Abstract
Purpose
Altered redox status has been implicated in pathogenesis of alcoholic liver disease (ALD) as well as in nonalcoholic fatty liver disease (NAFLD). This study was planned to find the relative role of redox status in these two diseases.
Methods
A total of 44 patients with ALD and 32 patients with NAFLD and 25 apparently healthy controls were included in the study. Redox status was estimated by measuring oxidative stress (superoxide dismutase (SOD) and lipid peroxidation products as thiobarbituric acid reactive substances (TBARS)) and antioxidant status (ferric reducing ability of plasma (FRAP) and vitamin C).
Results
TBARS level was raised significantly in both ALD (3.5 (2.3–9.4) vs. 1.8 (0.5–4.1) nmol/ml; P = 0.0001) and NAFLD (5.1 (1–10.2) vs. 1.82 (0.51–4.1) nmol/ml; P = 0.0001) as compared with controls, but was not different between ALD and NAFLD. SOD was significantly higher in ALD as compared to NAFLD (2.4 (1.3–7.8) vs. 0.68 (0.05–19.1) U/ml; P = 0.0001) and controls (1.12 (0.01–3.5) U/ml; P = 0.001). FRAP was lower in ALD as compared with NAFLD (345.4 (56–615.9) vs. 434.1 (197.6–733.3) μmol of Fe+2 liberated; P = 0.001) but similar to that of controls (340.9 (141.5–697.5) μmol of Fe+2 liberated).
Conclusions
ALD patients have a higher degree of redox imbalance as compared with NAFLD patients
Keywords: Alcoholic liver disease, Antioxidant status, Nonalcoholic fatty liver disease, Oxidative stress
Introduction
Despite being etiologically distinct entities, nonalcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD) have several common pathophysiological and histological features [1–3]. Recent studies have demonstrated oxidative stress (OS) as an important pathophysiological factor in a variety of diseases [4]. While the information on the pathogenesis of NAFLD is limited, that of ALD is supported by numerous studies pointing toward OS. [5, 6]. The liver injury in ALD is dependent on the metabolic product of ethanol (acetaldehyde) causing functional and metabolic derangements [7] along with induction of cytochrome P450 2E1 (CYP450 2E1) that results in increased generation of reactive oxygen species (ROS) and development of OS [8]. In NAFLD also, oxidative damage has been proposed as a mechanism of lipid peroxidation and inflammatory recruitment resulting in progression of disease along with antioxidant enzyme modification [9–13]. Biochemical and histological improvement after treatment with antioxidants in NAFLD is an indirect evidence of OS-induced pathogenesis [14]. Increased levels of ROS and products of lipid peroxidation have also been demonstrated in this condition [15, 16]. But most of the studies included small number of patients and the results in many of these studies are conflicting and show only weak associations. Except for the etiological agent, the strikingly similar histological features may suggest similar pathogenesis. Since OS is an important pathogenetic factor in both the diseases, we planned to evaluate the role of OS in ALD, relative to that in NAFLD.
Patients and methods
Patients
Alcoholic liver disease
All consecutive patients diagnosed as ALD attending outpatient clinic of the Department of Gastroenterology at the All India Institute of Medical Sciences were included in the study. Diagnosis was made on the basis of history of alcohol intake and clinical and biochemical findings. Patients with definitive evidence of other liver diseases, such as viral diseases, were excluded.
Nonalcoholic fatty liver disease
Consecutive patients presenting to the outpatient clinic of the Department of Gastroenterology of the All India Institute of Medical Sciences (AIIMS), with raised transaminases at least 1.5 times the upper limit of normal on at least two occasions, who had either histologically confirmed NAFLD or an evidence of fatty liver on ultrasound were included. Patients in whom alcohol intake exceeded 20 g/day, or who had definitive evidence for other liver diseases and patients who were on medications known to induce fatty liver or known to affect oxidant and/or antioxidant levels were also excluded. The history of duration and amount of drinking and smoking was confirmed from two close relatives of the patient. Thirty-two patients of NAFLD were included, of which 21 have also been described earlier [13].
Controls
Twenty-five age- and sex-matched apparently healthy, nonsmoking, nonalcoholic subjects, who were not taking any medications were included as controls. The controls were recruited from among hospital staff and patients’ relatives.
An informed consent in writing was obtained from each patient after describing the details of the investigations to be done. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Institute.
Methods
All patients were evaluated thoroughly at inclusion with anthropometric measurements (body mass index and waist–hip ratios) and complete blood counts, and biochemical tests (liver function profile, serum insulin, fasting, and postprandial blood sugar). Viral hepatitis markers (HBsAg (ELISA; Orgnanon teknika, Boxtel, The Netherlands), total anti-HBc (ELISA; Biorad, France) anti-HCV (ELISA; Xcyton, Bangalore, India), IgM anti-HEV (In house ELISA), IgM anti-HAV (ELISA; Medical biological Science, Milano, Italy)) were done to exclude virus-induced liver diseases. An ultrasound of abdomen was done in all patients to look for the presence of fatty liver and to exclude any other hepatobiliary disease.
Measurements of redox status markers
Ten milliliters of blood was drawn after an overnight fast and it was divided into two aliquots for separation of plasma and serum. This was done within 2 h of sample collection. Plasma and sera samples were then stored at −80°C until they were analyzed. All the analyses were done by colorimetric methods. The OS was measured by estimating lipid peroxidation products (LPO) and superoxide dismutase (SOD) enzyme, and antioxidant status was estimated by measuring plasma vitamin C levels and the total antioxidant capacity (ferric reducing ability of plasma; FRAP). All estimations were done using manual spectrophotometric methods. The samples were estimated in duplicates and quality control assays were taken and techniques were standardized in our lab.
Lipid peroxidation products were measured as thiobarbituric acid reactive substances (TBARS) that gives pink chromophore measurable at 530 nm [17]. Briefly, to 500 μl blank, standard and serum, 500 μl of 40% trichloroacetic acid was added and centrifuged for 10 min at 2,500 rpm. To the 500 μl supernatant, 500 μl of 0.67% thiobarbituric acid was added and incubated at 95°C for 1 h. The reaction assay was then cooled and read at 530 nm, and results (in nmol/ml) were calibrated using the standard graph. Superoxide dismutase was assessed by the method given by Marklund and Marklund [18] with slight modifications. A 50-μL sample was incubated with 1,400 μl TRIS buffer (50 mM, with 1 mM EDTA, pH 8.5). To this was added 50 μl pyragallol (20 mM, ΔOD/minute = 0.03–0.035). The change in absorbance (ΔOD) was noted from 1 min and 30 s to 3 min and 30 s at 420 nm. The standard enzyme (Sigma, India) was used to calibrate and calculate the results in U/l. Plasma vitamin C was measured by the method described by Okamura [19]. For this, 500 μl plasma was mixed with freshly prepared metaphosphoric acid (6 g/dl). After centrifugation, clear supernatant (1.2 ml) was separated and 400 μl reagent mix was added 2 g/dl of 2,4-DNPH in 4.5 mM/l sulphuric acid (100 ml) + 5 g/dl thiourea (5 ml) + 0.6 g/dl copper sulphate (5 ml). The tubes were incubated at 60°C for 1 h. These were then cooled and 2 ml of 12 mM/l sulphuric acid was added. The tubes were then cooled and absorbance was read at 520 nm. The standard ascorbic acid from Sigma, India was used (solution in metaphosphoric acid) for preparation of standard graph. The results were calibrated using standard vitamin C (Sigma). Total antioxidant capacity was measured by the method of Benzie and Strain [20]. For this, FRAP reagent was prepared freshly by mixing 10 volumes of acetate buffer (300 mM/l, pH 3.6), 1 volume of tripyridyl tetrazine (TPTZ; 10 mM/l in 40 mM/l HCl), and 1 volume ferric chloride hexahydrate (20 mM/l). Ferrous sulphate heptahydrate was taken as standard. One milliliter FRAP reagent was mixed with 33 μl of sample and change in absorbance was noted at 37°C for 30 s and 3 min and 30 s at 593 nm. The results were calibrated against the standard graph obtained by ferrous sulfate. This method shows total antioxidant capacity of the body that may not be reflected while estimating individual antioxidants and thus is a better indicator of the same because antioxidants always work in conjunction and are supplementary to each other.
Statistical analysis
The categorical parameters are expressed as proportion, and continuous parameters were expressed as median (range). Mann–Whitney U-test was applied for comparing the continuous variables between the two groups. Spearman rank correlation was used to assess the correlation between variables. Two sided P value of <0.05 was considered as significant. All data were analyzed using SAS version 8.0 statistical package.
Results
There were 44 patients with ALD, 32 patients with NAFLD, and 25 healthy controls. The baseline characteristics of the patients with ALD and NAFLD are given in Table 1. The patients with NAFLD had significantly higher BMI as compared to both the patients with ALD and healthy controls. All the controls were lean with a median BMI of 22.8 (20.4–24.3) kg/m2 and had normal liver function profile. Among patients with ALD, the median consumption of alcohol was 72 (28.57–428.5) g/day over a median duration of 10 (2–30) years and all were abstaining for a median duration of 6 (1–120) months. Of the 45 patients with ALD, 24 were also smokers, who were smoking 10 (2–100) bidis/cigarettes per day for the last 11 (1–35) years. All these patients had stopped smoking for 7 (1–36) months at the time of study. Patients with NAFLD and healthy controls were all nonsmokers and nonalcoholics.
Table 1.
Baseline characteristics of patients with ALD and NAFLD
| Variable | ALD | NAFLD | P value* |
|---|---|---|---|
| Age | |||
| N | 44 | 32 | 0.001 |
| Median | 40 | 33 | |
| Range | 26–73 | 25–50 | |
| Gender (m/f) | 44 | 29/3 | |
| Serum bilirubin (mg/dl) | |||
| N | 44 | 32 | 0.001 |
| Median | 3.85 | 0.77 | |
| Range | 0.5–15.4 | 0.6–2.6 | |
| AST (IU/L) | |||
| N | 39 | 29 | NS |
| Median | 64 | 58 | |
| Range | 25–230 | 37–128 | |
| ALT (IU/L) | |||
| N | 37 | 29 | 0.001 |
| Median | 48 | 100 | |
| Range | 16–300 | 56–219 | |
| Alkaline phosphatase (IU/L) | |||
| N | 39 | 28 | 0.011 |
| Median | 229 | 148 | |
| Range | 104–566 | 65–372 | |
| Albumin (g/dl) | |||
| N | 38 | 24 | 0.001 |
| Median | 3.05 | 4.75 | |
| Range | 2.1–4.7 | 3.8–5.4 | |
* Compared using Mann–Whitney test, data given in median (range)
Markers of oxidative stress
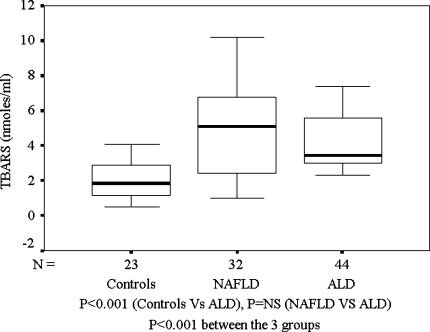
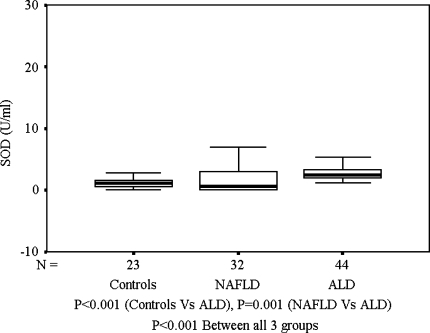
As compared with healthy controls, TBARS were significantly higher among ALD patients and in patients with NAFLD (Fig. 1). However, both ALD and NAFLD groups had similarly high levels of TBARS even after correcting for age, which was significantly higher in the ALD group. SOD levels were significantly higher among ALD patients as compared to patients with NAFLD or healthy controls (Fig. 2). Among patients with ALD, the TBARS levels correlated positively with SOD (P = 0.0002) and duration of alcohol intake (P = 0.001). On the other hand, the levels of TBARS correlated negatively with duration of abstinence from alcohol (P = 0.0001) and smoking (P = 0.017). Amongst patients with NAFLD, bilirubin correlated positively with TBARS (Spearman’s ρ = 0.421, P = 0.016). However, aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) showed no correlation with any of the markers of redox status. Among patients with ALD, TBARS showed a positive correlation with AST (Spearman’s ρ = 0.340, P = 0.034).
Fig. 1.
TBARS (nmol/ml): Controls, NAFLD, and ALD
Fig. 2.
SOD(U/ml): Controls, NAFLD, and ALD
Markers of antioxidant capacity/status
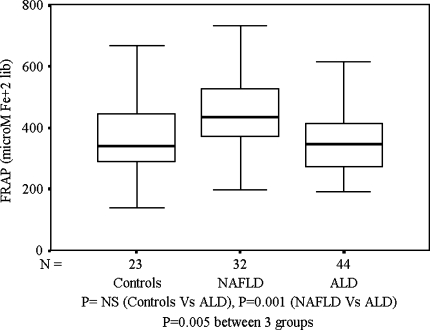
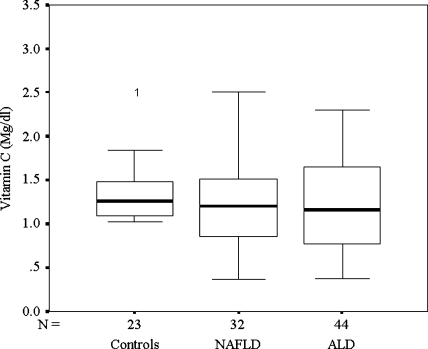
TAC was not significantly different between patients with ALD and healthy controls. It was however, significantly higher among patients with NAFLD when compared to patients with ALD or controls (Fig. 3). Plasma vitamin C levels were similar in all the three groups (Fig. 4). FRAP showed a negative correlation with TBARS (Spearman’s ρ = −0.425; P = 0.01) and SOD (Spearman’s ρ = −0.374; P = 0.03) in patients with NAFLD only.
Fig. 3.
FRAP (μmol Fe+2 liberated): Controls, NAFLD, and ALD
Fig. 4.
Vitamin C (mg/dl): Controls, NAFLD, and ALD
Discussion
Although, OS has been implicated in the pathogenesis of ALD for the last many years, only recently it has been shown to have a role in pathogenesis of NAFLD. The OS in ALD may result from ethanol-mediated free radical damage. This may occur not only due to alcohol-related malnutrition, but also secondary to ethanol’s hepatotoxicity via alcohol dehydrogenase and CYP450 2E1 pathways and the resulting production of toxic acetaldehyde [8, 21, 22]. Reactive oxygen species and LPOs in turn increase the generation of several cytokines having a key role in cell death, inflammation, and fibrosis [23]. Although, diabetes and obesity were initially held responsible for the pathogenesis of NAFLD, the basic underlying mechanism seems to be insulin resistance (IR) [24]. IR is probably the first hit in NAFLD and OS may be the elusive second of the multiple hits responsible for progression of this disease. In both ALD and NAFLD, there is excess fat deposited in the liver in the form of triglycerides. This provides the substrate for microsomal fatty acid oxidation. CYP2E1 and CYP4A are the microsomal oxidases involved in fatty acid oxidation. Both enzymes can reduce molecular oxygen to produce prooxidant species, which if not eliminated efficiently by antioxidants, can result in OS [25].
In the present study, LPO were significantly elevated among patients with ALD and NAFLD as compared with controls. This indicates that the free radical-mediated injury causing cell damage is common for both diseases, similar to the report by Kojima et al. [26]. This may be due to almost equivalent induction of microsomal oxidases either by ethanol or fatty acids and hence their similar potential of producing free radical-mediated damage.
In patients with ALD, it was found that LPO correlated positively with duration of alcohol consumption and negatively with duration of alcohol abstinence, which suggests that in ALD alcohol remains the major factor inducing OS. This has been supported by studies, demonstrating that chronic alcohol consumption independent of the presence of liver disease is associated with increased LPO [27]. Similarly, it was observed that quitting smoking decreased LPO, as indicated by the negative correlation between TBARS and duration of abstinence from smoking. The TBARS were still higher despite quitting both alcohol and smoking, indicating that the source of LPO now is the ongoing injury in the liver. We also found increased levels of TBARS among patients with NAFLD. High OS has been described previously to be associated with obesity [28, 29]. Also, there is accumulating evidence that mitochondrial dysfunction (more particularly respiratory chain defects) plays an important role in the pathophysiology of advanced forms of NAFLD, whatever its initial cause [29, 30]. Mitochondrial beta-oxidation of fatty acids can be increased, such as in insulin resistance-associated NAFLD, or decreased, as in drug-induced NAFLD [31, 32]. In either situation, generation of reactive oxygen radicals is augmented, which in a lipid-rich environment leads to lipid peroxidation and subsequent cascade of inflammation and necrosis [33, 34].
SOD, another marker of OS, was found to be significantly raised among patients with ALD as compared to patients with NAFLD or healthy controls. It is possible that chronic alcohol consumption may lead to an overexpression of SOD enzyme as a compensatory defense mechanism, something that has also been observed in chronic pancreatitis patients and several other free radical-mediated diseases [35]. However, SOD levels were not raised among NAFLD patients. Another study has shown that Mn-SOD levels were higher in alcohol-mediated liver injury as compared to nonalcoholic liver injury, suggesting the potential of alcohol to generate a higher level of OS [36]. Thus, higher SOD levels among ALD patients may represent direct induction of SOD by alcohol [37]. Earlier studies on SOD levels among NAFLD patients have reported conflicting results [38, 39].
Among markers of antioxidant status, FRAP was similar in patients with ALD and controls. This is surprising, since the antioxidant capacity is expected to be reduced in alcoholics. But this can be explained with the fact that the bilirubin levels were high in patients with ALD, and bilirubin contributes toward FRAP activity [40]. Increased reducing power has been shown in patients with ALD earlier [41]. We observed that FRAP was significantly raised in NAFLD group. This can be explained on several accounts. It has been suggested that 60% of FRAP activity is contributed by uric acid and hyperuricemia as part of the metabolic syndrome [2, 42–44] may be responsible for higher FRAP in the NAFLD group. The increase in FRAP levels may occur as a compensatory phenomenon in NAFLD patients to protect against OS. Similar increase in antioxidant capacity is also seen in other conditions associated with excess free radical-mediated injury [45]. But in the NAFLD group, FRAP correlated negatively with TBARS, suggesting that it is ineffective in protecting against OS. An increased dietary intake of fruits and vegetables has been associated with increased FRAP [46]. In our socioeconomic milieu, people suffering from any illness are given lot of fresh fruits and fruit juices irrespective of the illness or medical advice. Despite an increased reducing power of the plasma, the TBARS levels were still higher in patients with ALD as well as NAFLD. Hence, it appears that an increased FRAP in these cases may not be able to completely protect against the injury.
One of the major drawbacks of the present study is the absence of histological data among ALD patients. Consent for an invasive procedure solely for the purpose of study was difficult to receive from the patients, especially when the diagnosis was apparent based on clinical evidence and noninvasive testing. Furthermore, in our earlier report among NAFLD patients, we did not find any correlation between histology and markers of OS (21 of the NAFLD patients in the present study had a liver biopsy, the findings of which have been described previously) [13], so histological examination was not planned in the present study.
Even though the baseline serum bilirubin levels were raised among patients with ALD as compared with NAFLD, no correlation was found between serum bilirubin levels and TBARS among ALD patients. AST correlated positively with TBARS in patients with ALD while bilirubin was correlated positively with TBARS in patients with NAFLD, suggesting that at least one marker for liver injury increased with increasing OS. This further suggested that OS plays an important role in the pathophysiology of both there diseases.
In conclusion, patients with ALD have higher OS as demonstrated by high lipid peroxidation in presence of low antioxidant defenses. At the same time, even NAFLD patients have high lipid peroxidation, but they have higher antioxidant levels, which seem to be partly beneficial. Further work is needed with molecular markers of OS that may probably more reliably indicate the true OS at the cellular level.
Contributor Information
Payal Bhardwaj, Email: payalyad@gmail.com.
Kaushal Madan, Email: kaushalmona@gmail.com.
Sandeep Thareja, Email: sandeepthareja@gmail.com.
Yogendra Kumar Joshi, Email: ykj2511@usa.net.
Anoop Saraya, Email: ansaraya@yahoo.com.
References
- 1.Sugimoto M, Sadamoto T, Nonaka H. Clinical and pathological differences between alcoholic hepatitis and non-alcoholic steatohepatitis. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2003;38(1):34–45. [PubMed]
- 2.Nakano M, Fukusato T. Histological study on comparison between NASH and ALD. Hepatol Res. 2005;33(2):110–5. [DOI] [PubMed]
- 3.Nakano M. Histological study on the resemblance and difference between non-alcoholic steatohepatitis (NASH) and alcoholic liver diseases (ALD). Alcohol Clin Exp Res. 2005;29(Suppl 12):230S–5S. [DOI] [PubMed]
- 4.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1(5):358–64. [PubMed]
- 5.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–90. [DOI] [PubMed]
- 6.Masalkar PD, Abhang SA. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta. 2005;355(1–2):61–5. [DOI] [PubMed]
- 7.Hirnwich HE, Nahum LH, Pakieten N, Fazekas JF, Bots DU. Effects of alcohol on metabolism. Am J Physiol. 1982;101:57–68.
- 8.Morimotto M, Hagbjork AI, Nariji AA. Role of cytochrome P450 2E1 in alcoholic liver disease pathogenesis. Free Rad Biol Med. 1992;10:459–64.
- 9.Seki S, Kitada T, Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res. 2005;33(2):132–4. [DOI] [PubMed]
- 10.Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed]
- 11.Perlemuter G, Davit-Spraul A, Cosson C, Conti M, Bigorgne A, Paradis V, et al. Increase in liver antioxidant enzyme activity in non-alcoholic fatty liver disease. Liver Int. 2005;25(5):946–53. [DOI] [PubMed]
- 12.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Non-alcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. [DOI] [PubMed]
- 13.Madan K, Bhardwaj P, Thareja S, Dattagupta S, Saraya A. Oxidant stress and antioxidant status among patients with non-alcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. 2006;40(10):930–5. [DOI] [PubMed]
- 14.Harrison SA, Torgerson S, Hayashi P, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with non alcoholic steatohepaitis. Am J Gastroenterol. 2003;98:2485–90. [DOI] [PubMed]
- 15.Yang SQ, Zhu H, Li Y, et al. Mitochondrial adaptations to obesity related oxidant stress. Arch Biochem Biophys. 2000;378:259–68. [DOI] [PubMed]
- 16.Letteron P, Fromenty B, Terris B, et al. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–8. [DOI] [PubMed]
- 17.Belch JJF, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Brit Heart J. 1991;65:215–8. [DOI] [PMC free article] [PubMed]
- 18.Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyragallol and a convenient assay for SOD. Eur J Biochem. 1974;47:469–74. [DOI] [PubMed]
- 19.Okamura M. An improved method for determination of l-ascorbic acid and l-dehydroascorbic acid in blood plasma. Clin Chem Acta. 1980;103:259–68. [DOI] [PubMed]
- 20.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239:70–6. [DOI] [PubMed]
- 21.Lieber CS. Metabolism and metabolic effects of alcohol. Med Clin North Am. 1984;68(1):3–31. [DOI] [PubMed]
- 22.Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol induced cellular injury. Free Rad Biol Med. 1992;12:219–40. [DOI] [PubMed]
- 23.Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29(Suppl 11):110S–15S. [DOI] [PubMed]
- 24.Chitturi S, George J. Interaction of iron, insulin resistance and non-alcoholic steatohepatitis. Curr Gastroenterol Rep. 2003;5(1):18–25. [DOI] [PubMed]
- 25.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1135–9. [DOI] [PubMed]
- 26.Kojima H, Sakurai S, Uemura M, Takekawa T, Morimoto H, et al. Difference and similarity between non-alcoholic steatohepatitis and alcoholic liver diseases. Alcohol Clin Exp Res. 2005;29(Suppl 12):259S–63S. [DOI] [PubMed]
- 27.Suematsu T, Matsumura T, Sato N, Miyamoto T, Ooka T, Kamada T, et al. Lipid peroxidation in alcoholic liver disease in humans. Alcohol Clin Exp Res. 1981;5(3):427–30. [DOI] [PubMed]
- 28.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond). 2006;30(3):400–18. [DOI] [PubMed]
- 29.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90(12):6454–9. [DOI] [PubMed]
- 30.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42(6):928–40. [DOI] [PubMed]
- 31.Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Pract Res Clin Endocrinol Metab. 2005;19(4):625–35. [DOI] [PubMed]
- 32.Letteron P, Sutton A, Mansouri A, Fromenty B, Pessayre D. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38(1):133–40. [DOI] [PubMed]
- 33.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1–28. [DOI] [PubMed]
- 34.Moriel P, Plavnik FL, Zanella MT, Bertolami MC, Abdalla DS. Lipid peroxidation and antioxidants in hyperlipidemia and hypertension. Biol Res. 2000;33(2):105–12. [DOI] [PubMed]
- 35.Szuster-Ciesielska A, Daniluk J, Kandefer-Szerszen M. Alcohol-related cirrhosis with pancreatitis. The role of oxidative stress in the progression of the disease. Arch Immunol Ther Exp (Warsz). 2001;49(2):139–46. [PubMed]
- 36.Kubota S, Sato N, Matsumura T, Kamada T. Chemiluminescence and superoxide dismutase in the plasma in patients with alcoholic and non-alcoholic liver injuries. Alchol. 1985;2(3):469–72. [DOI] [PubMed]
- 37.Koch OR, De Leo ME, Borrello S, Palombini G, Galeotti T. Ethanol treatment up-regulates the expression of mitochondrial manganese superoxide dismutase in rat liver. Biochem Biophys Res Commun. 1994;201(3):1356–65. [DOI] [PubMed]
- 38.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci. 2004;106:261–268. [DOI] [PubMed]
- 39.Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed]
- 40.MacLean PD, Drake EC, Ross L, Barclay C. Bilirubin as an antioxidant in micelles and lipid bilayers: its contribution to the total antioxidant capacity of human blood plasma. Free Radic Biol Med. 2007;43(4):600–9. [DOI] [PubMed]
- 41.Hagymási K, Blázovics A, Lengyel G, Kocsis I, Fehér J. Oxidative damage in alcoholic liver disease. Eur J Gastroenterol Hepatol. 2001;13(1):49–53. [DOI] [PubMed]
- 42.Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett. 1999;446:305–8. [DOI] [PubMed]
- 43.Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: implications for definitions of overweight and obesity for Asians. Am J Clin Nutr. 2004;79:31–39. [DOI] [PubMed]
- 44.Ogura T, Matsuura K, Matsumoto Y, Mimura Y, Kishida M, Otsuka F, et al. Recent trends of hyperuricemia and obesity in Japanese male adolescents, 1991 through 2002. Metabolism. 2004;53:448–53. [DOI] [PubMed]
- 45.Notas G, Miliaraki N, Kampa M, Dimoulios F, Matrella E, Hatzidakis A, et al. Patients with primary biliary cirrhosis have increased serum total antioxidant capacity measured with the crocin bleaching assay. World J Gastroenterol. 2005;11(27):4194–8. [DOI] [PMC free article] [PubMed]
- 46.Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68(5):1081–7. [DOI] [PubMed]