Abstract
Objectives
We retrospectively compared the antiviral effect of tenofovir disoproxil fumarate (TDF) with that of adefovir dipivoxil (ADV) for patients with chronic hepatitis B (CHB) who developed resistance to lamivudine (LAM).
Materials and methods
One hundred nine patients (86 males), all Asian-American except 1 Caucasian male, with LAM resistance received TDF or ADV. HBV DNA levels were measured every 3 months. The HBeAg loss and ALT normalization were assessed at 12 months on therapy.
Results
Forty-four patients (37 males) received TDF (12 with LAM) and 65 (49 males) received ADV (18 with LAM). Median ages (years) for TDF and ADV were 49 (32–68) and 45 (22–68), respectively. Median duration of therapy was 13 months (6–38) and 17 months (6–34) for the TDF and ADV groups. Baseline HBV DNA levels (log10 copies/ml) were 6.2 ± 1.7 for the TDF and 6.5 ± 1.6 for ADV groups. Baseline ALT (IU/l) levels were 77.0 ± 86.0 and 100 ± 195 for the TDF and ADV (P = 0.46) groups, respectively. At 12 months, mean levels of log10 HBV DNA were 1.5 ± 1.0 and 4.3 ± 2.2 for TDF and ADV (P = 0.01). HBeAg loss and ALT normalization at 12 months showed no differences. Using a single factor, ANOVA (2-tailed P value), 4 groups, TDF (n = 32), TDF + LAM (12), ADV (47), and ADV + LAM (18), were compared. HBV DNA reduction at 12 months was the greatest for TDF + LAM (P < 0.001).
Conclusions
Our results suggest that for LAM-resistant HBV, TDF, alone or combined with LAM exerts greater viral reduction than ADV. However, no difference in HBeAg loss was observed. It appears that stronger HBV DNA reduction may not necessarily accelerate HBeAg loss.
Keywords: HBV, Adefovir, Tenofovir, Lamivudine, Viral resistance
Introduction
Lamivudine (LAM) was the first oral antiviral drug effective against hepatitis B virus. The drug became available in 1998 and has been used alone until the second anti-HBV drug, adefovir dipivoxil (ADV), was approved in 2002. We have witnessed significant improvement of liver diseases worldwide among patients with chronic hepatitis B with LAM therapy [1]. However, high incidence of virologic breakthrough (VBT) that results from viral resistance to LAM has been a major disadvantage of LAM treatment. Resistance to LAM was attributed to substitution of methionine in the tyrosine-methionine-aspartate-aspartate (YMDD) motif in the HBV polymerase with valine or isoleucine rtM204V/I [2, 3]. LAM resistance was observed in 15–30% after 1 year and reached 70% after 5 years of LAM treatment [4], although lower incidence of LAM resistance was observed in recent studies [5–8]. ADV, which was FDA-approved in 2002, is effective for both wild-type and YMDD mutant HBV and has been a standard rescue treatment for patients with LAM-resistant HBV [9, 10]. Nonetheless, ADV has a few drawbacks including nephrotoxicity for those who are at risk for renal dysfunction [11, 12], less potency toward HBV DNA suppression compared with that of tenofovir disoproxil fumarate (TDF) [13], and recent observations of the emergence of resistance to ADV [14, 15]. TDF was known for its effectiveness against LAM-resistant HBV among patients coinfected with HIV and HBV before ADV was approved in 2002 [16]. The primary aim of this study was to compare the therapeutic efficacy between TDF and ADV against LAM-resistant HBV in chronic hepatitis B patients. The secondary aim was to examine if combining TDF or ADV with LAM would enhance therapeutic efficacy.
Patients and methods
Patients
A total of 109 patients (86 males, 23 females, 108 Asian-Americans, and 1 Caucasian male) were included in this study. Patients coinfected with hepatitis C virus were excluded. Charts were reviewed for patients with CHB and LAM resistance who visited Liver Disease Prevention Center (LDPC), Division of Gastroenterology and Hepatology of Thomas Jefferson University Hospital, Philadelphia, during the period from August 2001 through March 2005. They were under the care of one physician (H.W.H.). In this retrospective analysis, the criteria of LAM resistance and the decision to treat with TDF or ADV were based on virologic breakthrough (VBT) (≥1 log10 copies/ml increase in HBV DNA above nadir after initially achieving virologic response) [1]. All patients received LAM as the first-line drug at a dose of 150 mg or 100 mg daily for longer than 9 months (mean 40 months). The patients who developed VBT (often accompanied by an increase in ALT) on LAM were subsequently treated with TDF or ADV. Time for TDF treatment was dated prior to that of ADV to rescue patients who developed LAM resistance before FDA approval of ADV. Thereafter, ADV was used for the rescue of LAM resistance. In addition, following the earlier reports (personal communication with several hepatologists at one of the HBV National Advisory Board meetings in the US in 2003) that demonstrated better results with add-on than switching to ADV, some patients had either ADV or TDF added-on LAM treatment.
The patients were routinely seen every 3–4 months at the clinic. Evaluation of the responses was made after the patients were treated with TDF or ADV for 6 months or longer. Liver panel, HBV markers, and quantitative HBV DNA levels were measured. To document the genotypic mutation during the time of VBT, search was made for stored serum samples. Fourteen samples were available and they were tested for HBV polymerase genotyping.
Methods
Serum HBV DNA was measured before and during the treatment. Hybridization assay was used in early period from 2000 to 2002 (Digene Hybrid Capture II test, Digene Corp, MD) [17, 18]. The lower limit of detection (LLOD) of the hybridization assay that we used in the early period was 1500 copies/ml. The baseline HBV DNA was measured by this method in 8 patients (18%) of TDF group (n = 44) and 24 patients (37%) in ADV group (n = 65). The follow-up HBV DNA was measured by PCR assay, since these 32 patients started to take either TDF or ADV from the second half of 2002.
From 2003, PCR assay (Roche PCR-Amplicor) was introduced to Thomas Jefferson University Hospital (Quest Diagnostics, Horsham, PA), with an LLOD of 500 copies/ml. The values below this cutoff were assigned a value of 1 log instead of 2.7 logs. Serial dilutions were performed for samples exceeding 5.3 log10 copies/ml. For assays done outside of Jefferson, a conversion formula was used to assess HBV DNA copies/ml. We converted pg/ml to corresponding HBV DNA copies/ml, using a conversion factor of 280,000 copies/ml per 1 pg/ml HBV DNA. Nonetheless, for patients who were referred from outside for consultation for possible LAM resistance, their HBV DNA levels were measured again at Jefferson to confirm the resistance and were used as the baseline before starting TDF or ADV.
HBV polymerase genotypes were investigated using a YMDD PCR-RFLP assay and sequencing of the HBV polymerase gene. The PCR-RFLP assay examined the presence of mutations at 2 sites (rtL180 and rtM204). The sequencing assay assessed mutations at codons rt180, rt181, rt204, and rt236. Analytes of the sequencing assay included genotype and PMUL and PMUA, polymerase mutants for LAM and for ADV, respectively.
Statistical analyses
Statistical testing was performed using SPSS version 13.0 (SPSS Inc, Chicago, IL) and Prism ver. 5, GraphPad Software (San Diego, CA). The results were reported as mean ± SD or median (range). For clarity, graphs show mean values ± SEM and the number of individuals. HBV DNA levels were logarithmically transformed for analysis. Continuous variables were compared using t-test with the Welch correction for unequal variances. For comparisons of 3 or more groups, a single factor ANOVA was used. Bonferroni’s correction for multiple comparisons was applied. The value of P was derived from a 2-tailed curve. Categorical data were compared using a 2-tailed Chi-square test or Fisher’s Exact test.
This study was approved by Institutional Review Board of Thomas Jefferson University.
Results
Baseline characteristics of patients
Detailed information of baseline characteristics is shown in Table 1. There were no significant differences in baseline characteristics between the TDF and ADV groups with regard to gender distribution, age, HBeAg, baseline ALT, platelets, or mean HBV DNA levels. Mean duration of LAM treatment was similar; 42 ± 25 months and 38 ± 22 months for TNF or ADV, respectively. At the start of treatment with TDF or ADV, all patients had HBV DNA levels >3 log10 copies/ml and 83 (76%) of those had HBV DNA >5 log10 copies/ml. HBV DNA (log10 copies/ml) at baseline for TDF group was 6.2 ± 1.7 and 6.5 ± 1.6 for ADV group. Forty-four patients received TDF; 12 (27%) of them received combined LAM. Sixty-five patients received ADV; 18 (27%) of them also received LAM (see Table 3).
Table 1.
Baseline characteristics of patients
| All patients (n = 109) |
TDF (n = 44) |
ADV (n = 65) |
P value* | |
|---|---|---|---|---|
| Mean age, years (SD)† | 46 (11) | 49 (11) | 45 (12) | 0.08 |
| % Male | 79 | 85 | 76 | 0.27 |
| % Asian | 99 | 97 | 100 | 0.22 |
| % HBeAg-positive | 78 | 75 | 84 | 0.19 |
| Mean ALT, IU/l (SD) | 91 (161) | 77 (86) | 100 (195) | 0.46 |
| Mean HBV DNA, log10 copies/ml (SD) | 6.4 (1.6) | 6.2 (1.7) | 6.5 (1.6) | 0.41 |
| Mean platelet count × 103/ml (SD) | 185 (68) | 186 (67) | 183 (70) | 0.84 |
| Mean duration of LAM therapy in months (SD) prior to LAM resistance | 40 (23) | 42 (25) | 38 (22) | 0.35 |
Note: Data are given as mean (SD)
* Student’s t-test (NS) = P < 0.05
† Difference in age between the tenofovir group and the adefovir group was not significant (P = 0.08)
Abbreviations: ADV, adefovir dipovoxil; ALT, alanine aminotransferase; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus; LAM, lamivudine; TDF, tenofovir disoproxil fumarate
Table 3.
The comparison of therapeutic effect among four treatment groups
| TDF (n = 32) |
TDF LAM (n = 12) |
ADV (n = 47) |
ADV + LAM (n = 18) |
|
|---|---|---|---|---|
| Mean HBV DNA log reduction (at 6 mos) | 3.4 ± 1.6 | 4.4 ± 2.1 | 2.2 ± 2.2 | 1.5 ± 1.5 |
| Mean HBV DNA log reduction (at 12 mos) | 4.7 ± 1.5 | 5.3 ± 1.8 | 2.4 ± 2.5 | 2.2 ± 1.6 |
| No. (%) patients with HBeAg loss (at 12 mos) | 2 (6) | 0 (0) | 4 (4) | 0 (0) |
| No. (%) patients with ALT normalization (at 12 mos) | 5 (55) | 5 (63) | 26 (65) | 8 (89) |
HBV DNA log reduction 6 mos; 12 mos
TDF + LAM vs. ADV + LAM: P < 0.001; P < 0.001
TDF alone vs. ADV + LAM: P < 0.01; P < 0.001
TDF + LAM vs. ADV alone: P < 0.01; P < 0.001
TDF alone vs. ADV alone: P < 0.01; P < 0.001
No. of follow-up patients at 12 mos: TDF (n = 9), TDF + LAM (n = 8), ADF (n = 40), ADV + LAM (n = 9)
Abbreviations: ADV, adefovir dipovoxil; ALT, alanine aminotransferase; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus; LAM, lamivudine; TDF, tenofovir disoproxil fumarate; mos, months; No., number
Virologic and biochemical responses
The results are shown in Table 2. At 6 months on therapy, HBV DNA levels (log10 copies/ml) were significantly reduced for the TDF group (2.7 ± 1.6) than for the ADV group (4.7 ± 2.1) (P = 0.01). At 6 months on therapy, 50% of TDF group had <3 log10 HBV DNA while 20% had <3 log HBV DNA in ADV group (P = 0.01).
Table 2.
The comparison of therapeutic effects between two groups
| TDF (n = 44) |
ADV (n = 65) |
P value | |
|---|---|---|---|
| Treatment duration (mos) | 14 ± 9 | 19 ± 8 | |
| Mean level of log10 HBV DNA (at baseline) | 6.2 ± 1.7 (n = 44) |
6.5 ± 1.6 (n = 65) |
0.41 |
| Mean level of log10 HBV DNA (at 3 mos) | 3.4 ± 1.9 (n = 35) |
5.3 ± 2.0 (n = 45) |
0.01 |
| Mean level of log10 HBV DNA (at 6 mos) | 2.7 ± 1.6 (n = 30) |
4.7 ± 2.1 (n = 45) |
0.01 |
| Mean level of log10 HBV DNA (at 9 mos) | 1.5 ± 1.1 (n = 22) |
4.5 ± 2.0 (n = 35) |
0.01 |
| Mean level of log10 HBV DNA (at 12 mos) | 1.5 ± 1.0 (n = 15) |
4.3 ± 2.2 (n = 42) |
0.01 |
| Mean level of log10 HBV DNA (at 15 mos) | 1.5 ± 1.1 (n = 13) |
4.5 ± 2.0 (n = 27) |
0.01 |
| No. (%) patients with <3 log10 DNA (at 6 mos) | 50 | 20 | 0.01 |
| No. (%) patients with <3 log10 DNA (at 12 mos) | 87 | 21 | 0.01 |
| No. (%) patients with HBeAg loss (at 12 mos) | 9 | 5 | 0.6 |
| No. (%) patients with ALT normalization (at 12 mos) | 59 | 69 | 0.43 |
Abbreviations: ADV, adefovir dipovoxil; ALT, alanine aminotransferase; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus; TDF, tenofovir disoproxil fumarate; mos, months; No., number
At 12 months on therapy, mean HBV DNA levels (log10 copies/ml) were reduced to 1.5 ± 1.0 for the TDF group and 4.3 ± 2.2 for the ADV group and the difference was significant (P = 0.01).
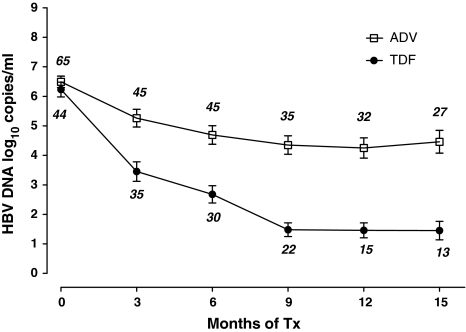
As shown in Fig. 1, TDF group showed a mean log HBV DNA reduction of 2.78, 3.55, 4.75, and 4.77, respectively, at months 3, 6, 9, and 12 compared with 1.23, 1.80, 2.14, and 2.24 at each time point in ADV group (P < 0.01). The log HBV DNA levels between the 2 groups were all significantly different at each time point.
Fig. 1.
Comparison of the level of HBV DNA in patients undergoing therapy with either adefovir (ADV) or tenofovir (TDF). HBV DNA reduction was significantly (P < 0.001) greater than in TDF group at all periods except at baseline. Baseline showed no difference (P = 0.4133). Within each treatment group, at months 3, 6, 9, and 12, TDF group showed a mean reduction (copies/ml) of HBV DNA of 2.78, 3.55, 4.75, and 4.77, respectively, compared with 1.23, 1.80, 2.14, and 2.24 at corresponding months in ADV group (P = 0.01). The month 15 levels were the same for TDF group and rose slightly (0.25 copies/ml) for the ADV group. Data are presented as mean values; the bars depict standard errors. The number above or below error bar represents the number of patients treated for that interval
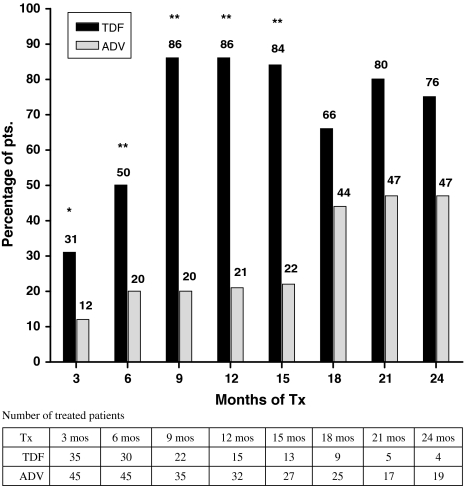
Figure 2 shows the percent of patients with HBV DNA reduction (<3 log10 copies/ml) observed at three monthly intervals between the TDF and ADV groups. TDF group showed greater percent reduction than ADV group at each time point from month 3 to month 15.
Fig. 2.
Percentage of TDF- and ADV-treated patients whose HBV DNA declined to a level of ≤1,000 copies/ml at the specified time points. Statistically significant differences (P < 0.01) between TDF and ADV are marked. No significant difference was noted at months 18, 21, or 24 (P = 0.160, 0.150, and 0.055, respectively). The number above the bars indicates the percentage. * P < 0.05, ** P < 0.01; HBV, hepatitis B virus
HBeAg loss in 12 months on therapy showed no difference between 2 groups; 9% and 5% for TDF and ADV, respectively. Also, ALT normalization at 12 months was 59% and 69% for TDF and ADV showing no significant difference (Table 2). No patient developed viral breakthrough (either to TDF or ADV) during the 6–38 months observation period.
Stored serum samples from 14 patients showed that all had HBV DNA levels greater than 105 copies/ml at the time of VBT. All were HBV genotype C and contained YMDD mutant HBV (data not shown).
Virologic and biochemical responses among four treatment groups
In some patients with VBT, LAM was continued with either TDF or ADV added. Table 3 shows the comparison of four groups. Using a single factor, 2-tailed ANOVA, the four groups, TDF (n = 32), TDF + LAM (n = 12), ADV (n = 47), and ADV + LAM (n = 18), were compared. No significant difference between the four groups was detected with respect to demographic features or baseline laboratory values. However, HBV DNA reduction at 6 months and 12 months was greater for TDF and TDF + LAM combination therapy group than either ADV group (P < 0.01). Using a single factor, ANOVA (2-tailed P value), the four groups, TDF (n = 32), TDF + LAM (n = 12), ADV (n = 47), and ADV + LAM (n = 18), were compared. HBV DNA reduction at 12 months was the greatest for TDF + LAM (Table 3). Again, no significant differences among the four treatment groups were detected in either HBeAg loss or ALT normalization.
Discussion
Our study shows that TDF is highly effective for LAM-resistant HBV as reported earlier by Kuo et al. [19] and exerts stronger anti-HBV activity than ADV. The mean reduction in the 12th month was significantly greater (5.0 ± 1.6 log10 copies/ml) in TDF group than the ADV group (2.4 ± 1.4 log10 copies/ml). When the TDF and ADV treatment groups were further divided by LAM combination, and analyzed by ANOVA, HBV DNA reduction in TDF with or without LAM combination was significantly greater than either ADV group. TDF + LAM group was superior to other groups (Table 3).
Van Bommel et al. [13] not only found the higher potency of TDF over ADV in treatment naive patients with hepatitis B, but also among patients with LAM resistance who showed incomplete response to ADV in patients [20]. Furthermore, Del Poggio et al. [21] found that low-dose TDF (75 mg) was more potent than adefovir (10 mg) in chronic HBeAg-negative hepatitis B. Although further studies are needed in a larger population and in HBeAg-positive patients, with the current understanding that HBV treatment may well be life long, the potential usage of low-dose TDF with a high potency could ease the financial burden in low-income HBV endemic regions.
HBeAg loss in 12 months was not different between TDF and ADV groups (9% vs. 5%, P = 0.6). It appears that stronger HBV DNA reduction may not necessarily accelerate HBeAg loss. ALT normalization at 24 weeks was 55% in TDF group and 65% in ADV group (P = 0.39); and at 48 weeks, the ALT normalization was increased to 60% and 69% for TDF and ADV, respectively, but without significant difference (P = 0.55). Earlier, Van Bommel et al. [13] observed significant difference in ALT normalization at 48 weeks but not at 24 weeks.
The slope of HBV DNA decline curve (Fig. 1) was steeper in TDF group than ADV group. This phenomenon was observed up to 15 months. After 15 months of treatment, this difference became less obvious due to many drop-out patients in TDF group. This rapid and persistent viral response was observed from the virus dynamic study. While treating HIV-HBV coinfected patients with TDF, Lacombe et al. [16] examined the hepatitis B virus dynamics and noted an early rapid and late slow decline, that is, the biphasic pattern of HBV clearance by TDF. This biphasic phenomenon reflected the clearance of free virions followed by the elimination of infected cells. TDF appears to have a potent and durable effect on HBV replication.
Our results suggest that for LAM-resistant HBV, TDF, alone or combined with LAM exerts greater viral reduction than ADV. However, we found no significant difference in HBeAg loss or ALT normalization. It appears that stronger HBV DNA reduction may not necessarily induce speedier loss of the HBeAg.
Abbreviations
- ADV
Adefovir dipovoxil
- ALT
Alanine aminotransferase
- CHB
Chronic hepatitis B
- DNA
Deoxyribonucleic acid
- HBeAg
Hepatitis B e-antigen
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HDV
Hepatitis D virus
- INR
International normalized ratio
- IU
International units
- LLOD
Lower limit of detection
- log
log10 copies/ml
- PCR
Polymerase chain reaction
- PT
Prothrombin time
- TDF
Tenofovir disoproxil fumarate
- VBT
Virologic breakthrough
Footnotes
The author (H.W.H.) who has taken part in this study has declared a relationship with the manufacturers of the drugs involved either in the past or present.
References
- 1.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31. [DOI] [PubMed]
- 2.Allen MI, Deslauries M, Andrew CW, Tipples GA, Walters KA, Tyrrell DL, et al. Identification and characterization of mutations in hepatitis B virus resistance to lamivudine. Hepatology. 1998;27:1670–7. [DOI] [PubMed]
- 3.Stuyver LJ, Locarnini SA, Lok A, Richmann DD, Carman WF, Dienstag JL, et al. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology. 2001;33:751–7. [DOI] [PubMed]
- 4.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology. 2000;119:172–80. [DOI] [PubMed]
- 5.Lai CL, Gane E, Liaw YF, Hsu CW, Thongssawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–88. [DOI] [PubMed]
- 6.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. [DOI] [PubMed]
- 7.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20. [DOI] [PubMed]
- 8.Chae HB, Hann HW. Baseline HBV DNA level is the most important factor associated with viral breakthrough (BT) during lamivudine (LVD) therapy for chronic hepatitis B (CHB). Gastroenterology. 2006;130:A846. [DOI] [PMC free article] [PubMed]
- 9.Perrillo RP, Hann HW, Multimer D, Williams B, Leung N, Lee WM, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. [DOI] [PubMed]
- 10.Peters M, Hann HW, Martin P, Heathocote EJ, Buggisch P, Rubin R, et al. Adefovir dipivoxil (ADV) alone and in combination with lamivudine in patients with lamivudine resistance and chronic hepatitis B. Gastroenterology. 2004;126:91–101. [DOI] [PubMed]
- 11.Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, et al. Adefovir dipivoxil therapy for lamivudine resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–27. [DOI] [PubMed]
- 12.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–16. [DOI] [PubMed]
- 13.Van Bommel F, Wunsche T, Mauss S, Reinke R, Bergk A, Schurmann D, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–5. [DOI] [PubMed]
- 14.Locarnini S, Qi X, Arterburn S, Snow A, Brosgart CL, Currie G, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB) [Abstract]. J Hepatol. 2005;42 Suppl 2:A36.
- 15.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marreo J, Oberhelman K, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–90. [DOI] [PubMed]
- 16.Lacombe K, Gozlan J, Boelle P-Y, Serfaty L, Zoulim F, Valleron AJ, et al. Longterm hepatitis B virus dynamics in HIV-hepatitis B virus coinfected patients treated with tenofovir disoproxil fumarate. AIDS. 2005;19:907–15. [DOI] [PubMed]
- 17.Ho SK, Chan TM, Cheng IK, Lai KN. Comparison of the second-generation digene hybrid-capture assay with the branched-DNA assay for measurement of hepatitis B virus DNA in serum. J Clin Microbiol. 1999;37:2461–5. [DOI] [PMC free article] [PubMed]
- 18.Yuan H-J, Yuen M-F, Wong DK-H, Sum SS-M, Lai C-L. Clinical evaluation of the Digene hybrid capture II test and the COBAS Amplicor monitor test for determination of hepatitis B virus DNA levels. J Clin Microbiol. 2004;42:3513–7. [DOI] [PMC free article] [PubMed]
- 19.Kuo A, Dienstag JL, Chung RT. Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin Gastroenterol Hepatol. 2004;2:266–72. [DOI] [PubMed]
- 20.van Bommel F, Zolliner B, Sarrazin C, Spengler U, Huppe D, Moller B, et al. Tenofovir for patients with lamivudine-resistant hepatitis B (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318–25. [DOI] [PubMed]
- 21.Del Poggio P, Zaccanelli M, Oggionni M, Colombo S, Jamoletti C, Puhalo V. Low-dose tenofovir is more potent than adefovir and is effective in controlling HBV viremia in chronic HBeAg-negative hepatitis B. World J Gastroenterol. 2007;13:4096–9. [DOI] [PMC free article] [PubMed]