144 completed the 12-week blinded treatment phase. One patient who was randomized to placebo discontinued blinded treatment at week 10 owing to adverse events, but was granted an exception to start open-label entecavir. Of the 145 patients who entered the open-label phase of the study, 141 completed 36 weeks of open-label entecavir treatment.
Demographic and pretreatment baseline characteristics were comparable between the two treatment arms (Table 1). Overall, the majority of patients were HBeAg(+) males. The mean baseline HBV DNA level by PCR assay was 8.79 log10 copies/ml, and 52% of patients had ALT >1 × ULN. Sixteen percent of patients had a history of prior treatment with interferon-α. At baseline, 42% of patients exhibited mutations associated with lamivudine resistance detected by Inno-LiPA HBV DR assay. Approximately 70% of patients were infected with HBV genotype C and 30% were infected with HBV genotype B.
Table 1.
Patient demographics and clinical characteristics at baseline
| Characteristic | Entecavir 1 mg (n = 116) | Placebo (n = 29) | Total (n = 145) |
|---|---|---|---|
| Age (y), mean (range) | 34 (16, 66) | 38 (19, 57) | 35 (16, 66) |
| Male (%) | 87 (75) | 22 (76) | 109 (75) |
| HBeAg(+) (%) | 106 (91) | 25 (86) | 131 (90) |
| HBV DNA by PCR, mean ± SD, log10 copies/ml | 8.84 ± 0.88 | 8.60 ± 0.80 | 8.79 ± 0.87 |
| Range (log10 copies/ml) | 4.89, 10.78 | 6.39, 9.79 | 4.89, 10.78 |
| ALT, mean ± SD, U/L | 85.04 ± 96.6 | 104.24 ± 91.5 | 88.89 ± 95.6 |
| Range, U/L | 10, 760 | 15, 350 | 10, 760 |
| >1.0 × ULN (%) | 59 (51) | 16 (55) | 75 (52) |
| Documented lamivudine-resistance mutations (%) | 48 (41) | 13 (45) | 61 (42) |
| HBV genotype (%) | |||
| B | 37 (32) | 6 (21) | 43 (30) |
| C | 78 (67) | 22 (76) | 100 (69) |
| D | 1 (<1) | 0 | 1 (<1) |
| Indeterminate | 0 | 1 (3) | 1 (<1) |
| Prior interferon-α treatment | 17 (15) | 6 (21) | 23 (16) |
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; ULN, upper limit of normal
Virologic response
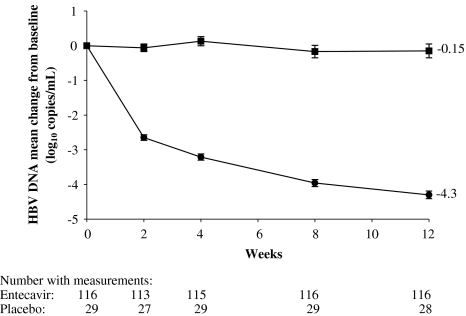
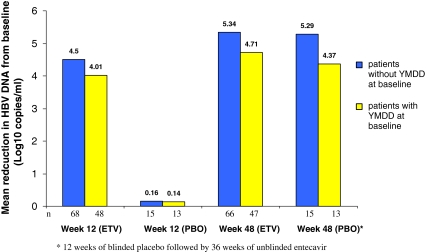
At week 12, the mean change from baseline in HBV DNA level by PCR assay was –4.30 log10 copies/ml for entecavir-treated patients compared to –0.15 log10 copies/ml for patients who received placebo (P < .0001; Table 2). As early as week 2, entecavir reduced HBV DNA levels by a mean of 2.65 log10 copies/ml, and the mean HBV DNA level for entecavir-treated patients continued to decline through the remainder of the blinded dosing period (Fig. 2). At week 12, the mean change from baseline in HBV DNA by PCR assay was significantly greater in the entecavir group compared with placebo in patients with lamivudine-resistance mutations at baseline (–4.01 vs. –0.14 log10 copies/ml, P < .0001) and in patients without lamivudine-resistance mutations at baseline (–4.50 vs. –0.16 log10 copies/ml, P < .0001; Fig. 4). At week 12, the proportion of patients with HBV DNA <0.7 MEq/ml by bDNA assay was 74% for entecavir patients and 10% for placebo patients (P < .0001). The proportion of patients with HBV DNA <300 copies/ml by PCR assay at week 12 was 8% for entecavir patients and 0% for placebo patients (P = 0.12). The virologic response to entecavir was consistent across baseline HBV genotypes.
Table 2.
Efficacy results at week 12 and week 48
| Initial treatment regimen | ||
|---|---|---|
| Endpoints | Entecavir 1 mg (n = 116) | Placebo (n = 29) |
| HBV DNA by Roche PCR assay | ||
| Mean change from baseline at week 12, log10 copies/ml (SE) | –4.30 (0.11)† | –0.15 (0.20)† |
| Mean change from baseline at week 48, log10 copies/ml (SE) | –5.08 (0.13) | –4.86 (0.25) |
| HBV DNA <300 copies/ml at week 12 (%) | 9/116 (8)‡ | 0/29 (0)‡ |
| HBV DNA <300 copies/ml at week 48 (%) | 31/116 (27) | 12/29 (41)a |
| HBV DNA by bDNA assay | ||
| HBV DNA <0.7 MEq/ml at week 12b (%) | 85/115 (74)† | 3/29 (10)† |
| HBV DNA <0.7 MEq/ml at week 48b (%) | 85/115 (74) | 22/29 (76)a |
| Normalization of ALTc (%) | ||
| Week 12 (%) | 40/59 (68)† | 1/16 (6)† |
| Week 48 (%) | 50/59 (85) | 15/17 (88)a |
| HBeAg seroconversiond | ||
| Week 48 (%) | 6/106 (6) | 2/23 (9)a |
For all analyses, with the exception of mean reduction in HBV DNA by PCR, patients with a missing measurement for an endpoint were considered as having nonresponse for that endpoint
ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; SE, standard error of the mean
†P < .0001; ‡not significant
aPatients in both treatment arms who completed 12 weeks of blinded dosing started 36 weeks of open-label entecavir. Hence, all observations at week 48 relate to patients receiving open-label entecavir but are presented according to treatment arm during the initial blinded dosing period
bIn patients with baseline HBV DNA ≥0.7 MEq/ml by bDNA assay
cALT ≤1 × ULN in patients with baseline ALT >1 × ULN
dIn patients who were HBeAg(+) at baseline
Fig. 2.
Mean change from baseline in HBV DNA by PCR assay through 12 weeks of blinded treatment with entecavir 1 mg/d (●) or placebo (■). Error bars represent the standard error of the mean
Fig. 4.
Mean change from baseline in HBV DNA by PCR assay at weeks 12 and 48 for patients with and without YMDD mutations at baseline
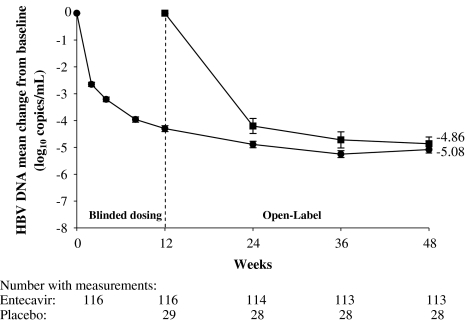
Patients who were initially randomized to entecavir (for the12-week blinded dosing phase) achieved a mean change in HBV DNA by PCR assay of –5.08 log10 copies/ml at week 48 (Table 2; Fig. 3). Patients initially randomized to placebo for 12 weeks and switched to entecavir for the next 36 weeks had a mean change in HBV DNA by PCR assay of –4.86 log10 copies/ml. After 48 weeks of entecavir (12 weeks blinded plus 36 weeks open-label), patients with lamivudine-resistance mutations at baseline achieved a mean change in HBV DNA of –4.71 log10 copies/ml and patients without lamivudine-resistance mutations at baseline achieved a mean change in HBV DNA of –5.34 log10 copies/ml (Fig. 4). Entecavir treatment for 48 weeks achieved consistent mean reductions in HBV DNA in both patients infected with HBV genotypes B (–4.96 log10 copies/ml) and C (–5.12 log10 copies/ml). Among patients who received 48 weeks of entecavir treatment, the proportion achieving HBV DNA <0.7 MEq/ml was 74% and the proportion achieving HBV DNA <300 copies/ml by PCR assay was 27%.
Fig. 3.
Mean change from baseline in HBV DNA by PCR assay on entecavir through 48 weeks. Circles represent patients initially randomized to entecavir and squares represent patients initially randomized to placebo. Error bars represent the standard error of the mean
Serologic response
Among patients who were positive for HBeAg at study entry, 12/129 (9%) patients lost HBeAg and 8/129 (6%) patients developed HBeAg seroconversion by week 48.
Biochemical response
Entecavir was superior to placebo for achievement of ALT normalization at week 12 among patients with baseline ALT >1 × ULN. Sixty-eight percent of patients receiving entecavir versus 6% of patients receiving placebo achieved ALT normalization by week 12 (P < .0001; Table 2). By week 48, the proportions of patients achieving ALT normalization had increased to 85% in patients initially randomized to entecavir and to 88% in those initially randomized to placebo.
Resistance
Thirteen patients demonstrated virologic breakthrough during 48 weeks of entecavir treatment. None of these 13 patients experienced an ALT flare in association with the virologic breakthrough. Previous studies have shown that among patients harboring lamivudine-resistance mutations rtL180M and rtM204V, an additional substitution at residue rtT184, rtS202, or rtM250 may be associated with reduced susceptibility to entecavir [25]. Genotypic analyses on paired baseline and on-treatment samples from the 13 patients in the current study found no emerging substitutions at residues rtT184, rtS202, or rtM250, suggesting that the observed virologic breakthroughs were not due to the emergence of genotypic resistance to entecavir.
Safety
Mean exposure to entecavir during the double-blind dosing phase was 12 weeks. Proportions of patients experiencing any adverse event during the blinded dosing phase were comparable in the two treatment arms: 33% and 28% of patients receiving entecavir and placebo, respectively. The most frequently reported adverse events (occurring in ≥5% of patients in any treatment arm) are shown in Table 3. Upper respiratory tract infections were more frequent in patients receiving entecavir; however, all upper respiratory tract infections were mild to moderate in severity and none was considered related to the study drug by the investigator. One patient receiving placebo discontinued treatment at week 10 owing to grade 4 elevated ALT, AST (aspartate aminotransferase), and total bilirubin levels. ALT flares occurred in 2 (2%) patients receiving entecavir and 3 (10%) patients receiving placebo during the blinded dosing period. The two ALT flares in entecavir patients were self-limited and temporally associated with marked declines in HBV DNA by PCR assay. The ALT flares in the placebo group were associated with persistently elevated or rising HBV DNA by PCR assay. No deaths were reported on blinded treatment.
Table 3.
Summary of adverse events during 12-week blinded-dosing phase
| Entecavir 1 mg (n = 116) | Placebo (n = 29) | |
|---|---|---|
| Any adverse event (%)a | 38 (33) | 8 (28) |
| Most frequent adverse events (%) | ||
| Fatigue | 8 (7) | 2 (7) |
| Upper respiratory tract infection | 8 (7) | 0 |
| ALT increased | 2 (2) | 3 (10) |
| AST increased | 2 (2) | 3 (10) |
| Abdominal discomfort | 2 (2) | 2 (7) |
| Dizziness | 1 (<1) | 4 (14) |
| Serious adverse event (%) | 0 (0) | 1 (3) |
| Discontinuation due to adverse event (%) | 0 (0) | 1 (3) |
| Death | 0 (0) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase
aIncluding laboratory abnormalities reported by investigator as an adverse event
Mean exposure to entecavir during the open-label phase was 40.1 weeks. The most frequently reported adverse events for this phase are listed in Table 4. No adverse events during open-label entecavir resulted in discontinuation of study drug. There were no ALT flares during the open-label phase. Two patients had serious adverse events during open-label entecavir treatment; both had received placebo during the blinded-dosing phase. One of these patients had elevated ALT and AST at the last study visit of the blinded-dosing phase, and these events resolved on open-label entecavir treatment. The second patient had discontinued placebo during week 10 of the blinded-dosing phase with elevated ALT (1,540 U/L), AST (1,081 U/L), and total bilirubin (32 mg/dl) and received supportive care and traditional Chinese medications. A protocol exception was granted for open-label entecavir. The patient subsequently developed hepatorenal syndrome, peritonitis, and hepatic failure; and the patient subsequently died of hepatic failure during week 15.
Table 4.
Summary of adverse events during 36-week open-label phase
| Entecavir 1 mg (n = 145) | |
|---|---|
| Any adverse event (%)a | 79 (54) |
| Most frequent adverse events (%) | |
| Upper respiratory tract infection | 17 (12) |
| Fatigue | 10 (7) |
| Hepatic pain | 10 (7) |
| Serious adverse event (%) | 2 (1) |
| Discontinuation due to adverse event (%) | 0 (0) |
| Death | 1 (<1)b |
aIncluding laboratory abnormalities reported by investigator as an adverse event.
bConsidered unrelated to study medication by investigator
The proportion of patients with hematologic abnormalities on entecavir treatment was low. Grade 3 or 4 hematologic abnormalities were reported for three patients, all of whom had abnormal baseline values. Most patients had normal renal function tests at baseline and on entecavir treatment. Elevations in amylase were reported for 28% of patients on treatment, but grade 3 or 4 abnormalities were rare (one patient). No patients were reported to have a diagnosis of clinical pancreatitis.
Discussion
Chronic hepatitis B is highly prevalent in the Asia-Pacific region. This study evaluated the role of entecavir in lamivudine-refractory chronic hepatitis B within the Chinese patient population. As early as week 2, entecavir-treated patients demonstrated potent and rapid suppression of HBV DNA, with a mean reduction of 2.65 log10 copies/ml, and by week 12 of entecavir treatment, the mean reduction in HBV DNA was 4.30 log10 copies/ml. The magnitude of viral load reduction observed after 48 weeks of entecavir in this study (mean of 5.08 log10 copies/ml) was consistent with that observed in patients receiving entecavir for 48 weeks in a multinational phase III trial in lamivudine-refractory patients [24]. This level of HBV DNA suppression also compares favorably to that reported in patients with lamivudine-resistant HBV receiving adefovir in which the mean reduction in HBV DNA was 4.00 log10 copies/ml after 1 year of treatment [26].
Patients included in this study were lamivudine-refractory and had stopped lamivudine treatment at least 12 weeks prior to enrollment. This study design reflects the real-world situation in which lamivudine treatment is often stopped when resistance emerges. Because the replicative capacity of lamivudine-resistant virus may be less than that of wild-type virus, the reemergence of wild-type virus (and archiving of mutant virus) after cessation of lamivudine could be anticipated [19, 27]. Although the detection of lamivudine-resistant virus at baseline was not a requirement for randomization, 42% of randomized patients had HBV DNA polymerase mutations at baseline. Among patients both with and without documented lamivudine-resistance mutations at baseline, entecavir suppressed HBV DNA over 5.0 log10 copies/ml after 48 weeks. The InnoLipa HBV DR assay is capable of detecting lamivudine-resistant subpopulations of virus when they exceed 10% of the sample [28], so it is possible that a larger proportion of patients meeting the study inclusion criteria as lamivudine-refractory were infected with a subpopulation of lamivudine-resistant virus, but in a lower fraction than would be detected by this assay.
Among HBeAg(+) patients enrolled in this study, the rate of HBeAg seroconversion after 1 year of treatment was low (6%). Eighty percent of patients who enrolled in this study had ALT <2 × ULN and 16% of patients had received interferon therapy, which may help explain the low rates of seroconversion. Furthermore, lower serologic response rates in patients who are refractory to lamivudine are to be expected given the likelihood that patients who have remained HBeAg(+) despite prior lamivudine treatment may have a decreased ability to mount an immune-mediated seroconversion response. These findings suggest that long-term therapy may be needed to achieve HBeAg seroconversion in the lamivudine-refractory patient population.
Serum ALT levels, which serve as a marker for hepatic inflammation in patients with chronic hepatitis B patients, normalized in 85% of patients after 48 weeks of entecavir treatment. In the multinational phase III trial of entecavir in lamivudine-refractory patients, normalization of ALT was observed in 61% of entecavir-treated patients with histologic improvement (≥2 point improvement in Knodel necroinflammatory score, with no worsening of Ishak fibrosis) and a reduction in fibrosis being observed in 55% and 34% of patients, respectively [24]. Liver biopsy was not included in the study protocol as the standard of care in China at that time precluded doing so.
Entecavir’s potent suppression of viral replication and high genetic barrier helps to minimize the emergence of virologic resistance. Tenney and coworkers have shown that resistance to entecavir requires the presence of lamivudine-resistance mutations rtL180M and/or rtM204V plus additional substitution in the HBV reverse transcriptase at positions rtT184, rtS202, or rtM250 [25]. In this study, there was no evidence of emerging substitutions associated with virologic resistance to entecavir. Extended treatment and observation through 2 years in a similar trial with entecavir in this patient population showed that virologic breakthrough due to entecavir resistance was observed in 9% of patients [29].
During 12 weeks of blinded dosing and a further 36 weeks of open-label treatment, entecavir was well tolerated and no significant safety issues were identified. Fewer ALT flares were observed in patients on blinded entecavir treatment compared to patients receiving placebo, and ALT flares on entecavir were infrequent, self-limited, and temporally associated with declining HBV DNA levels.
Treatment for lamivudine-refractory patients often poses a challenge, and results of this study are encouraging. In summary, this study demonstrates and confirms the antiviral activity and safety of entecavir in adults with lamivudine-refractory chronic hepatitis B infection.
Acknowledgments
The authors thank Dr. Ronald Rose, Pharmaceutical Research Institute, Bristol-Myers Squibb, Wallingford, Connecticut, USA, for his assistance with the resistance analysis.
Footnotes
This clinical trial was sponsored by Bristol-Myers Squibb Company, 5 Research Parkway, Wallingford, CT, USA.
References
- 1.Khan M, Dong JJ, Acharya SK, Dhagwahdorj Y, Abbas Z, Jafri W, et al. Hepatology issues in Asia: perspectives from regional leaders. J Gastroenterol Hepatol 2004;19:S419–30 [DOI]
- 2.Hepatitis B Foundation [accessed 2005 Jan 19]. Available at: http://www.hepb.org/02-0360.hepb
- 3.Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol 2002;67:447–50 [DOI] [PubMed]
- 4.Lau GKK. Hepatitis B infection in China. Clin Liver Dis 2001;5:361–79 [DOI] [PubMed]
- 5.Liaw YF, Leung N, Guan R, Lau GKK, Merican I, McCaughan G, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int 2005;25:472–89 [DOI] [PubMed]
- 6.Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 2000;32(4 Pt 1):828–34 [DOI] [PubMed]
- 7.Liaw YF. Impact of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. Antivir Chem Chemother 2001;12(Suppl 1):67–71 [PubMed]
- 8.Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, et al. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest 2001;107:449–55 [DOI] [PMC free article] [PubMed]
- 9.Zoulim F. Detection of hepatitis B virus resistance to antivirals. J Clin Virol 2001;21:243–53 [DOI] [PubMed]
- 10.Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis. J Gastroenterol Hepatol 2004;19:1276–82 [DOI] [PubMed]
- 11.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. for the Asia Hepatitis Lamivudine Study Group. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology 2000;119:172–80 [DOI] [PubMed]
- 12.Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001;33:1527–32 [DOI] [PubMed]
- 13.Leung N. Clinical experience with lamivudine. Semin Liver Dis 2002;22 Suppl 1:15–21 [DOI] [PubMed]
- 14.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. for the Asia Hepatitis Lamivudine Study Group. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998;339:61–8 [DOI] [PubMed]
- 15.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology 1999;30:567–72 [DOI] [PubMed]
- 16.Papatheodoridis GV, Dimou E, Laras A, Papadimitropoulos V, Hadziyannis SJ. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology 2002;36:219–26 [DOI] [PubMed]
- 17.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003;125:1714–22 [DOI] [PubMed]
- 18.Chen CH, Lee CM, Lu SN, Wang JH, Tung HD, Hung CH, et al. Comparison of clinical outcome between patients continuing and discontinuing lamivudine therapy after biochemical breakthrough of YMDD mutants. J Hepatol 2004;41:454–61 [DOI] [PubMed]
- 19.Liaw YF, Chien RN, Yeh CT. No benefit to continue lamivudine therapy after emergence of YMDD mutations. Antivir Ther 2004;9:257–62 [PubMed]
- 20.Dienstag JL, Goldin RD, Heathcote EJ, Hann HWL, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology 2003;124:105–17 [DOI] [PubMed]
- 21.Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother 1997;41:1444–8 [DOI] [PMC free article] [PubMed]
- 22.Levine S, Hernandez D, Yamanaka G, Zhang S, Rose R, Weinheimer S. Efficacies of entecavir against lamivudine-resistant hepatitis B virus replication and recombinant polymerases in vitro. Antimicrob Agents Chemother 2002;46:2525–32 [DOI] [PMC free article] [PubMed]
- 23.Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, et al. A dose-ranging study of the efficacy and tolerability of entecavir in lamivudine-refractory chronic hepatitis B patients. Gastroenterology 2005;129:1198–209 [DOI] [PubMed]
- 24.Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006;130:2039–49 [DOI] [PubMed]
- 25.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother 2004;48:3498–507 [DOI] [PMC free article] [PubMed]
- 26.Peters MG, Hann HW, Martin P, Heathcote EJ, Buggisch P, Rubin R, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2004;126:91–101 [DOI] [PubMed]
- 27.Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology 1998;27:1711–6 [DOI] [PubMed]
- 28.Pas SD, de Man RA, Fries E, Osterhaus A, Niesters H. The dynamics of mutations in the YMDD motif of the hepatitis B virus polymerase gene during and after lamivudine treatment as determined by reverse hybridisation. J Clin Virol 2002;25:63–71 [DOI] [PubMed]
- 29.Tenney DJ, Rose RE, Baldick CJ, Levine SM, Pokornowski KA, et al. Two-year assessment of Entecavir resistance in Lamivudine-refractory Hepatitis B Virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51:902–11. [DOI] [PMC free article] [PubMed]