Abstract
Purpose
Transcriptional silencing of tumor suppressor genes associated with DNA hypermethylation has been known as a hallmark of human cancer. In this study, we revealed that a local anesthetic, procaine (PCA), possessed growth-inhibitory and demethylating effect on human hepatoma cells in vitro and in vivo.
Methods
The viability of PCA-treated cells with or without trichostatin A (TSA) was investigated. To clarify the mechanism of the antiproliferating effect of PCA, TUNEL assay, FACS analysis, and morphological observation of PCA-treated cells were performed. The expression levels and epigenetic alterations of 4 genes inactivated by DNA hypermethylation in hepatocellular carcinoma (HCC) were examined in hepatoma cells with or without PCA treatment. The growth-inhibitory and demethylating effect of PCA in vivo was tested in nude mice bearing xenograft.
Results
The viability of HLE, HuH7, and HuH6 cells was significantly decreased by PCA treatment. In these cells, the combination treatment with TSA and PCA exhibited stronger reduction of the viability. Inhibition of S/G2/M transition, morphological changes such as vacuolation and no increase in apoptosis rate were observed in the PCA-treated HLE cells. All the genes transcriptionally suppressed by DNA hypermethylation were demethylated and reactivated with PCA treatment. PCA treatment led to partial demethylation and significant reduction in tumor volume in vivo.
Conclusions
These data indicated that PCA had growth-inhibitory and demethylating effects on human hepatoma cells in vitro and in vivo. PCA may be a candidate agent for future therapies for HCC.
Keywords: Hepatocellular carcinoma, Procaine, Methylation, Growth-inhibitory effect, Nonnucleoside analog
Introduction
Genetic alterations, including loss of heterogeneity (LOH), deletion, and mutation, are a hallmark of human cancer. In addition to these genetic alterations, epigenetic alterations, such as promoter hypermethylation and histone modifications, have recently been reported to be another hallmark of human cancer [1]. In normal tissues, CpG island methylation is limited to exceptional situations, such as imprinted alleles [2], genes on the inactive X chromosome in women [3] and germline- and tissue-specific genes [4, 5], while most human genes with CpG islands in their 5′-promoter region are usually unmethylated. On the other hand, in malignancies, global DNA hypomethylation and localized hypermethylation involving CpG islands occur [5]. Aberrant CpG island hypermethylation is clearly associated with transcriptional silencing of gene expression and plays an important role in the mechanism by which tumor suppressor genes are inactivated in cancer [5, 6]. Actually, many reports have indicated that aberrant methylation of tumor suppressor genes might be involved in the development of malignancies.
As described above, CpG island methylation is not a common finding in normal cells, so cytosine methylation of tumor suppressor genes in cancer cells seems to provide a highly specific target for pharmacologic therapy. Indeed, it has been reported that the reexpression of silenced tumor suppressor genes with a demethylating drug, 5-aza-2′-deoxycytidine (DAC), led to strong inhibitory effects on cancer cell growth in vitro and in vivo [7]. Although DAC has the most potent hypomethylating effect among several cytidine analog and was used for the early clinical trials in malignant hematologic diseases [8, 9], its mutagenic or carcinogenic properties possibly induced by its incorporation into DNA, resulting in blockage of DNA synthesis, prevent its widespread clinical use [10]. Thus, demethylation drugs that are nonnucleoside analog and not incorporated into DNA have been the target of investigations.
The antiarrhythmic drug procainamide (PCAA) has been known as an inhibitor of DNA methylation in human T cells for some time, and its demethylating effect on T cells leads to the overexpression of lymphocyte function-associated antigen 1 that makes T cells autoreactive [11, 12]. PCAA was also reported to restore the expression of tumor suppressor genes—glutathione S-transferase P1 (GSTP1), estrogen receptor (ER), retinoic-receptor (RAR) β, and p16INK4a—in cancer cells in which they had been silenced by promoter hypermethylation, showing mild inhibition of xenograft tumor growth [13, 14].
Recently, the local anesthetic procaine (PCA), an analog of procainamide, was also revealed to be a DNA-demethylating agent with growth-inhibitory effect in a human breast cancer cell line, MCF-7 [15]. However, there has been only this one report regarding PCA having a demethylating effect on cancer cells, and in this report, PCA was shown to have such effect only in vitro and in a certain cell type. We therefore investigated the properties of PCA, such as growth-inhibitory and demethylation effect, on hepatoma cells in vitro and in vivo.
Materials and methods
Cell culture and drug treatment
The human hepatoma and hepatoblastoma cell lines HLE, HuH7, HepG2, HuH6, and PLC/PRF/5 were obtained from the Health Science Research Bank (Osaka, Japan), Hep3B from the Cell Resource Center for Biomedical Research (Sendai, Japan), and primary human hepatocytes from ACBRI (Kirkland, WA). The hepatoma and hepatoblastoma cell lines were grown in Dulbecco’s modified Eagle medium (DMEM) (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Invitrogen). Primary human hepatocytes were cultured in CS-C complete medium (Cell Systems, Kirkland, WA) containing 10% serum and CS-C growth factor. For demethylation experiments, cells were plated at a density of 5.0 × 105 cells/100-mm dish and cultured for 24 h, followed by 96 h’ culture with DAC (Sigma Chemical Co., St. Louis, MO), or procaine hydrochloride (PCA) (Sigma), or procainamide hydrochloride (PCAA) (Sigma) at concentrations of 1 μM, 1 mM, and 1 mM, respectively. Culture medium with or without drug was changed every 24 h.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay
The viable cell numbers of hepatoma, hepatoblastoma, and primary human hepatocytes treated with 1 mM of PCA were determined by MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay; Promega Corp., Madison, WI). For the cell lines sensitive to PCA treatment, time-dependent (72, 96, and 120 h) and dose-dependent (0.5, 1, 1.5, and 2 mM) reduction of the viability by PCA were also examined by MTS assay. Next, to compare the cell viability-reduction effect of PCA with other drugs that elicit epigenetic alterations, hepatoma cells and primary human hepatocytes were treated with 1 μM of DAC, or 1 mM of PCAA, or 0.67 μM of trichostatin A (TSA) alone or in combination, and then the viable cell numbers were determined by MTS assay. TSA was added 24 h before the measurement by MTS assay. We also treated the cells with no drug as a control. All assays were carried out in triplicate.
TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay
For apoptosis analysis, we performed the TUNEL assay on HLE cells treated with no drug (control), or 1 μM of DAC, or 1 mM of PCA, or 1 mM of PCAA for 96 h according to the manufacturer’s instructions (Takara Bio Inc., Otsu, Japan). The proportion of TUNEL-positive cells was calculated by counting at least 500 cells randomly. All assays were carried out in triplicate.
Fluorescence-activated cell sorter (FACS) analysis
For cell cycle analysis, HLE cells and primary human hepatocytes were treated with or without 1 mM of PCA for 96 h, fixed in ice-cold 70% ethanol, and then subjected to DNA staining in PI buffer containing 0.05 mg/ml of propidium iodide (Wako Pure Chemical Industries Ltd., Osaka, Japan), 0.1% sodium citrate (Wako), 0.02 mg/ml of ribonuclease A (Wako), 0.3% polyoxyethylene (9) octylphenyl ether (NP-40) (Wako). DNA fluorescence of 20,000 cells was analyzed by using FACS Calibur and CellQuest software (BD Biosciences, San Jose, CA). All assays were carried out in triplicate.
Morphological observation
HLE cells were cultured for 5 days in medium with or without 1 mM of PCA. The culture medium was replaced daily. The morphological changes in HLE cells were then observed by microscope.
Sodium bisulfite DNA sequencing
Genomic DNA extracted from each hepatoma and hepatoblastoma cell line and primary human hepatocytes treated with no drug (control) was subjected to sodium bisulfite sequencing for 5′ CpG island of promoter region of p16INK4a, hepatocyte growth factor activator inhibitor 2/placental bikunin (HAI-2/PB), 14-3-3-sigma, and NADP (H): quinone oxidoreductase 1 (NQO1) genes, which were reported to be methylated in HCC [16–19]. To confirm DNA demethylation by the drugs, genomic DNA from the drug-treated cells was also subjected to bisulfite sequencing when they showed DNA hypermethylation of these genes. In brief, genomic DNA was modified by sodium bisulfite using a CpGenome DNA Modification Kit (Intergen, Purchase, NY) according to the manufacturer’s instructions. The bisulfite-modified DNA was amplified by nested PCR using the specific primers for these four genes. PCR products were then subcloned into the pCR2.1-TOPO vector using a TA cloning kit (Invitrogen) according to the manufacturer’s instructions. To determine the CpG methylation status of the 5′ CpG island of these genes, 10 clones from each cell line were sequenced using an ABI PRISM Dye Deoxy Terminator Cycle Sequencing Kit and analyzed on an ABI 377 DNA Sequencer (Applied Biosystems, Tokyo, Japan). Primer sequences were available on request.
Quantitative real-time RT-PCR
To examine the change of mRNA expression level of the above four genes with or without treatment of the drug, quantitative real-time RT-PCR was performed. About 3 μg of total RNA from drug-treated or untreated cell lines was subjected to RT reaction by using random oligonucleotide primers and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed in 96-well plates on the ABI PRISM 7000 Sequence Detection System, and data were collected using Taqman Universal Master Mix and Taqman Gene Expression Assays Inventoried (Applied Biosystems). The standard curve method was used to calculate target gene expression normalized to β-actin gene. Each sample was analyzed in triplicate. All experiments were repeated twice.
In vivo experiment
HLE cells were propagated in vivo by inoculation of 2.0 × 106 cells in 0.4 ml of PBS solution admixed with 75% Matrigel (BD Biosciences, Billerica, MA) into the subcutaneous region of the flanks of male nude mice (nu/nu) of BALB/c background, 5 weeks old, purchased from CLEA Japan (Tokyo, Japan). Mice carrying visible HLE xenograft tumors 2 weeks after inoculation were treated with weekly intravenous injections of PCA at a dose of 0.5 mg (low dose) or 1 mg (high dose) (in 0.2 ml of saline) or DAC at a dose of 17.5 μg (in 0.2 ml of saline) or PCAA at a dose of 1 mg (in 0.2 ml of saline) or saline alone (0.2 ml) as a control for 6 weeks. To assess the effects of PCA and other drugs on tumor size, the mice were killed 2 weeks after the last treatment injection. For each animal, the tumor was measured with a caliper, and tumor volume was determined using the following formula: volume = 0.5 × (width)2 × (length). Each experimental group consisted of six animals.
Genomic DNAs isolated from xenograft tumors of three saline-treated mice and three mice treated with 1 mg of PCA were also subjected to methylation analysis for p16INK4a, HAI-2/PB, and 14-3-3-sigma genes in the same way as our cell line analysis. To determine the CpG methylation status of the 5′ CpG islands of these genes, three clones from each mouse were sequenced. The study was conducted in accordance with the guidelines of our institute for the care and use of laboratory animals.
Statistical analysis
Differences in mean values were evaluated by unpaired t test and differences in frequencies were evaluated by Fisher exact test. Results were considered statistically significant at P < .05.
Results
PCA reduced the viable number of hepatoma cells
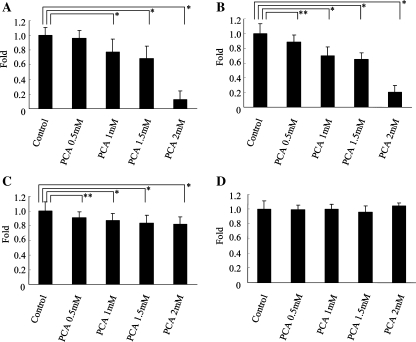
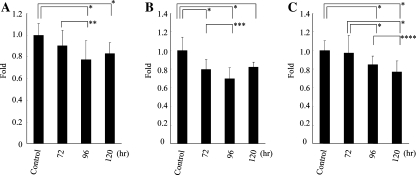
Assessment of the viable cell number after 96 h culture using the MTS assay revealed that the viability of HLE, HuH7, and HuH6 cells was significantly reduced by the treatment with 1 mM of PCA (77.4 ± 11.0%, 70.1 ± 11.4%, and 86.9 ± 9.2% relative to control, respectively; P < .0001) (Fig. 1A–C), while PCA did not reduce the viability of HepG2, Hep3B, PLC/PRF/5 cells, and primary human hepatocytes (data not shown) (Fig. 1D). MTS assay also revealed that PCA showed dose-dependent reduction of HLE and HuH7 cell viability. Especially, the 96 h treatment of 2 mM of PCA extremely reduced the viable number of HLE and HuH7 cells (12.3 ± 11.8% and 20.1 ± 9.4% relative to control, respectively), while primary human hepatocytes with the same treatment showed no reduction in viability at all (Fig. 1A–D). PCA also reduced the viable number of HLE, HuH7, and HuH6 cells in a time-dependent manner (Fig. 2A–C).
Fig. 1.
Dose-dependent antiproliferative effect of PCA on hepatoma cells. HLE (A), HuH7 (B), HuH6 (C) cells, and primary human hepatocytes (D) were cultured for 96 h with several concentrations of PCA. Shown are mean ± SD values from the results of three independent experiments. *P < .0001, **P = .0015
Fig. 2.
Time-dependent antiproliferative effect of PCA on hepatoma cells. HLE (A), HuH7 (B), and HuH6 (C) cells were cultured with 1 mM of PCA for 72, 96, and 120 h. Shown are mean ± SD values from the results of three independent experiments. *P < .0001, **P = .0005, ***P = .0072, ****P = .018
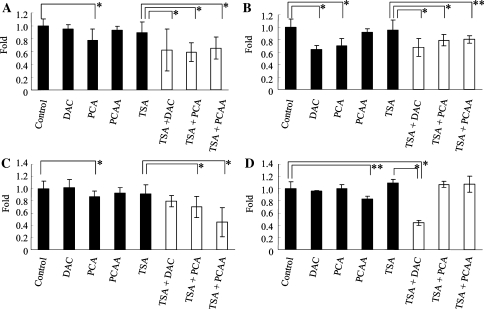
The MTS assay revealed that single doses of the other drugs that elicited epigenetic alterations, such as DAC, PCAA, and TSA, showed no reduction of the viability of HLE, HuH7, and HuH6 cells, with the exception of the treatment of HuH7 cells with 1 μM of DAC (Fig. 3A–C). The combination treatment with TSA and 1 mM of PCA significantly decreased the viability of HLE, HuH7, and HuH6 cells as well as the combination treatment with TSA and 1 μM of DAC or 1 mM of PCAA as compared with the effect of the treatment with TSA alone (P < .0001) (Fig. 3A–C). On the other hand, primary human hepatocytes treated with TSA and 1 mM of PCA showed no decrease in viable cell number, while the treatment with TSA and 1 μM of DAC was associated with significantly decreased viability (P < .0001). A single dose of 1 mM of PCAA also showed significant reduction in viable primary human hepatocytes (P = .0025) (Fig. 3D).
Fig. 3.
Antiproliferative effect of several drugs on hepatoma cells and primary human hepatocytes. HLE (A), HuH7 (B), and HuH6 (C) cells, and primary human hepatocytes (D) were cultured for 96 h with DAC, PCA, PCAA, or TSA. Shown are mean ± SD from the results of three independent experiments. *P < .0001, **P = .0025. Closed bars, single-dose treatment; open bars, combination treatment
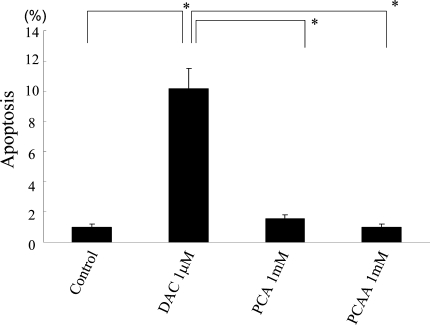
PCA has no apoptotic effect on HLE cells
No increase in the apoptosis rate was observed as a consequence of 96 h treatment with PCA and PCAA, compared to control, while 96 h treatment with DAC led to a significant increase of TUNEL-positive cells (P < .0001) (Fig. 4).
Fig. 4.
Apoptotic effect of several drugs. HLE cells were cultured for 96 h with no drug (control), 1 mM of DAC, 1 mM of PCA, or 1 mM of PCAA. Shown are mean ± SD values from the results of three independent experiments. *P < .0001
PCA inhibits S/G2/M transition of HLE cells
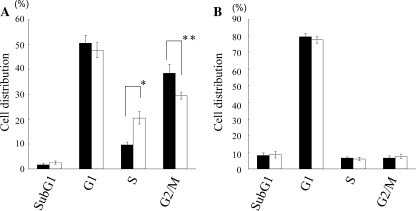
FACS analysis was performed to examine cell cycle changes in these PCA-treated HLE cells. As a control, we also analyzed the cell cycle of PCA-treated primary human hepatocytes on which PCA had no growth-inhibitory effect. We found that PCA treatment significantly increased S-phase populations and decreased G2/M-phase populations, compared with those of non-PCA-treated HLE cells (P = .0024 and .00138, respectively) (Fig. 5A), but no change in the cell cycle was seen in primary human hepatocytes with or without PCA treatment (Fig. 5B).
Fig. 5.
Cell cycle analysis of HLE cells (A) and primary human hepatocytes (B) with or without PCA treatment. Histograms represent percentages of cell distributions in indicated stages of cell cycles. Closed bars, without PCA; open bars, with PCA. Shown are mean ± SD values from the results of three independent experiments. *P = .0024, **P = .00138
PCA treatment leads to morphological changes in HLE cells
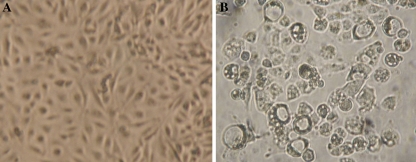
About 5 days of treatment with PCA led to morphological changes such as vacuolation, cell rounding, and retraction from the surface on which they were growing, while no spherical change or vacuolation was seen in HLE cells without PCA treatment (Fig. 6).
Fig. 6.
Morphological changes in HLE cells. (A) HLE cells cultured for 5 days without drug as control. (B) HLE cells cultured for 5 days with 1 mM of PCA
PCA has a demethylating effect on hepatoma cells and restores gene expression
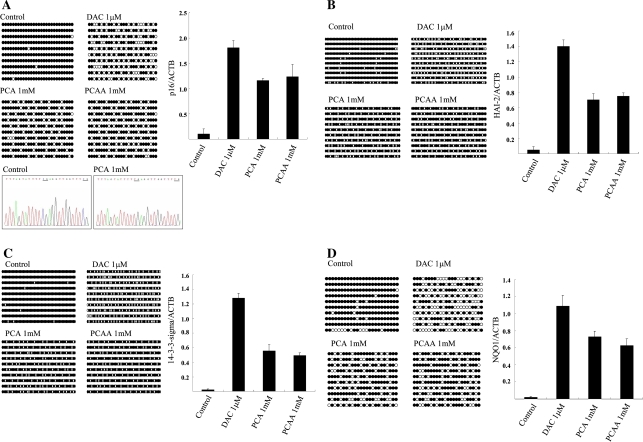
The occurrence of hypermethylation of 5′ CpG islands and the mRNA expression of p16INK4a, HAI-2/PB, 14-3-3-sigma, and NQO1 genes were analyzed by sodium bisulfite DNA sequencing and quantitative real-time RT-PCR. The 5′ CpG island of p16INK4a gene was hypermethylated in HLE and HuH7 cells, HAI-2/PB gene in HLE and HuH7 cells, 14-3-3-sigma gene in HLE cells, and NQO1 gene in HuH6 cells, and without drug treatment the transcription level of these genes was very low in each cell. This analysis revealed that PCA treatment led to the partial demethylation and restoration of mRNA expression of all four genes in these cells, respectively, similar to that on treatment with DAC and PCAA. Representative results are shown in Fig. 7A–D.
Fig. 7.
Methylation status of 5′ CpG island (left panel) and the ratio of mRNA expression (right panel) of p16 INK4A gene in HLE cells (A), HAI-2/PB gene in HuH7 cells (B), 14-3-3-sigma gene in HLE cells (C), and NQO1 gene in HuH6 cells (D) to that of β-actin with no drug (control), DAC, PCA, or PCAA detected by sodium bisulfite sequencing or quantitative real-time RT-PCR, respectively. Representative sequence chromatograms of the 5′ CpG islands of the promoter region of p16 INK4A in HLE cells treated with no drug or PCA were also shown at the bottom of Fig. 7A. Underlining indicates methylated CpG dinucleotide (Control) and unmethylated CpG dinucleotide (PCA 1 mM). For methylation analysis, each circle indicates a CpG site in the primary DNA sequence, and each line of circles represents the analysis of a single cloned allele. Closed circles, methylated CpG dinucleotides; open circles, unmethylated CpG dinucleotides. For real-time PCR, mean ± SD from the results of three independent experiments are shown
PCA has a growth-inhibitory effect against xenograft in vivo
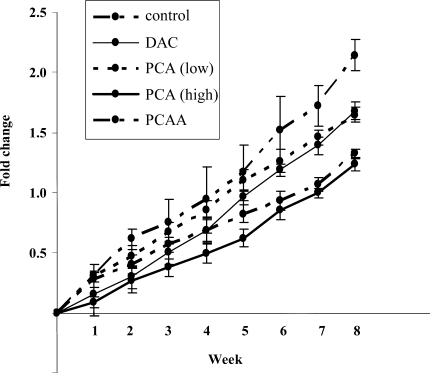
We investigated the effect of PCA on the growth of HLE cells that had been transplanted into nude mice. Weekly treatment with a high dose of PCA (1 mg/mouse, once a week) for 6 weeks showed significant tumor growth-inhibitory effect (P < .0001); tumor volume was reduced by as much as 42.2 ± 4.3% relative to the tumor volume of the control, and no obvious adverse effects occurred (Fig. 8). By the treatment with DAC, PCAA, and a low dose of PCA, tumor volume was also reduced by as much as 21.5 ± 5.8%, 38.2 ± 4.2%, and 23.2 ± 5.5%, respectively, relative to the tumor volume of control (Fig. 8). A high dose of PCA was significantly more effective than a low dose in the inhibition of HLE xenograft, and among the several treatments tested, the high dose of PCA was the most effective.
Fig. 8.
Change in HLE xenograft volume in mice treated with several drugs for 6 weeks. Shown are mean ± SD values from the results of six mice in each group
PCA has a demethylating effect on xenograft tumors
The 5′ CpG islands of p16INK4a, HAI-2/PB, and 14-3-3-sigma gene were aberrantly hypermethylated in xenograft tumors of control mice, whereas these genes were partially demethylated in the tumors of PCA-treated mice (Fig. 9).
Fig. 9.
Methylation status of 5′ CpG islands of p16INK4a (A), HAI-2/PB (B), and 14-3-3-sigma (C) genes in xenografts. Mouse 1, 2, and 3 were treated with saline (control) and mouse 4, 5, and 6 were treated with 1 mg of PCA. Each circle indicates a CpG site in the primary DNA sequence, and each line of circles represents the analysis of a single cloned allele. Closed circles, methylated CpG dinucleotides; open circles, unmethylated CpG dinucleotides
Discussion
Abnormal patterns of DNA methylation in cancer have been recognized for more than 20 years, and the inactivation of tumor suppressor genes through the hypermethylation of the 5′ CpG islands in gene promoter regions is one of the major features of most human cancers. As opposed to genetic alteration, epigenetic gene silencing through DNA hypermethylation is potentially reversible, and this presents new opportunities for the clinical management of malignant tumors. Two demethylating agents, nucleoside analog 5-azacytidine and DAC, are currently being tested in clinical trials of cancer treatment. However, the clinical utility of these drugs has not been fully realized because of their adverse effects, such as mutagenicity and myelotoxicity [20]. Recently, PCA, known as a local anesthetic, was revealed to be a nonnucleoside inhibitor of DNA methylation and have a growth-inhibitory effect in the human breast cancer cell line, MCF-7 [15]. In the current study, we demonstrated that a single dose of PCA also had a growth-inhibitory effect both in vitro and in vivo and that this agent was an inhibitor of DNA methylation in hepatoma cells.
At first, using the MTS assay, we investigated whether PCA reduced the viability of human hepatoma and hepatoblastoma cells. Treatment with 1 mM of PCA led to a decrease of viable HLE, HuH7, and HuH6 cells, and PCA reduced the cell viability in a dose- and time-dependent manner in these cells, while no reduction was seen in the viability of HepG2, Hep3B, PLC/PRF/5 cells, and primary human hepatocytes. In particular, 2 mM of PCA treatment showed considerable (less than 25% relative to control) decrease in viable HLE and HuH7 cell numbers, while primary human hepatocytes were entirely unaffected by such high-dose PCA treatment. On the other hand, mono-treatment with the drugs exploring epigenetic change, such as DAC, PCAA, and TSA, resulted in reduction of the viability in at most one cell line. These data might indicate that PCA exclusively possesses a growth-inhibitory effect in hepatoma cells among the drugs that elicited epigenetic change.
The combination treatment of TSA and DAC induces more potent apoptosis than the treatment with TSA alone [21] and has synergistic antineoplastic action on cancer cells through the reactivation of tumor suppressor genes silenced by aberrant DNA hypermethylation [22]. In our study, the combination treatment of TSA and PCA led to a significant decrease in the number of viable hepatoma cells as well as that of TSA and DAC, with no reduction in the viability of primary human hepatocytes, while the combination treatment of TSA and DAC exhibited a significant reduction in the number of viable primary human hepatocytes. These results suggest that the combination treatment of TSA and PCA appears to be a more promising strategy when considering clinical applications than the combination treatment of TSA and DAC [23].
To explore the mechanisms of the growth-inhibitory effect of PCA, we performed a TUNEL assay, FACS analysis, and morphological analysis of PCA-treated cells. By the TUNEL assay, no increase in the apoptotic rate was observed in HLE cells treated with 1 mM of PCA, concordant with previous observation [15]. FACS analysis revealed that 1 mM of PCA treatment increased S-phase populations and decreased G2/M in HLE cells, while no cell cycle change was found in primary human hepatocytes treated with PCA. These results might indicate that cell cycle inhibition in S phase, but not an apoptotic effect, is one of the mechanisms of the growth inhibition effect of PCA in HLE cells. This is in disagreement with a previous report that showed G2/M cell cycle arrest by PCA treatment [15]. The reasons for this discrepancy are unclear, but the difference in histological origin of the cell lines might be one. Morphological observation showed that, compared to control, 5 days of PCA treatment led to vacuolation and cell rounding in HLE cells, corresponding with a previous report [24]. In that report, Yang et al. [24] stated that PCA could penetrate the cell membrane through the presence of a nonionized weak base and that this led to the induction of vacuolization.
To further investigate the mechanisms of the growth-inhibitory effect of PCA, we explored whether PCA had any DNA-demethylating effect on the genes that were previously demonstrated to be inactivated in HCC through DNA hypermethylation—p16INK4a, HAI-2/PB, 14-3-3-sigma, and NQO1 genes [16–19]—and reactivated these genes. We demonstrated that 1 mM of PCA treatment led to partial DNA demethylation and reactivation of all the genes transcriptionally suppressed through DNA hypermethylation, as did 1 μM of DAC and 1 mM of PCAA treatment as previously reported [13, 14, 20], although the potency of PCA is weaker than that of DAC, a more potent demethylating agent compared with nonnucleoside DNA-methylation inhibitors [25]. Recently, it was reported that treatment with PCA did not induce demethylation in TK6 and HCT116 cells [26]. On the other hand, PCA treatment led to DNA demethylation in MCF7 cells [15]. These discrepancies might depend on cell type.
To investigate whether PCA also has growth-inhibitory effect on hepatoma cells in vivo, PCA was administered to nude mice bearing HLE xenograft. PCA showed a significant dose-dependent growth-inhibitory effect. The single administration of DAC or PCAA also had tumor growth-inhibitory effect as previously reported [13, 27, 28]. PCAA exhibited a growth-inhibitory effect in hepatoma cells to an extent similar to high-dose PCA treatment, but PCAA also significantly reduced viable primary human hepatocytes in vitro. PCA also led to partial demethylation of tumor suppressor genes in xenograft tumors similarly to that in the cell lines. These findings suggest that PCA is probably safer than PCAA as a new demethylating agent.
Local anesthetics, including PCA, alter surface membrane characteristics that lead to permeability of large molecules [29, 30]. In addition, these drugs have been shown to induce vacuoles within the cell cytoplasm [24] and interfere with intracellular microtubule function [31]. It has been reported that PCA could enhance the antitumor activity of chemotherapeutic agents such as bleomycin and cisplatin, as well as of hyperthermia [32–34], and this effect might be due to the above-stated property of PCA.
Although these properties of PCA, such as demethylation, cell cycle arrest, and inhibition of microtubule function, are universal and not specific to certain cell lines, PCA showed growth-inhibitory effect on only three cell lines and no effect on three other hepatoma cell lines, HepG2, Hep3B, and PLC/PRF/5, nor on primary human hepatocytes, in our study. Our data using cDNA microarray showed that the number of upregulated (ratio > 2.0) genes in primary human hepatocytes with PCA treatment is significantly fewer than that of PCA-treated HLE, HuH7, and HuH6 cells. In addition, the number of downregulated (ratio < 0.5) genes in primary human hepatocytes with PCA treatment is also significantly fewer than that of PCA-treated HLE, HuH7, and HuH6 cells (unpublished data). These data indicated that treatment with PCA has little influence on gene expression change in primary human hepatocytes. Although the expression profiles of HepG2, Hep3B, and PLC/PRF/5 treated with PCA were not investigated, the differences in influence on gene expression change might be one of the reasons why the sensitivities to PCA differed among cell lines.
Most HCC cases arise in the setting of chronic hepatitis virus infection [35], but the molecular pathogenesis of HCC is complex and has not yet been fully elucidated. Epigenetic alteration, such as aberrant DNA methylation, is a hallmark of cancer [1], and inactivation of several tumor suppressor genes, such as E-cadherin, p16INK4a, SOCS, 14-3-3sigma, and GSTP1, through DNA hypermethylation, was also reported in HCC [16, 18, 36–39]. So, the antitumor effect of PCA in vitro and in vivo might be exerted through the reactivation of tumor suppressor genes by DNA demethylation in addition to several other functions, such as alteration of membrane permeability and interference of microtubule function. Moreover, PCA may modulate immune systems by the demethylation of T cells as well as PCAA [11] and it may lead to reinforcement of immune surveillance by T cell reactivation. Concentrations of PCA similar to those employed in our study are achievable in humans [40].
In conclusion, PCA, used as a local anesthetic for a long time, may be a safe candidate for future HCC therapies based on epigenomics.
References
- 1.Herman JG. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol 1999;9:359–67 [DOI] [PubMed]
- 2.Barlow DP. Gametic imprinting in mammals. Science 1995;270:1610–13 [DOI] [PubMed]
- 3.Goto T, Monk M. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol Mol Biol Rev 1998;62:362–78 [DOI] [PMC free article] [PubMed]
- 4.Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol 2002;196:1–7 [DOI] [PubMed]
- 5.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998;72:141–96 [DOI] [PubMed]
- 6.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet 1999;21:163–7 [DOI] [PubMed]
- 7.Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2′-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res 1998;58:95–101 [PubMed]
- 8.Petti MC, Mandelli F, Zagonel V, De Gregoris C, Merola MC, Latagliata R, et al. Pilot study of 5-aza-2′-deoxycytidine (Decitabine) in the treatment of poor prognosis acute myelogenous leukemia patients: preliminary results. Leukemia 1993;7 Suppl 1:36–41 [PubMed]
- 9.Schwartsmann G, Fernandes MS, Schaan MD, Moschen M, Gerhardt LM, Di Leone L, et al. Decitabine (5-aza-2′-deoxycytidine; DAC) plus daunorubicin as a first line treatment in patients with acute myeloid leukemia: preliminary observations. Leukemia 1997;11 Suppl 1:S28–31 [PubMed]
- 10.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980;20:85–93 [DOI] [PubMed]
- 11.Scheinbart LS, Johnson MA, Gross LA, Edelstein SR, Richardson BC. Procainamide inhibits DNA methyltransferase in a human T cell line. J Rheumatol 1991;18:530–4 [PubMed]
- 12.Yung R, Chang S, Hemati N, Johnson K, Richardson B. Mechanisms of drug-induced lupus. IV. Comparison of procainamide and hydralazine with analogs in vitro and in vivo. Arthritis Rheum 1997;40:1436–43 [DOI] [PubMed]
- 13.Lin X, Asgari K, Putzi MJ, Gage WR, Yu X, Cornblatt BS, et al. Reversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide. Cancer Res 2001;61:8611–6 [PubMed]
- 14.Segura-Pacheco B, Trejo-Becerril C, Perez-Cardenas E, Taja-Chayeb L, Mariscal I, Chavez A, et al. Reactivation of tumor suppressor genes by the cardiovascular drugs hydralazine and procainamide and their potential use in cancer therapy. Clin Cancer Res 2003;9:1596–603 [PubMed]
- 15.Villar-Garea A, Fraga MF, Espada J, Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res 2003;63:4984–9 [PubMed]
- 16.Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene 1999;18:789–95 [DOI] [PubMed]
- 17.Fukai K, Yokosuka O, Chiba T, Hirasawa Y, Tada M, Imazeki F, et al. Hepatocyte growth factor activator inhibitor 2/placental bikunin (HAI-2/PB) gene is frequently hypermethylated in human hepatocellular carcinoma. Cancer Res 2003;63:8674–9 [PubMed]
- 18.Iwata N, Yamamoto H, Sasaki S, Itoh F, Suzuki H, Kikuchi T, et al. Frequent hypermethylation of CpG islands and loss of expression of the 14-3-3 sigma gene in human hepatocellular carcinoma. Oncogene 2000;19:5298–302 [DOI] [PubMed]
- 19.Tada M, Yokosuka O, Fukai K, Chiba T, Imazeki F, Tokuhisa T, et al. Hypermethylation of NAD(P)H: quinone oxidoreductase 1 (NQO1) gene in human hepatocellular carcinoma. J Hepatol 2005;42:511–9 [DOI] [PubMed]
- 20.Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med 2001;134:573–86 [DOI] [PubMed]
- 21.Zhu WG, Lakshmanan RR, Beal MD, Otterson GA. DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res 2001;61:1327–33 [PubMed]
- 22.Primeau M, Gagnon J, Momparler RL. Synergistic antineoplastic action of DNA methylation inhibitor 5-aza-2′-deoxycytidine and histone deacetylase inhibitor depsipeptide on human breast carcinoma cells. Int J Cancer 2003;103:177–84 [DOI] [PubMed]
- 23.Chiba T, Yokosuka O, Arai M, Tada M, Fukai K, Imazeki F, et al. Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. J Hepatol 2004;41:436–45 [DOI] [PubMed]
- 24.Yang WC, Strasser FF, Pomerat CM. Mechanism of drug-induced vacuolization in tissue culture. Exp Cell Res 1965;38:495–506 [DOI] [PubMed]
- 25.Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, et al. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2′-deoxycytidine. Mol Cancer Ther 2005;4:1515–20 [DOI] [PubMed]
- 26.Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res 2006;66:2794–800 [DOI] [PubMed]
- 27.Laird PW, Jackson-Grusby L, Fazeli A, Dickinson SL, Jung WE, Li E, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell 1995;81:197–205 [DOI] [PubMed]
- 28.Lantry LE, Zhang Z, Crist KA, Wang Y, Kelloff GJ, Lubet RA, et al. 5-Aza-2′-deoxycytidine is chemopreventive in a 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone-induced primary mouse lung tumor model. Carcinogenesis 1999;20:343–6 [DOI] [PubMed]
- 29.Tsong TY, Greenberg M, Kanehisa MI. Anesthetic action of membrane lipids. Biochemistry 1977;16:3115–21 [DOI] [PubMed]
- 30.Carlsen SA, Till JE, Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta 1976;455:900–12 [DOI] [PubMed]
- 31.Eichhorn JH, Peterkofsky B. Local anesthetic-induced inhibition of collagen secretion in cultured cells under conditions where microtubules are not depolymerized by these agents. J Cell Biol 1979;81:26–42 [DOI] [PMC free article] [PubMed]
- 32.Mizuno S, Ishida A. Selective enhancement of bleomycin cytotoxicity by local anesthetics. Biochem Biophys Res Commun 1982;105:425–31 [DOI] [PubMed]
- 33.Esposito M, Fulco RA, Collecchi P, Zicca A, Cadoni A, Merlo F, et al. Improved therapeutic index of cisplatin by procaine hydrochloride. J Natl Cancer Inst 1990;82:677–84 [DOI] [PubMed]
- 34.Mizuno S, Ishida A. Selective enhancement of the cytotoxicity of the bleomycin derivative, peplomycin, by local anesthetics alone and combined with hyperthermia. Cancer Res 1982;42:4726–9 [PubMed]
- 35.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol 1978;24:40–69 [PubMed]
- 36.Esteller M, Corn PG, Urena JM, Gabrielson E, Baylin SB, Herman JG. Inactivation of glutathione S-transferase P1 gene by promoter hypermethylation in human neoplasia. Cancer Res 1998;58:4515–8 [PubMed]
- 37.Tchou JC, Lin X, Freije D, Isaacs WB, Brooks JD, Rashid A, et al. GSTP1 CpG island DNA hypermethylation in hepatocellular carcinomas. Int J Oncol 2000;16:663–76 [DOI] [PubMed]
- 38.Kanai Y, Ushijima S, Hui AM, Ochiai A, Tsuda H, Sakamoto M, et al. The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer 1997;71:355–9 [DOI] [PubMed]
- 39.Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001;28:29–35 [DOI] [PubMed]
- 40.Chlebowski RT, Block JB, Cundiff D, Dietrich MF. Doxorubicin cytotoxicity enhanced by local anesthetics in a human melanoma cell line. Cancer Treat Rep 1982;66:121–5 [PubMed]