Abstract
Purpose
To analyze the prognostic factors for the patients with lung metastases from hepatocellular carcinoma (HCC).
Methods and materials
One hundred and five patients with lung metastases from HCC were analyzed retrospectively. We analyzed the impact factors, including the gender, age, liver function, serum AFP and γ-GT level, the status of intrahepatic tumor and pulmonary metastases and treatment for them, the distant metastases beyond the lung, as well as the causes of death. The overall cumulative probability of survival was calculated by the Kaplan–Meier method, and the difference between the groups was compared using the Log-rank test. Univariate and multivariate analyses using the Cox-regression proportional hazard model were performed to evaluate the prognostic parameters for survival.
Result
The survival after the lung metastases was influenced by clinical parameters, such as the status and the treatment for both the intrahepatic tumor and the pulmonary lesions. The causes of death were respiratory failure due to metastatic lesions from HCC in 16 patients (20.0%), liver failure caused by the progressive intrahepatic lesions in 54 (67.5%). The mean and median survival times were 684 and 487 days after HCC diagnosis and 264 and 179 days after lung metastases, respectively.
Conclusion
It was very important to treat the intrahepatic tumor because its worsening was still the major cause of death. The progressive treatment for pulmonary metastases may also be advised for possible prolongation of survival.
Keywords: Hepatocellular carcinoma (HCC), Pulmonary metastases, Survival analysis
Introduction
Hepatocellular carcinoma (HCC) is one of the common malignant tumors worldwide. Especially in China, its mortality ranks second after stomach carcinoma, accounting for 18.8% of the total cancer deaths [1]. 66–77% of patients with HCC die from the progression of intrahepatic tumor [2]. With the development of the diagnostic modalities and the multidisciplinary application of the surgical resection, transplantation, transcatheter arterial chemoembolization (TACE), and percutaneous ablation [2, 3], the intrahepatic tumor and its recurrence have been well controlled and the survival is prolonging, hence the distant metastasis is increasing. The lung is the most commonly affected organ of the extrahepatic metastases. Once the pulmonary metastases occur, the tumor is considered automatically to have entered the advanced stage. But there are few reports about the prognostic analysis of the pulmonary metastases from HCC. The aim of this study is to provide some evidence on the prognosis of pulmonary metastases from HCC for clinical doctors.
Materials and methods
Patients
We conducted a retrospective chart review of 105 patients with pulmonary metastases from HCC hospitalized in Zhongshan Hospital, Fudan University (Shanghai, China) from January 1999 to September 2004. During this period, 2,301 patients with HCC were hospitalized at the Liver Cancer Institute, Zhongshan Hospital, including 105 patients with the pulmonary metastases. The proportion of HCC with pulmonary metastases was only 4.56%. But the incidence of lung metastases observed by others was up to 41.6–43.6% in the autopsy of HCC [4, 5]. This low incidence was attributable to the following up of the hospitalized patients. And whether the patients received treatment for pulmonary metastases from HCC was a matter of physician preference in most cases.
The baseline characteristics of all patients are summarized in Table 1. The median age of the patients including 88 males and 17 females was 46 years. The median diameter of intrahepatic tumor was 8.49 cm (range, 2.6–17 cm) at the time of diagnosis of HCC. The primary HCC was multiple or diffuse in 30 patients and unifocal in 75 patients. HBsAg was positive in 89 patients (84.76%). All patients were in Child-Pugh Class A or B. The 64 patients who did not receive treatment for pulmonary metastases were classified as non-treatment group, and the 41 patients who underwent treatment for pulmonary metastases were classified as treatment group.
Table 1.
Baseline characteristics of groups with and without treatment of pulmonary metastasis
| Cases of patients | Non-treatment group | Treatment group | P value | |
|---|---|---|---|---|
| Sex | Male/Female | 55/9 | 33/8 | 0.460 |
| HBsAg | Negative/Positive | 9/55 | 7/34 | 0.675 |
| AFP (μg/l) | ≤20/20–400/≥400 | 13/14/37 | 5/12/24 | 0.469 |
| γ-GT (IU/l) | ≤75/75–150/≥150 | 21/14/29 | 12/7/22 | 0.689 |
| Child-Pugh classification | Class A/Class B | 61/3 | 39/2 | 0.651a |
| Intrahepatic tumor status | ||||
| Size | ≤8/>8cm | 27/37 | 23/18 | 0.164 |
| Type | Unifocal/diffuse | 47/17 | 28/13 | 0.569 |
| Extrahepatic tumor status | ||||
| Tumor thrombus | No/Yes | 46/18 | 25/16 | 0.244 |
| Lymph Metastases | No/Yes | 57/7 | 40/1 | 0.145a |
| Therapeutic models for liver tumor | ||||
| Surgical resection | No/Yes | 35/29 | 23/18 | 0.887 |
| TACE | No/Yes | 8/56 | 2/39 | 0.309a |
| Radiotherapy | No/Yes | 35/29 | 34 /7 | 0.003 |
| Pulmonary metastasis status | ||||
| Type | Solitary/Multiple | 7/57 | 8/33 | 0.221 |
| Pleural fluid | No/Yes | 55/9 | 40/1 | 0.084a |
| Other metastasis except lung | No/Yes | 54/10 | 33/8 | 0.606 |
All data were acquired when the pulmonary metastases occurred
aP values were obtained in Fisher’s exact test, the others in Chi-square test
Thirteen patients were diagnosed with metastatic pulmonary lesion first, and HCC was diagnosed in the next examination; the other 92 patients were on the verse. There were 19 patients with other distant metastases concurrently beyond the lung, including 7 adrenal metastases, 6 bone metastases, and 6 brain metastases.
Examination and follow-up observation
Pretreatment evaluation included a medical history and physical examination, complete blood cell count, serum chemistries, liver function tests involving γ-glutamyltransferase (γ-GT), serum alpha-fetoprotein (AFP), chest X-ray, abdominal ultrasonography, and enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Clinical monitoring was performed monthly. Hepatic angiography was obtained in patients who received TACE. The Abdominal and liver status was followed up using ultrasonography, and enhanced CT or MRI, the chest status using the X-ray or CT imaging monthly.
Diagnosis
In 52 patients, the diagnosis of HCC was confirmed by histological testing (47 surgical specimen and 5 biopsy) and in 53 patients by the clinical diagnosis (34 AFP ≥ 400 μg/l, 16 AFP 20–400 μg/l, 3 AFP ≤ 20 μg/l). The diagnosis of pulmonary metastases mainly depended on the imaging of CT, with or without clinical symptoms such as coughing, chest stuffiness, coughed up blood, chest pain, pleural fluid, and even dyspnea if multiple metastases occurred in both lung fields.
For clinical diagnosis, the patients had to meet the following criteria, issued by the Chinese Liver Cancer Association in 2001: (1) AFP level of 400 μg/l or more, to rule out patients with active liver disease, embryonic malignant teratoblastomas of testes and ovary, or other malignant tumors metastasizing to the liver, and liver tumor should have a characteristic intrahepatic appearance on one of the HCC images. (2) If level is less than 400 μg/l, the characteristic intrahepatic lesion should be confirmed by two of the HCC imaging methods listed next. The status of carcinoembryonic antigen or carbohydrate antigen 19-9 should be negative for patients with negative AFP level, to exclude those with metastatic tumors from the digestive system or intrahepatic cholangiocarcinoma [6]. AFP was tested by the electrochemiluminescence immunoassay from Roche Diagnostics Corporation. The imaging modalities of HCC include the CT, MRI scan, ultrasonography, and hepatic angiography.
The plain CT scan of HCC presents the hypodensity or homodensity. Dual-phase contrast CT is obtained during the arterial and portal venous phase of a single i.v. injection of contrast. During the arterial phase, HCC presents an enhancing lesion. During the portal-venous phase, HCC is usually hypodense or almost invisible. The central necrosis in the large HCC is less hypodense and is not enhanced in the contrast scan. There is a filling-defect in the portal or inferior vena cava if tumor thrombus occurs in them. In MRI, multiplanar scan and reconstruction were used to present the lesion with the low signal intensity and high signal intensity in T1WI and T2WI imaging, respectively. Dynamic gadolinium-enhanced MRI can improve the detection of HCC, which usually appears as a markedly enhancing lesion in arterial phase that disappears quickly in portal and delayed phase.
In ultrasonography, the majority of lesions less than 3 cm in size were hypoechoic, probably because of their homogeneously cellular structure. Larger HCC tumors usually have a heterogeneously hyperechoic appearance. The pattern of intralesional flow might be helpful in distinguishing HCC from other hepatic tumors by color Doppler ultrasound.
Angiography is performed during TACE. Most focal lesions have a characteristic angiographic appearance, with hypervascularity caused by abnormal arteries and a dense tumor blush during the capillary and late phases.
In the CT scan, most pulmonary metastases were described as multiple nodular or mass lesions of different sizes with the smooth margin scattering in one or both lung fields or in one or more lung lobes, occasionally with the pleural fluid. In the follow-up, the lesions may become larger and the number may increase gradually if they were not treated.
Treatment
The intrahepatic tumor received surgical resection in 47 patients with the post-surgery TACE in 40 patients; TACE in 55 patients combined with radiofrequency ablation (RF) in 9 patients and percutaneous ethanol injection in 6 patients. Three patients got no treatment. TACE was a combination of targeting chemotherapy with 5-fluorouracil 1 g, cisplatin 80 mg, mitomycin C 10 mg, and arterial embolization with iodized oil (lipiodol ultra fluid) 10 ml mixed with 10-mg mitomycin C, which had both a selective ischemic, necrotic and chemotherapeutic effect on the intrahepatic lesions.
Thirty-six patients received external beam radiation treatment (EBRT) on the lymph involvements and tumor thrombus of the portal vein or the inferior vena cava. EBRT was a limited-field radiotherapy on the local lesions with a linear accelerator of 6 or 15 MV photons, depending on tumor location and depth. The design and delivery of radiotherapy was based on the CT scan during the simulation process. The radiation portals encompassed the tumor lesions with 1–2 cm margins around the imaging presentation, sparing the key tissues and organs. The median tumor dose was 50 Gy (range, 36–54 Gy) in daily 1.8–2.0 Gy fraction, five times a week.
Of the 15 patients identified with solitary pulmonary lesion, 4 patients underwent surgical resection, 2 patients received radiotherapy, and 9 patients received none. Of the 90 patients identified with multiple pulmonary lesions, 35 patients received pulmonary-artery infusion (PAI), bronchial artery infusion + embolization (BAI + BAE), or pulmonary artery port-catheter system (PA-PCS, intermittent continuous pulmonary infusion of chemotherapy, for 6–12 months. The regimens of the chemotherapy were 5-FU 0.5–1.0 g + DDP 40–80 mg + MMC 10–20 mg or ADM 30–60 mg), the other 55 patients got no treatment. Many treatments for the pulmonary metastases depended on the physician preference because there were no guidelines for patients with pulmonary metastases. We always considered the patients with solitary lesion for surgical resection unless the patients had other distant metastases, otherwise the patients received local radiation therapy. If the patients had multiple lung metastases, we considered them to receive PAI, BAI + BAE, or PA-PCS.
Statistical analysis
Kaplan–Meier curves were generated for overall survival. Differences between curves were assessed by the Log-rank test. The Chi-square test was used to compare the prevalence of baseline characteristics between two groups. The Cox regression proportional hazard model was used to detect the association between the survival and all the variables such as the AFP and γ-GT levels, the status of the intrahepatic tumor and pulmonary metastases and treatment for them. In this study, all data were obtained on lung metastases and we analyzed the prognosis from two-time start point-at the HCC and pulmonary metastases diagnosis, respectively, using the Cox regression model. For the multivariate analysis, all variables were entered in a single step. P < 0.05 was considered statistically significant. All calculations were performed using SPSS11.5 for Windows XP (SPSS, Inc., Chicago, IL).
Result
The diagnostic meaning of AFP and γ-GT
AFP is useful not only for diagnosis, but also as a prognostic indicator for HCC patients. AFP mRNA has been proposed as a predictive marker of HCC cells disseminated into the circulation and for metastatic recurrence [7]. The γ-GT is one of the liver functional and HCC markers. It is an important predictive marker for disease-free survival (DFS) of HCC patients [7]. Higher γ-GT level shows a significant effect on lower survival [2]. Two factors are thought to demonstrate the occurrence and development of HCC and affect the survival. In this study, we combined the factors to observe if they could affect the survival.
AFP level had increased beyond the normal range (0–20 μg/l) in 85 of the 105 patients at the diagnosis of HCC and in 87 patients at the diagnosis of pulmonary metastases. In a multivariate Cox regression analysis, increased AFP level was associated with survival after the HCC diagnosis (P = 0.007), but not after the diagnosis of pulmonary metastases. In a univariate Cox regression analysis, increased AFP level was also associated with the survival after the diagnosis of HCC (P = 0.029), and not after the diagnosis of pulmonary metastases.
γ-GT level had increased beyond the normal range (0–75 IU/l) in 67 of the 105 patients at the diagnosis of HCC and in 72 patients at the diagnosis of pulmonary metastases. In both multivariate and univariate Cox regression analyses, increased γ-GT level was associated with survival after the HCC diagnosis (P = 0.021 and P < 0.001, respectively). After the diagnosis of pulmonary metastases, increased γ-GT level could affect the survival in a univariate Cox regression analysis (P = 0.003), but it was not associated with the survival in a multivariate Cox regression analysis.
The distribution of pulmonary lesions
Of all the 105 patients with pulmonary metastases from HCC examined in the present study, the distribution of pulmonary lesions was 85.71% (90 of 105) in both the lung fields, and 14.28% (15 of 105) in one lung field including 1 in upper left, 12 in the lower left, 2 in the lower right, concurrently 10 patients with pleural fluid.
The overall survival of the HCC with pulmonary metastases
The mean survival time ± SE was 684 ± 68 days, the median time was 487 days after the diagnosis of HCC; and 264 ± 28 and 179 days after the diagnosis of pulmonary metastases, respectively.
By the Kaplan–Meier method, the 1, 2, 3 year survival rates were 64.8, 33.6, 15.0% after the diagnosis of HCC, and 29.7, 10.8, 0% after the diagnosis of pulmonary metastases, respectively.
The prognostic analysis and impact factor of the HCC with pulmonary metastases
The univariate and multivariate Cox regression analyses of survival showed the prognostic factors after the diagnosis of HCC (seen Table 2). The favorable parameters for survival were the Child-Pugh Class A, the resection of intrahepatic lesion.
Table 2.
Prognostic risk factors for the survival after the diagnosis of HCC
| Multivariate analysis | Univariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P value | RR | 95% CI | P value | ||
| γ-GT (IU/l) | ≤75 | 1 | 1 | ||||
| 75–150 | 1.425 | 0.736–2.758 | 0.293 | 1.839 | 0.997–3.392 | 0.051 | |
| ≥150 | 2.157 | 1.125–4.135 | 0.021 | 3.667 | 2.129–6.315 | <0.001 | |
| AFP (μg/l) | ≤20 | 1 | 1 | ||||
| 20–400 | 3.226 | 1.383–7.523 | 0.007 | 1.732 | 0.843–3.560 | 0.135 | |
| ≥400 | 1.954 | 0.952–4.011 | 0.068 | 2.005 | 1.073–3.746 | 0.029 | |
| Child-Pugh classification | |||||||
| Class B versus A | 23.138 | 6.925–77.314 | <0.001 | 30.306 | 9.680–94.882 | <0.001 | |
| Intrahepatic tumor status | |||||||
| Size | >8 vs. ≤8 cm | 1.728 | 0.962–3.102 | 0.067 | 3.054 | 1.897–4.918 | <0.001 |
| Tumor thrombus | Yes versus no | 1.219 | 0.677–2.192 | 0.509 | 1.427 | 0.889–2.288 | 0.141 |
| Lymph metastasis | Yes versus no | 2.776 | 1.122–6.867 | 0.027 | 1.055 | 0.484–2.299 | 0.893 |
| Resection | Yes versus no | 0.278 | 0.140–0.552 | <0.001 | 0.252 | 0.150–0.424 | <0.001 |
| TACE | Yes versus no | 0.602 | 0.213–1.700 | 0.338 | 0.759 | 0.362–1.590 | 0.465 |
| Pulmonary metastasis status | |||||||
| Type | Solitary versus multiple | 0.527 | 0.263–1.057 | 0.071 | 1.815 | 0.904–3.643 | 0.094 |
| Pleural fluid | Yes versus no | 3.646 | 1.508–8.811 | 0.004 | 1.514 | 0.743–3.083 | 0.253 |
| Treatment | Yes versus no | 0.583 | 0.344–0.990 | 0.046 | 0.622 | 0.384–1.008 | 0.054 |
The univariate and multivariate Cox regression analyses of survival showed the prognostic factors after the diagnosis of the pulmonary metastases (seen Table 3). The Child-Pugh Class A, normal AFP level, resection of intrahepatic lesion, sole pulmonary metastasis without pleural fluid, and treatment for the pulmonary metastases could benefit the survival after the diagnosis of pulmonary metastases.
Table 3.
Prognostic factors for the survival after the diagnosis of pulmonary metastasis
| Multivariate analysis | Univariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P value | RR | 95% CI | P value | ||
| γ-GT (IU/l) | ≤75 | 1 | 1 | ||||
| 75–150 | 1.146 | 0.544–2.413 | 0.720 | 1.760 | 0.957–3.236 | 0.069 | |
| ≥150 | 1.722 | 0.843–3.518 | 0.136 | 2.267 | 1.329–3.870 | 0.003 | |
| AFP (μg/l) | ≤20 | 1 | 1 | ||||
| 20–400 | 1.827 | 0.815–4.098 | 0.143 | 1.123 | 0.545–2.316 | 0.753 | |
| ≥400 | 2.269 | 0.842–6.117 | 0.105 | 1.726 | 0.902–3.304 | 0.099 | |
| Child-Pugh classification | |||||||
| Class B versus A | 12.283 | 4.193–35.981 | <0.001 | 10.350 | 3.750–28.565 | <0.001 | |
| Intrahepatic tumor status | |||||||
| Size | ≤8 versus >8cm | 1.023 | 0.498–2.099 | 0.951 | 1.958 | 1.239–3.095 | 0.004 |
| Tumor thrombus | Yes versus no | 1.580 | 0.915–2.728 | 0.101 | 1.771 | 1.105–2.840 | 0.018 |
| Lymph metastasis | Yes versus no | 2.590 | 1.057–6.345 | 0.037 | 1.375 | 0.631–2.997 | 0.423 |
| Resection | Yes versus no | 0.434 | 0.251–0.751 | 0.003 | 0.487 | 0.304–0.779 | 0.003 |
| TACE | Yes versus no | 0.848 | 0.304–2.364 | 0.752 | 0.866 | 0.394–1.907 | 0.722 |
| Pulmonary metastases status | |||||||
| Type | Solitary versus multiple | 3.124 | 1.471–6.633 | 0.003 | 2.525 | 1.250–5.099 | 0.010 |
| Pleural fluid | Yes versus no | 4.464 | 1.992–10.000 | <0.001 | 3.040 | 1.495–6.185 | 0.002 |
| Treatment | Yes versus no | 0.345 | 0.196–0.608 | <0.001 | 0.355 | 0.214–0.588 | <0.001 |
The survival status in two groups with and without treatment for pulmonary metastases
The baseline characteristics of patients in two groups are summarized in Table 1. Sex, distribution of AFP and γ-GT level, the intrahepatic tumor status and its modalities, and the pulmonary metastases status do not seem to differ in the two groups.
In the treatment group, the mean survival time ± SE was 1,066 ± 155 days, the median time was 709 days from the diagnosis of HCC; and 620 ± 105 and 547 days from the diagnosis of pulmonary metastases, respectively. In the non-treatment group, the mean survival time ± SE was 837 ± 152 days, the median time was 483 days from the diagnosis of HCC; and 210 ± 26 and 161 days from the diagnosis of pulmonary metastases, respectively.
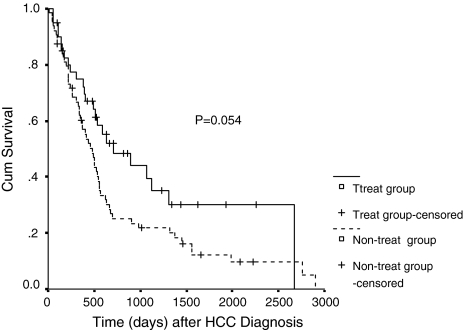
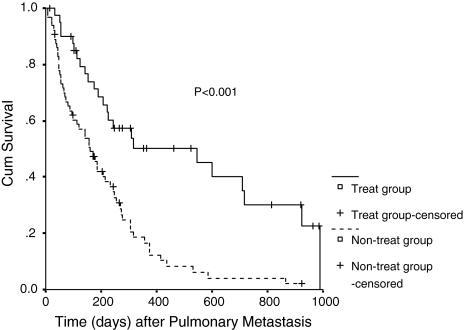
In a univariate Cox regression analysis, the treatment of the pulmonary metastases was not an influential parameter for the survival rate after the diagnosis of HCC (P = 0.054, Fig. 1). Despite a P value of 0.054 which was not significant statistically, the survival rate was a little higher in the treatment group (RR = 0.622). But in a multivariate Cox regression analysis, the treatment of the pulmonary metastases was associated with survival after the diagnosis of HCC (P = 0.046). And it was a beneficial factor for the survival rate after the diagnosis of pulmonary metastases (P < 0.001, Fig. 2), which showed that the survival can be prolonged if the pulmonary metastases were treated by PAI, BAI + BAE, or PA-PCS after the diagnosis of pulmonary metastases.
Fig. 1.
Survival curve for two groups according to risk factors after HCC diagnosis
Fig. 2.
Survival curve for two groups according to risk factors after pulmonary metastasis
Failure patterns
At the time of analysis, 25 patients were still alive and 80 had died. The death was caused by liver failure in 54 patients (67.5%), by lung metastases in 16 patients (20.0%), by gastrointestinal bleeding in 2 patients, by brain metastases in 8 patients (10.0%). In the further stratification 6 out of 24 (25.0%) and 11 out of 56 (19.6%) patients died of lung metastases in the treatment and non-treatment group of pulmonary metastases, respectively.
Discussion
Lung metastases have been found in 25–30% of the patients with malignant tumors at autopsy [8]. The incidence of lung metastases was especially high, up to 41.6–43.6%, in hepatocellular carcinoma [4, 5]. Lung is the most commonly affected organ of the extrahepatic metastases from HCC because all blood must pass through and flow slowly into the pulmonary circulation; hence the tumor cells in the blood easily settle down in the lung. In the HCC patients, the survival time was 5.5 months with bone metastases [9], 10 months with adrenal gland metastasis [10], 8 and 4 months with tumor thrombus of the treatment group and non-treatment group, respectively [11]. In this study the survival time was 264 days (8.8 months), which was longer than that of the bone metastases and tumor thrombus.
In the present study, the treatment for the pulmonary metastases was not an influential parameter after the diagnosis of HCC (P = 0.054) using a univariate Cox regression analysis. That may mean the treatment for the pulmonary metastases could not prolong the overall survival even if the intrahepatic tumors were controlled and did not progress. Despite a P value of 0.054, the survival rate was a little higher in the treatment group (RR = 0.622), which meant the treatment can benefit the survival. But, the treatment was associated with the survival after the diagnosis of HCC (P = 0.046) in a multivariate Cox regression analysis. And after the diagnosis of pulmonary metastases, treatment for pulmonary metastases was a beneficial factor for the survival rate (P < 0.001, statistically evident). That meant the survival can be prolonged if the pulmonary metastases were treated by PAI, BAI, or PA-PCS after the diagnosis of pulmonary metastases. In conclusion, the treatment for the pulmonary metastases was advised if they occurred.
At present, the modalities for the pulmonary metastases include interventional chemotherapy (PAI, BAI + BAE, and PA-PCS), surgical resection, and radiation therapy.
Interventional chemotherapy is applied widely in clinical practice because it is performed anatomically and technologically, and can be concurrently implemented with the TACE for the intrahepatic lesions. Cheng et al. [12] reported an obvious decrease in lung tumor size and stable disease in 35.5 and 32.3% of cases in the 6-month follow-up after 62 patients underwent PA-PCS, and hence concluded that the percutaneous implantation techniques of PA-PCS could be a good method in the treatment of metastatic lung cancer from HCC because of its simple and positive effects with few complications. Wu et al. [13] compared the group with or without BAI (n = 67, P < 0.001). BAI is a very effective method for prevention of the lung metastases from primary liver cancer (PLC) and can eliminate the lesions that are 1 mm or less in size, and the dose of the chemotherapeutic agents is one-fourth or one-fifth of the conventional doses. Combined use of TACE and BAI is superior to TACE alone for reducing the metastatic rate of the lung and increasing the survival rate for patients with PLC.
Lam et al. [14] report that 9 in 48 patients were suitable for curative pulmonary resection. The median survival after the pulmonary resection was 42 months, and the 1-, 2-, and 5-year survival rates were 100, 78, and 67%, respectively. This high survival rate was due to the patients selected only with a solitary pulmonary metastasis and no concomitant intrahepatic recurrence after hepatectomy for HCC. Davidson et al. [8] summarized the established criteria for the surgical management of pulmonary metastases which were limited and strict. But most pulmonary metastases of HCC were multiple and were not amenable to surgical resection.
Three-dimensional conformal radiation therapy (3-DCRT) can be applied to treat the pulmonary lesions. In this study, only 2 patients received it and one could not finish because of the progressive intrahepatic lesion. And the 3-DCRT can induce the radiation lung injury too. So it was seldom applied in the clinical treatment for the pulmonary metastases, especially from HCC, like the surgical resection.
As a conclusion, it is very important to treat the intrahepatic tumor because its progression is the major cause of death. Once pulmonary metastases is diagnosed, the progressive treatment such as PAI, BAI + BAE, or PA-PCS for pulmonary metastases may also be advised for possible prolongation of survival.
References
- 1.Zhang S, Li L, Lu F. Mortality of primary liver cancer in China from 1990 through 1992. Zhonghua Zhong Liu Za Zhi. 1999; 21:245–9 (in Chinese). [PubMed]
- 2.Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10:307–16. [DOI] [PubMed]
- 3.Livraghi T. Guidelines for treatment of liver cancer. Eur J Ultrasound. 2001;13:167–76 Review. [DOI] [PubMed]
- 4.Kakumu S. Trends in liver cancer researched by the Liver Cancer Study Group of Japan. Hepatol Res. 2002;24(Suppl 1):S21–7. [DOI]
- 5.Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28(1):21–9. [DOI] [PubMed]
- 6.Chinese Liver Cancer Association. The diagnostic criteria and stages of primary hepatoma in the 4th National Conference of Liver Cancer: problem and discussion. Chin J Gen Surg. 2000:15:238–9 (in Chinese).
- 7.Qin LX, Tang ZY. The prognostic significance of clinical and pathological feature in HCC. World J Gastroenterol. 2002;8:193–9. [DOI] [PMC free article] [PubMed]
- 8.Davidson RS, Nwogu CE, Brentjens MJ, Anderson TM. The surgical management of pulmonary metastasis: current concept. Surg Oncol. 2001;10:35–42. [DOI] [PubMed]
- 9.He J, Zeng ZC, Tang ZY, Wang FY, Yang P. Radiotherapy for primary liver neoplasm with bone metastases and analysis of related prognostic factors. Tumor. 2002;22:421–3 (in Chinese).
- 10.Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol. 2005;35:61–7. [DOI] [PubMed]
- 11.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–43. [DOI] [PubMed]
- 12.Cheng JM, Wang JH, Yan ZP, Wang XL, Gong GQ, Liu QX. Implantation port-catheter permanent indwelling of pulmonary artery in treating lung metastasis from HCC. J Interv Radiol. 2000;9:158–60 (in Chinese).
- 13.Wu XJ, Hou NP, Huang Y. Combined TACE and BAI to prevent lung metastases from PLC. J Interv Radiol. 2002;11:431–2 (in Chinese).
- 14.Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198–200. [DOI] [PubMed]