Abstract
The natural course of hepatitis B virus (HBV) infection is variable, and chronic hepatitis B (CHB) disease exhibits itself through a spectrum of clinical manifestations. These factors contribute to the challenges faced when managing patients who live with HBV infection. Furthermore, conventional treatment options (e.g., interferon alfa-2a, lamivudine, and adefovir) are moderately effective and can be associated with problems, such as poor tolerability (interferon alfa-2a) and the development of drug resistance (lamivudine). Over the last 5 years, several antiviral agents including entecavir, peginterferon alfa-2a, and telbivudine which are more efficacious and have improved tolerability over previous drugs have become available. The availability of novel antiviral agents and advances in understanding resistance patterns of antiviral agents has resulted in refinement of CHB treatment recommendations and guidelines. More recently, evidence from clinical trials suggests the central importance of virologic suppression as an indicator of treatment outcome and the predictive value of on-treatment HBV DNA levels in response to antiviral therapy. This review highlights the goals of therapy and clinical experience with therapies that are newly licensed or in the late stages of clinical development. Current approaches for treating CHB and new strategies for optimizing response to therapy are also discussed.
Keywords: Chronic hepatitis B, Human immunodeficiency virus, Hepatitis B e antigen
Introduction
Profound and sustained inhibition of viral replication is the most important goal of the management of chronic hepatitis B (CHB), as it can reduce the likelihood of subsequent disease progression and the emergence of viral resistance. Despite the availability of potent antiviral agents, response to therapy remains less than satisfactory, and the emergence of resistance remains a barrier to achieving the goals of therapy. Guidelines for the management of CHB address the criteria for patient selection, the objectives and timing of therapy, and the advantages and disadvantages of available treatment options, but little information is available on treatment monitoring and strategies for optimizing patient outcomes [1–5]. Emerging evidence from clinical studies of antiviral agents suggest that on-treatment serum levels of hepatitis B virus (HBV) DNA are predictive of treatment response [6–9]. Based on these findings, a panel of international experts has proposed an algorithm for optimizing treatment response that relies on the on-treatment monitoring of HBV DNA levels [10]. Combination antiviral therapy and add-on therapy have also been investigated as potential strategies for improving treatment outcomes in patients with CHB. This review highlights current opinion on when to start antiviral therapy and when to stop CHB treatment, citing clinical experience with newly available agents and agents in the late stages of development. New strategies for optimizing response to therapy are also discussed.
Initiation of therapy and the goal of treatment
The fundamental goal of CHB therapy is to reduce progression to cirrhosis, hepatic decompensation with liver failure, development of hepatocellular carcinoma, and the need for liver transplantation by achieving a sustained reduction of viral replication to undetectable levels [3–5].
During the initial evaluation of an individual at high risk of hepatitis B, physicians should obtain the patient’s history and the family history of liver disease, ask about alcohol use, and perform a physical examination [5]. Laboratory tests should include a complete blood cell count, assessment of liver transaminase levels, and the performance of other assays needed to assess liver disease. Serologic testing for the presence of HBV should include assays for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and HBV DNA levels. In addition, patients should be screened for the presence of coinfection with hepatitis A virus (HAV), hepatitis C virus, hepatitis D virus, and human immunodeficiency virus (HIV), as co-infection is associated with more severe liver disease [5]. Liver biopsy may be useful, especially in patients who do not clearly meet the criteria for starting treatment.
CHB follows a variable clinical course that can be characterized by four distinct phases: immune tolerance, immune clearance (HBeAg-positive CHB), inactive HBsAg carrier, and reactivation (HBeAg-negative CHB) [11]. The criteria used to define each phase of infection include the presence or absence of HBeAg, serum HBV DNA levels, alanine aminotransferase/aspartate aminotransferase (ALT/AST) levels, and necroinflammation on liver biopsy (Table 1) [11]. The persistence of HBsAg beyond 6 months is adequate for a diagnosis of CHB [1–5].
Table 1.
Phases of chronic HBV infection
| Phase | Diagnostic criteria |
|---|---|
| Immune tolerance | 1. HBsAg-positive >6 months |
| 2. HBeAg-positive, anti-HBe-negative | |
| 3. Serum HBV DNA >20,000 IU/ml | |
| 4. Persistently normal ALT levels | |
| 5. Liver biopsy normal or showing minor nonspecific changes | |
| Immune clearance (HBeAg- positive CHB) | 1. HBsAg-positive >6 months |
| 2. HBeAg-positive, anti-HBe-negative | |
| 3. Serum HBV DNA >20,000 IU/ml | |
| 4. Persistent or intermittent elevation of ALT levels | |
| 5. Liver biopsy showing chronic hepatitis (necroinflammatory score ≥4)a | |
| Inactive HBsAg carrier | 1. HBsAg-positive >6 months |
| 2. HBeAg-negative, anti-HBe-positive | |
| 3. Serum HBV DNA <2,000 IU/ml | |
| 4. Persistently normal ALT levels | |
| 5. Liver biopsy showing absence of significant hepatitis (necroinflammatory score <4)a | |
| Reactivation (HBeAg-negative CHBb) | 1. HBsAg-positive >6 months |
| 2. HBeAg-negative, anti-HBe-positive | |
| 3. Serum HBV DNA >2,000 IU/ml | |
| 4. Persistent or intermittent elevation of ALT levels | |
| 5. Liver biopsy showing chronic hepatitis (necroinflammatory score ≥4)a |
Note: From Yim and Lok [11]
aLiver biopsy optional
bMost of these patients have precore or core promoter variants
Treatment is currently recommended only for patients with the immune clearance and reactivation phases. However, all newly diagnosed patients infected with HBV should be followed for ≥6 months before the institution of antiviral therapy, because of the potential for spontaneous HBeAg seroconversion, particularly in patients with adult-acquired HBV infection. Current guidelines recommend initiating antiviral therapy in HBeAg-positive patients who have ALT levels ≥2 times the upper limit of normal (ULN) and HBV DNA levels ≥20,000 IU/ml. Antiviral therapy is also recommended for HBeAg-positive patients with mildly elevated ALT levels (<2 × ULN) and HBV DNA levels ≥20,000 IU/ml who have evidence of active necroinflammation and fibrosis, in the absence of another cause of liver injury, on histologic testing. In HBeAg-negative patients, the viral load for the threshold of treatment is lower, typically ≥2,000 IU/ml. Regardless of HBeAg status, patients with decompensated cirrhosis and HBV DNA levels ≥2,000 IU/ml are candidates for treatment. These patients should be started on oral nucleoside or nucleotide analog (NA) therapy and referred to a liver transplantation center. Other candidates for therapy include individuals receiving chemotherapy or immunosuppressive agents and HIV-coinfected individuals who are HBV seropositive, regardless of ALT and HBV DNA levels, as these individuals are at risk for reactivation of HBV.
Initial therapy
Current therapy for patients with CHB consists of two types of drugs: immunomodulators, such as interferon alfa, and NAs. Of the six available therapies for CHB, the preferred treatment options include adefovir, entecavir, peginterferon alfa, and, potentially, telbivudine (in patients in whom HBV DNA is undetectable after 24 weeks of therapy). Although lamivudine and interferon alfa are effective treatments for CHB, their use is limited by the rapid emergence of resistance and poor tolerability, respectively. Peginterferon alfa has largely supplanted standard interferon in the treatment of CHB because of its improved tolerability and convenience related to once-weekly dosing. Similarly, lamivudine is no longer considered a preferred first-line drug for CHB due to its association with rapid and high rates of resistance [1, 5]. Two additional agents, tenofovir and clevudine, have demonstrated promising anti-HBV activity and resistance profiles; they are in the late stages of clinical study as HBV treatment.
Peginterferon alfa
Peginterferon alfa is an injectable immunomodulator that differs from standard interferon by the conjugation of a single-branched bis-monomethoxy polyethylene glycol (PEG) molecule to interferon alfa. Two forms of peginterferon alfa—alfa-2a and alfa-2b—have been developed; they differ with respect to pharmacologic properties. The longer serum half-life of the peginterferons allows for better maintenance of effective interferon concentrations throughout the dosing interval, as compared with the conventional interferons.
The efficacy of peginterferon alfa-2a has been demonstrated in large, randomized studies comparing peginterferon alfa-2a with and without lamivudine in patients with HBeAg-positive [12] and HBeAg-negative CHB [13]. In these studies, patients were randomized to one of three treatments: peginterferon alone, lamivudine alone, or peginterferon plus lamivudine, for 48 weeks. Patients were then followed for 24 additional weeks. Peginterferon alfa-2a demonstrated superior efficacy to lamivudine alone, resulting in a greater incidence of HBeAg seroconversion, HBV DNA suppression, and HBsAg seroconversion in patients with HBeAg-positive and HBeAg-negative CHB. Among HBeAg-negative patients, baseline ALT and HBV DNA levels, patient age, gender, and infecting HBV genotype significantly influenced response at 24 weeks post treatment in patients treated with peginterferon alfa-2a, lamivudine, or both [14]. In a 3-year follow-up of the HBeAg-negative patients, one third of those treated with peginterferon had maintained normal ALT levels and HBV DNA levels of <10,000 copies/ml [8]. Similar findings have been reported in studies of peginterferon alfa-2b in patients with HBeAg-positive [15, 16] and HBeAg-negative CHB [17, 18].
Although peginterferon is not associated with resistance and induces a higher rate of HBsAg loss (3%) than does oral NA therapy, its use is limited by its parenteral administration, frequent side effects, potential risk for ALT flares, and cost. In addition, peginterferon is contraindicated in patients with decompensated liver cirrhosis. Evidence from clinical studies suggests that virologic response to peginterferon varies according to HBV genotype. Patients with genotypes A and B appear to respond (defined as ALT normalization and HBV DNA levels of <20,000 copies/ml) better than do patients with genotypes D and C, respectively [14]. If these findings are confirmed in larger clinical studies, genotype may be a useful tool for clinicians in determining treatment options in the management of patients with CHB.
Currently, peginterferon therapy is considered primarily for children and young adults (≤25 years of age) with recently acquired chronic infection, because the duration of treatment is defined and, if successful, the response is durable [1–5].
Adefovir
Adefovir dipivoxil is an NA of adenosine monophosphate that is active against wild-type HBV and lamivudine-resistant HBV. The efficacy of adefovir 10 mg/day has been demonstrated in large, randomized clinical trials of patients with HBeAg-positive and HBeAg-negative CHB [19, 20]. Long-term follow-up studies have shown that the virologic, biochemical, and histologic benefits of adefovir are maintained with the continuation of therapy through 5 years in HBeAg-negative patients, more than two thirds of whom maintained undetectable HBV DNA levels and ALT normalization [21]. One limitation of adefovir is the potential for renal toxicity with prolonged administration [21]. Due to this, renal function should be monitored regularly, particularly in patients with concomitant renal disease.
The incidence of adefovir resistance is low but increases gradually over time. In long-term follow-up studies, resistance to adefovir was observed in 3% of patients at 2 years, 11% of patients at 3 years, 18% of patients at 4 years, and 29% of patients at 5 years [21]. Adefovir monotherapy is effective for the treatment of lamivudine-resistant HBV [22]. However, the cumulative incidence of adefovir resistance was found to be higher in lamivudine-resistant patients than previously reported: 6.4% at 12 months and up to 25% after 2 years [23, 24]. In patients with pre-existing lamivudine resistance or breakthrough viremia, the emergence of adefovir resistance has been associated with significant viral rebound and hepatic decompensation [25]. Accordingly, recommendations favor the addition of adefovir to continued lamivudine in patients who develop lamivudine resistance, particularly those with cirrhosis [5, 26].
Entecavir
Entecavir, a novel deoxyguanosine analog, is a potent inhibitor of HBV replication in patients with HBeAg-positive and HBeAg-negative CHB [27–29]. Entecavir differs from the other NA reverse transcriptase inhibitors approved for HBV therapy in several ways: entecavir is more than 100-fold potent against HBV in culture and, in contrast to lamivudine and adefovir, entecavir halts HBV DNA elongation after incorporation of a few additional bases [30]. In phase III randomized controlled trials that involved patients with nucleoside-naïve and lamivudine-refractory CHB, entecavir treatment resulted in significantly higher rates of virologic, biochemical, and histologic outcomes than did treatment with lamivudine. Rates for achievement of histologic improvement, undetectable levels of HBV DNA by Polymerase Chain Reaction (PCR) assay, and normalization of ALT levels in nucleoside-naive HBeAg-positive and HBeAg-negative patients after 48 weeks of entecavir treatment were, respectively, 72% and 70%, 67% and 90%, and 68% and 78% [27, 28]. Similarly, in lamivudine-refractory patients, rates of response were higher for entecavir than for lamivudine with respect to histologic response (55% vs. 28%), virologic response (21% vs. 1%), and biochemical response (75% vs. 23%) [29]. Rates of HBeAg loss in patients treated with entecavir were 21% for nucleoside-naive patients and 10% for lamivudine-resistant patients [29]. Similar findings have been reported in large, randomized trials of entecavir involving Chinese patients with CHB [31].
Resistance surveillance from phase III studies shows that entecavir is associated with a low resistance rate in nucleoside-naïve HBsAg-positive and -negative patients treated for up to 4 years (<1%) [32–34]. In contrast, an increasing rate of genotypic resistance with virologic breakthrough (1%, 11%, 27% and 39% at 1, 2, 3, and 4 years, respectively) is observed in lamivudine-refractory patients [32–34]. The presence of lamivudine resistance mutation rtM204V/I and rtL180M in conjunction with one or more entecavir-specific mutations (rtT184, rtS202, or rtM250) results in decreased susceptibility to entecavir. Analysis at 2 years of entecavir resistance in patients with lamivudine-refractory CHB found that, although 6% of patients had HBV variants harboring entecavir-resistance mutations (rtT184, rtS202, or rtM250) at baseline, the majority of these patients had a decrease in HBV DNA levels in response to entecavir and virologic rebounds coinciding with the emergence of entecavir resistance mutations did not occur until 2 years [35]. These findings suggest that HBV variants harboring entecavir-resistance mutations may be replication deficient, and other host and viral factors must be required for development of entecavir resistance [35]. Although entecavir-resistant HBV has been shown to be susceptible to adefovir in vitro, limited clinical data are available on the efficacy of adefovir in patients with this condition.
The results of these studies suggest that entecavir would be a suitable first-line choice among the currently available anti-HBV NAs for patients with treatment-naïve CHB. Entecavir is an effective therapy for lamivudine resistance; however, lamivudine therapy should be discontinued when patients are switched to entecavir to reduce the risk of entecavir resistance.
Telbivudine
Telbivudine, an HBV-specific l-nucleoside analog of thymidine that especially targets the viral DNA polymerase enzyme responsible for HBV replication, is the most recent oral anti-HBV therapy for CHB. The superiority of telbivudine 600 mg over conventional lamivudine has been established in large, randomized, and controlled clinical trials [36, 37]. In the large, phase III GLOBE trial comparing telbivudine with lamivudine in HBeAg-positive and HBeAg-negative patients with CHB, telbivudine was associated with a greater mean reduction in HBV DNA levels and clearance of PCR-detectable HBV DNA than was lamivudine monotherapy, but not ALT normalization [36–38]. The clinical benefits of telbivudine have been shown to have sustained through 2 years of treatment. In the 2-year analysis of the GLOBE data, telbivudine proved to be superior to lamivudine in the reduction of absolute HBV DNA levels from baseline and in the time to achievement of undetectable HBV DNA viral load, regardless of the patient’s HBeAg status [38]. At 104 weeks, a higher proportion of telbivudine-treated than lamivudine-treated HBeAg-positive patients (61% telbivudine vs. 47% lamivudine; P < 0.05) and HBeAg-negative patients (74% telbivudine vs. 62% lamivudine; P < 0.05) achieved a therapeutic response (defined as HBV DNA level <5 log10 with HBeAg loss or ALT normalization) [38]. The safety of telbivudine and the durability of HBeAg loss and seroconversion were comparable to the findings with lamivudine.
Analysis of virologic response and outcomes in the GLOBE study showed a positive correlation between HBV DNA levels at week 24 and virologic and clinical efficacy response over 2 years of treatment [6]. In a multivariate analysis, a lower HBV DNA level at week 24 was the best predictor of clinical and virologic efficacy responses at week 52, irrespective of HBeAg serostatus [7]. Among patients who were HBV DNA-negative by PCR at week 24, 90% were HBV DNA-negative (<300 copies/ml) at week 52, and <1% developed resistance [9]. More recently, the 2-year analysis of GLOBE data demonstrated that week 24 viral suppression is correlated with maintained viral suppression at 2 years. In this analysis, viral suppression at week 24 with telbivudine and lamivudine was predictive of PCR negativity, ALT normalization, seroconversion in HBeAg-positive patients, and resistance at week 104. Moreover, the degree of HBV DNA suppression (analyzed in strata of <300 copies/ml, <3 log10 copies/ml, 3–4 log10 copies/ml, and >4 log10 copies/ml) at week 24 correlated with the virologic and clinical outcomes regardless of HBeAg status [6]. In HBeAg-positive patients, a higher degree of viral suppression at week 24 was associated with an increased rate of seroconversion (45%, 38%, 19%, and 6%, respectively, for the HBV DNA strata), ALT normalization (79%, 76%, 63%, and 43%, respectively) and PCR negativity (77%, 58%, 32%, and 12%, respectively) by week 104 [6].
Resistance to telbivudine is associated primarily with the rtM204I mutation in the HBV polymerase gene [38]. The resistance rate for telbivudine after 1 and 2 years has been found to be higher among HBeAg-positive patients (4.4% at 1 year and 21.6% at 2 years) than among HBeAg-negative patients (2.7% at 1 year and 8.6% at 2 years) [38]. However, among patients achieving undetectable HBV DNA levels at 24 weeks of therapy, resistance to telbivudine was substantially lower in those who were HBeAg negative than in those who were HBeAg positive (2% vs. 4%). Based on these findings, telbivudine appears to be an effective therapy for treatment-naïve patients with CHB. Achievement of serum HBV DNA levels that are undetectable after 24 weeks of therapy is associated with the absence of resistance or a very low rate of resistance at year 1 and year 2 of therapy.
Tenofovir
Tenofovir is an acyclic NA that is structurally related to adefovir and has activity against HIV and HBV. Tenofovir inhibits the replication of HIV and HBV through the competitive inhibition of the viral polymerase. Currently, tenofovir is approved for the management of HIV infection. Although it is not approved for the management of hepatitis B, it has demonstrated efficacy against wild-type and lamivudine-resistant HBV and is widely used in the setting of HBV–HIV co-infection.
Preliminary clinical data suggest that tenofovir 300 mg/day results in significantly greater serum HBV DNA suppression than does the approved dosage of adefovir, 10 mg/day [39, 40]. Additionally, the reversible nephrotoxicity observed using high doses of adefovir (≥30 mg/day) has not been reported with tenofovir 300 mg/day. In a study among such patients, who exhibited persistent viral replication (>104 copies/ml) after 15 months of adefovir monotherapy, treatment with tenofovir 300 mg/day led to undetectable HBV DNA levels (<400 copies/ml) in 19 of 20 patients [40]. ALT levels that were initially elevated had normalized in 10 of 14 patients by the end of follow-up (median, 12 months; range, 3–24 months). In addition, four patients lost HBeAg (after 3, 4, 5, and 16 months, respectively), and one patient seroconverted to anti-HBs after 16 months of tenofovir therapy.
Based on long-term safety data and initial efficacy data in patients with wild-type or lamivudine-resistant HBV infection, tenofovir is being studied in two larger phase III clinical trials, one in HBeAg-positive patients and the other in HBeAg-negative patients. Both studies are comparing tenofovir with adefovir and will provide 48-week efficacy and safety data.
Stopping antiviral therapy
Once antiviral treatment is initiated, patients should be monitored regularly to assess drug efficacy, safety, and tolerability, and also to detect disease flares and recognize the development of drug resistance; a further purpose for monitoring is to evaluate patient adherence [2]. HBV DNA and transaminase levels should be monitored at least every 3–6 months, while patients are receiving therapy with the oral agents. In addition, patients receiving peginterferon alfa should be monitored frequently for the development of side effects that might reduce tolerability including flu-like symptoms, depression, bone marrow suppression and neuropsychiatric disease. Peginterferon alfa-2a is administered for a fixed period of 1 year. Therapy is stopped prematurely only if intolerable side effects develop (these occur in <5% of patients). HBeAg, anti-HBe, and HBV DNA levels should be assessed at the time of discontinuation and every 6 months thereafter.
The traditional end point of treatment with oral agents for patients with HBeAg-positive CHB is HBeAg seroconversion to anti-HBe in association with very low or undetectable serum HBV DNA levels [1–5]. Treatment is typically continued for an additional 6–12 months to reduce the likelihood of relapse. Some clinicians favor the long-term treatment of patients with cirrhosis with oral agents, even after HBeAg seroconversion, to avoid the risk of relapse. At this point, too few data exist to determine the optimal point at which to discontinue NAs. Extended or indefinite treatment with oral antiviral agents may be appropriate for cirrhotic patients. It is not known what the best treatment end point is for HBeAg-negative patients. However, if HBV DNA levels become undetectable and treatment is discontinued, relapse rates are high. In this case, current data support long-term treatment with oral antiviral agents. The relapse rate may be lower, albeit still substantial, if undetectable HBV DNA levels have been maintained for several years. In one study, two thirds of patients who discontinued adefovir after 4–5 years of therapy maintained normal ALT levels for an additional 15–20 months [21].
Optimizing response to antiviral therapy
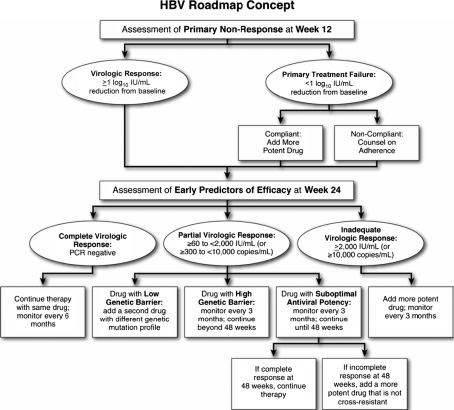
On-treatment HBV DNA levels may be useful in optimizing treatment response to oral antiviral therapy. Analysis of the GLOBE study found that week 24 HBV DNA levels were highly predictive of efficacy outcomes after 2 years of telbivudine treatment [6]. Similar findings have been reported for other oral NAs. Recently, a panel of international experts has proposed an algorithm for optimizing response to oral antiviral agents using on-treatment monitoring of HBV DNA levels [10]. Based on published data, the panel categorized virologic response at 24 weeks of therapy as complete, partial, or inadequate. Complete virologic response was defined as HBV DNA levels <60 IU/ml (<300 copies/ml), which is the lower limit of detection of standard PCR assays, while a partial virologic response was defined as residual HBV DNA levels less than 2,000 IU/ml (<4 log10 copies/ml) at week 24. Inadequate virologic responses were defined as residual HBV DNA levels of ≥2,000 IU/ml (≥4 log10 copies/ml) at week 24.
The strategy for optimizing a partial or inadequate response to oral antiviral therapy is presented in Fig. 1 [10]. For primary treatment failure, which is uncommon, the optimal strategy is to switch to a more potent drug or possibly a combination of drugs. For an inadequate virologic response, the panel recommended the addition of another drug (preferably one that is more efficacious or, if such a drug is not available, adding one that is not cross-resistant) and repeat monitoring at 3-month intervals. Monitoring after 48 weeks may be extended from 3 to 6 months if the virologic response becomes complete. For patients with inadequate response at week 24, the panel recommended consideration of intensified treatment, with a second or alternative agent.
Fig. 1.
HBV treatment roadmap: virologic responses and their management in patients receiving oral therapy for chronic hepatitis B. Adapted with permission from Keeffe et al. [10]
For patients with lamivudine resistance, which is the most common type of HBV antiviral drug resistance, options include switching to or adding adefovir, with recent data favoring the continuation of lamivudine with the addition of adefovir. The latter strategy is favored because the rate of subsequent adefovir resistance is considerably higher using the switch strategy in patients who are lamivudine-resistant [41, 42]. Other potential therapeutic options for patients who are resistant to adefovir, entecavir, and telbivudine are listed in Table 2.
Table 2.
Treatment options after NA failure
| Resistance to | Treatment options |
|---|---|
| Lamivudine | Add adefovir |
| Add tenofovir | |
| Switch to tenofovir plus emtricitabine (fixed-dose combination) | |
| Switch to entecavir | |
| Adefovir | Add lamivudine |
| Add entecavir | |
| Switch to entecavir | |
| Switch to tenofovir plus emtricitabine (fixed-dose combination) | |
| Switch to entecavir (if no lamivudine resistance) | |
| Entecavir | Add adefovir |
| Add tenofovir | |
| Switch to adefovir | |
| Switch to tenofovir | |
| Telbivudine | Add adefovir |
| Add tenofovir | |
| Switch to emtricitabine | |
| Switch to tenofovir |
Future directions
Combination therapy, the standard of care for HIV infection, HCV infection, and tuberculosis, may also have a place in the treatment of patients with CHB. Potential benefits of this strategy include increased treatment efficacy, either additive [43] or synergistic, and the suppression of viral replication, which may delay or prevent resistance [5]. Disadvantages include increased drug toxicity and an increased potential for drug–drug interactions. The use of two or more drugs would clearly cause an immediate increase in the cost of treatment, but the long-term cost implications are not known. The ideal time to initiate combination therapy for HBV (e.g., as a first-line option or in response to drug resistance) is also not known.
Although this is not currently recommended as a clinical option, combination therapy has been and continues to be an active area of research. The use of peginterferon plus lamivudine for treatment-naïve patients with HBeAg-positive and HBeAg-negative CHB failed to show that the combination yielded a better off-treatment virologic response and ALT normalization than did peginterferon alone [12, 13]. A study comparing lamivudine plus adefovir with adefovir monotherapy in nucleoside-naïve patients did not indicate any significant improvements in efficacy with combination treatment compared with lamivudine [44]. Finally, a fourth study found no benefit in treating nucleoside-naïve patients with lamivudine plus telbivudine instead of telbivudine alone [37].
On the other hand, a study of lamivudine-resistant patients found that adding adefovir to lamivudine, rather than switching to adefovir monotherapy, resulted in less adefovir resistance [22]. More recent trials support this finding [23, 42, 45]. For this reason, treatment guidelines recommend the use of combination therapy in patients with lamivudine resistance [5]. Patients with decompensated cirrhosis may also benefit from combination therapy with adefovir plus lamivudine or possibly entecavir [3]. A recent study of peginterferon monotherapy or peginterferon plus lamivudine found higher rates of HBV DNA suppression and lower ALT levels with combination therapy [46]. Whether these results endure beyond treatment completion will be addressed in follow-up analyses.
Conclusion
The ultimate goals of anti-HBV treatment are the prevention of liver disease and reduced incidence of liver-related complications and HCC. The most important factors in achieving these goals are maximizing the antiviral effect of therapy and minimizing the emergence of drug resistance. However, because antiviral therapy is complex and requires a long-term commitment, treatment should be initiated only in patients who meet the criteria for treatment. Treatment algorithms will continue to evolve as new antiviral agents become available and the role of combination therapy is clarified.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CHB
Chronic hepatitis B
- HBeAg
Hepatitis B e antigen
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HIV
Human immunodeficiency virus
- PCR
Polymerase chain reaction
- PEG
Polyethylene glycol
- NA
Nucleoside or nucleotide analog
- ULN
Upper limit of normal
References
- 1.ACT-HBV Asia-Pacific Steering Committee Members. Chronic hepatitis B: treatment alert. Liver Int. 2006;26 Suppl 2:47–58. [DOI]
- 2.de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, et al. EASL international consensus conference on hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol. 2003;39 Suppl 1:S3–25. [PubMed]
- 3.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4:936–62. [DOI] [PubMed]
- 4.Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472–89. [DOI] [PubMed]
- 5.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. [DOI] [PubMed]
- 6.Di Bisceglie A, Lai C-L, Gane E, Chen YC, Thongasawat S, Wang YM, et al. Telbivudine GLOBE trial: maximal early HBV suppression is predictive of optimal two-year efficacy in nucleoside-treated hepatitis B patients [abstract 112]. Hepatology. 2006;44:230A–1A.
- 7.Gane E, Lai C-L, Liaw Y-F. Phase III comparison of telbivudine vs lamivudine in HBeAg-positive patients with chronic hepatitis B: efficacy, safety, and predictors of response at 1 year [abstract 493]. J Hepatol. 2006;44:S183–4. [DOI]
- 8.Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, et al. Virological and biochemical response in patients with HBeAg-negative CHB treated with peginterferon alfa-2a (40 kD) ± lamivudine: 3-year follow-up results [abstract 53]. J Viral Hepat. 2007;46 Suppl 1:S25–6.
- 9.Zeuzem S, Lai CL, Gane E, Liaw YF, Thongsawat S, Wang Y, et al. Optimal virologic and clinical efficacy at one year is associated with maximal early HBV suppression in nucleoside-treated hepatitis B patients. J Hepatol. 2006;44 Suppl 2:S24. [DOI]
- 10.Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, et al. Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890–7. [DOI] [PubMed]
- 11.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173–81. [DOI] [PubMed]
- 12.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95. [DOI] [PubMed]
- 13.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–17. [DOI] [PubMed]
- 14.Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, et al. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699–705. [DOI] [PMC free article] [PubMed]
- 15.Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005;142:240–50. [DOI] [PubMed]
- 16.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomized trial. Lancet. 2005;365:123–9. [DOI] [PubMed]
- 17.Vassiliadis T, Tziomalos K, Patsiaoura K, Zagris T, Giouleme O, Sourfleris K, et al. Lamivudine/pegylated interferon alfa-2b sequential combination therapy compared with lamivudine monotherapy in HBeAg-negative chronic hepatitis B. J Gastroenterol Hepatol. 2007;22:1582–8. [DOI] [PubMed]
- 18.Kaymakoglu S, Oguz D, Gur G, Gurel S, Tankurt E, Ersoz G, et al. Pegylated interferon Alfa-2b monotherapy and pegylated interferon Alfa-2b plus lamivudine combination therapy for patients with hepatitis B virus E antigen-negative chronic hepatitis B. Antimicrob Agents Chemother. 2007;51:3020–22. [DOI] [PMC free article] [PubMed]
- 19.Hadziyannis S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–7. [DOI] [PubMed]
- 20.Marcellin P. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–16. [DOI] [PubMed]
- 21.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–51. [DOI] [PubMed]
- 22.Liaw YF, Lee CM, Chien RN, Yeh CT. Switching to adefovir monotherapy after emergence of lamivudine-resistant mutations in patients with liver cirrhosis. J Viral Hepat. 2006;13:250–5. [DOI] [PubMed]
- 23.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–90. [DOI] [PubMed]
- 24.Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil (ADV) in lamivudine- resistant chronic hepatitis B patients treated with ADV. Gut. 2006;55:1488–95. [DOI] [PMC free article] [PubMed]
- 25.Fung SK, Andreone P, Han SH, Rajender RK, Regev A, Keeffe EB, et al. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J Hepatol. 2005;43:937–43. [DOI] [PubMed]
- 26.Hadziyannis SJ. Treatment paradigms on hepatitis B e antigen-negative chronic hepatitis B patients. Expert Opin Investig Drugs. 2007;16:777–86. [DOI] [PubMed]
- 27.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. [DOI] [PubMed]
- 28.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20. [DOI] [PubMed]
- 29.Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–49. [DOI] [PubMed]
- 30.Langley DR, Walsh AW, Baldick CJ, Eggers BJ, Rose RE, Levine SM, et al. Inhibition of hepatitis B virus polymerase by entecavir. J Virol. 2007;81:3992–4001. [DOI] [PMC free article] [PubMed]
- 31.Yao G. Entecavir is a potent anti-HBV drug superior to lamivudine: experience from clinical trials in China. J Antimicrob Chemother. 2007;60:201–5. [DOI] [PubMed]
- 32.Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology. 2006;44:1656–65. [DOI] [PubMed]
- 33.Colonno R, Rose R, Pokornowski K, Baldick CJ, Klesczewski K, Tenney D. Assessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naive patients while resistance emergence increases over time in lamivudine refractory patients [abstract 110]. Hepatology. 2006;44:229A–30A. [DOI]
- 34.Colonno R, Rose R, Pokornowski K, Baldick CJ, Eggers B, Yu ES, et al. Four year assessment of ETV resistance in nucleoside-naive and lamivudine refractory patients [abstract 781]. Last update: 2007. Available at: http://www.easl.ch/easl2007/Program/session1.asp?SessionId=PS14&SSessionDate=4/14/2007. Accessed October 5, 2007.
- 35.Tenney DJ, Rose RE, Baldick CJ, Levine SM, Pokornowski KA, Walsh AW, et al. Two-year assessment of entecavir resistance in lamivudine-refractory hepatitis B virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51:902–11. Epub 2006 Dec 18. [DOI] [PMC free article] [PubMed]
- 36.Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine (LDT) vs. lamivudine for chronic hepatitis B: first-year results from the international phase III globe trial [abstract LB1]. Hepatology. 2005;42:748A. 16175614 [DOI]
- 37.Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528–36. [DOI] [PubMed]
- 38.Lai C-L, Gane E, Hsu CW, Thongsawat S, Wang Y, Chen Y, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [abstract 91]. Hepatology. 2006;44:22A.
- 39.van Bommel F, Wunsche T, Mauss S, Reinke P, Bergk A, Schurmann D, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–5. [DOI] [PubMed]
- 40.van Bommel F, Zollner B, Sarrazin C, Spengler U, Huppe D, Moller B, et al. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318–25. [DOI] [PubMed]
- 41.Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–91. [DOI] [PubMed]
- 42.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–13. [DOI] [PubMed]
- 43.Zhu Y, Qi X, Delaney W, Curtis M, Miller MD, Borroto-Esoda K. Anti-HBV activity of in vitro combinations of tenofovir with nucleoside analogs [abstract 172]. Hepatology. 2006;44:253a.
- 44.Sung JJY, Lai JY, Zeuzem S, et al. A randomized double-blind phase II study of lamivudine (LAM) compared with lamivudine plus adefovir dipivoxil (ADV) for treatment naive patients with chronic hepatitis B (CHB): week 52 analysis [abstract 69]. J Hepatol. 2003;38 Suppl 2:25–6. [DOI]
- 45.Lampertico P, Marzano A, Levrero M, Santantonio T, DiMarco V, Brunetto M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [abstract LB5]. Hepatology. 2006;44:693A–4A.
- 46.Piccolo P, Lenci I, DiPaolo D, Telesca C, DeMelia L, Sorbello O, et al. Peginterferon-alpha-2a plus adefovir vs. peginterferon alpha-2a for 48 weeks in HBeAg-negative chronic hepatitis B: preliminary 24 week results of the Peg for B randomized multicenter trial [abstract 54]. J Hepatol. 2007;46:S26. [DOI]