Abstract
Purpose
Adeno-associated virus (AAV) vectors can achieve long-term gene expression and are now feasible for use in human gene therapy. We constructed hepatocyte growth factor (HGF) expressing AAV (AAV5-HGF) and examined its effect in two mouse hepatic fibrosis models.
Methods
A model of hepatic fibrosis was established by carbon tetrachloride (CCl4) administration in Balb/c mice. After the establishment of liver fibrosis, AAV5-HGF was injected once into the portal vein. Mice were killed 3, 6, 9, and 12 weeks after injection. Another model was established by bile duct ligation (BDL). Seven weeks after AAV5-HGF injection, mice underwent BDL, and were then killed 2 weeks after BDL.
Results
Mice that received AAV5-HGF achieved stable HGF expression both in the serum and liver for at least 12 weeks. In both models, significant improvement of the liver fibrosis was found in all mice receiving AAV5-HGF based on Azan-Mallory staining. Suppression of hepatic stellate cells (HSC) was confirmed by immunohistochemistry. Fibrogenic markers were significantly suppressed and collagenase activity increased in the livers of mice receiving AAV5-HGF.
Conclusions
A single injection of AAV vector containing HGF gene achieved long-term expression of HGF and resulted in resolution of mouse liver fibrosis. HGF gene therapy mediated by AAV is feasible for the treatment of liver fibrosis.
Keywords: HGF, Liver fibrosis, AAV, CCl4, BDL
Introduction
Liver fibrosis is induced by the wound healing response to chronic liver injury caused by hepatitis virus infections, alcohol abuse, prolonged biliary obstruction, hepatotoxic drugs, or metabolic diseases [1]. It is a major cause of morbidity and mortality worldwide, with no effective therapy except for liver transplantation. The main characteristic of liver fibrosis is the excess production and deposition of extracellular matrix (ECM) caused by activated hepatic stellate cells (HSC), portal fibroblasts, and myofibroblasts of bone marrow origin. These cells are activated by fibrogenic cytokines, like transforming growth factor (TGF)-β [2]. Liver fibrosis was considered to be an irreversible end result, but recent studies have demonstrated that liver fibrosis is reversible after clearance of hepatitis C virus (HCV) with either interferon or pegylated interferon, with or without the addition of ribavirin [3–6]. These reports demonstrate the reversibility of human liver fibrosis.
Prior to these reports, the in vivo therapeutic effect of hepatocyte growth factor (HGF) against liver fibrosis was shown. The HGF identified and cloned as a 69-kDa α-chain and a 34-kDa β-chain, initially was characterized as a potent mitogen for hepatocytes [7, 8]. HGF also shows mitogenic, motogenic, and morphogenic activities in a wide variety of cell types [9]. Several in vivo approaches have shown that HGF plays an essential role in both the development and regeneration of liver [10] and have demonstrated antiapoptotic and cytoprotective effects in hepatocytes [11]. The first report demonstrating the effect of HGF on liver fibrosis used recombinant HGF injection [12]. However, a large amount of recombinant HGF was required, because HGF is unstable in blood, with a half-life of 3–5 min [13, 14]. In order to overcome this problem, we demonstrated HGF gene therapy using hemagglutinating virus of Japan (HVJ) liposomes [15]. The HGF gene transfection into rat skeletal muscle dramatically improves liver fibrosis; however, this strategy also requires repetitive transfections to achieve persistent expression because HVJ-liposome-mediated gene expression is transient [16]. From a clinical point of view, development of a novel gene transfer strategy to achieve long-term expression of HGF protein in vivo is crucial. Therefore, we assessed the therapeutic efficacy of adeno-associated virus (AAV) vector-mediated HGF gene therapy for liver fibrosis.
AAV includes a number of small single-stranded DNA viruses and members of the parvovirus family. A number of unique properties make AAV a very promising vector for gene therapy. The advantages of the use of AAV-based vectors are that they can transduce therapeutic genes into both dividing and nondividing cells and achieve long-term gene expression with no apparent adverse effect [17, 18]. In this study, we constructed a recombinant AAV vector coding the human HGF gene (AAV-HGF) and assessed its therapeutic effects for hepatic fibrosis using two mouse models of hepatic fibrosis.
Materials and methods
Cell culture
The human HCC cell line, HepG2, was grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin.
Animals
Male Balb/c mice (6-week-old) were purchased from Japan CLEA, and were maintained in a pathogen-free facility at Hyogo College of Medicine (Nishinomiya, Hyogo, Japan). The animal experiments were performed in accordance with the guidelines of the National Institutes of Health (Bethesda, MD, U.S.A.), as specified by the animal care policy of Hyogo College of Medicine.
Plasmid AAV5-HGF construction
We first evaluated vectors derived from AAV serotypes 1, 2, and 5. LacZ gene expression recombinant AAV vectors (AAV1-LacZ, AAV2-LacZ, or AAV5-LacZ) were transduced into mouse livers using the same method described below, and efficiency of gene expression was determined by LacZ staining. Among these vectors, AAV5-LacZ had the highest LacZ expression (unpublished result). On the basis of this result, we selected the AAV serotype 5 for the present study and constructed the AAV5-HGF vector. Plasmid AAV5-HGF was constructed by inserting the full-length cDNA of human HGF [19], representing about 2.2 kb, at the Hinc II site of the AAV5-MCS that included the CMV promoter and inverted terminal repeat sequence.
AAV5-HGF vector preparation
Plasmids for AAV vector production were purchased from Stratagene (La Jolla, CA, USA). pAAV5-CMV-LacZ, a plasmid encoding LacZ, and 5RepCapA, a helper plasmid for AAV serotype 5, were generous gifts from Dr J. A. Chiorini (National Institutes of Health, Bethesda, MD, USA) [20]. pAAV5-CMV-HGF containing the HGF sequence was prepared as previously described, with the inverted terminal repeat (ITR) sequences changed to those of the AAV5 vector. Recombinant AAV vector stocks were prepared in accordance with an adenovirus-free triple-plasmid transfection protocol [21]. After harvest, vector solutions were purified twice on a cesium chloride (CsCl) gradient and quantified by DNA dot blot hybridization. The same vector stock was used in the same series of experiments to minimize the variability that could occur as a result of the potential differences in vector potency.
AAV vector transduction in vitro
In order to confirm HGF expression in vitro, HepG2 cells (2 × 105) were plated in 6-cm plastic dishes. After 24 h, cells were infected with 106 or 2 × 106 vector genomes of AAV5-HGF. Forty-eight hours after transduction, culture medium and cell lysates were harvested. AAV5-LacZ was transfected into HepG2 cells using the same procedure as that for a control vector. Protein concentrations of human HGF were determined by enzyme-linked immunosorbent assay (ELISA) using an IMMUNIS human HGF enzyme immunoassay kit (Institute of Immunology, Tokyo, Japan).
Experimental animal models
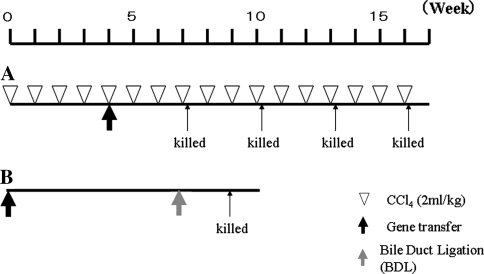
Liver fibrosis was induced by carbon tetrachloride (CCl4) [22] or bile duct ligation (BDL) [23]. In the first model, 40 BALB/c mice received CCl4 by intragastric administration of 5% CCl4 at a dose of 2 ml/kg body weight (Wako, Tokyo, Japan) dissolved in olive oil, once a week for 16 weeks. After the establishment of liver fibrosis, AAV5-HGF (n = 20) or AAV5-LacZ (n = 20), at a dose of 1011 vector genomes were transfected into the portal vein through the splenic hilum. Mice were killed at 3, 6, 9, or 12 weeks after transfection (n = 5, each point). The second model was created by BDL. Mice were transfected with AAV5-HGF (n = 5) or AAV5-CMV-LacZ (n = 5) at day 0 by the same procedure described above. Seven weeks after transfection, BDL was performed on all mice. Briefly, the common bile duct was double-ligated using 4-0 silk through a midline abdominal incision. All mice were killed 2 weeks after BDL, and liver and blood were collected for histologic and protein analyses. All mice were anesthetized with ether during AAV transduction, BDL, and at death. The time schedule is shown in Fig. 1.
Fig. 1.
Schedule of AAV5-HGF or AAV5-LacZ transduction (a: CCl4 mice model, b: BDL mice model)
Histologic examination and immunostaining
Paraffin-embedded tissues were fixed and embedded in paraffin. The sections were stained with Azan-Mallory for collagen visualization. Hepatic fibrosis was assessed in a blinded manner by image analysis, using a planimetric method and the Automatic Image Analysis System (Carl Zeiss, Oberkochem, Germany). For immunohistochemical analysis, the sections were pretreated through deparaffinization, antigen unmasking, and blocking with 1% H2O2 for 10 min and 1.5% goat normal serum for 60 min. The specimens were incubated with mouse antihuman α-smooth muscle antibody (α-SMA) (1:100 dilution; Thermo Fisher Scientific, Waltham, MA, USA) for 60 min. After washing, the sections were incubated with biotin-conjugated antimouse IgG secondary antibody (1:5000) for 60 min, then with DAB for 1 min and counterstained with hematoxylin for 10 s.
RNA isolation and cDNA synthesis
Total RNA was extracted from mice livers using ISOGEN (Nippon Gene, Tokyo, Japan). The concentration of RNA was spectrophotometrically determined, and the integrity of samples was confirmed by visualizing the 28S and 18S ribosomal RNA bands under ultraviolet light after agarose gel electrophoresis. One microgram of total RNA was reverse transcribed with random primers using a commercial cDNA kit (High-capacity cDNA Archive kit: Applied Biosystems, Foster City, CA, USA). The resulting synthesized cDNA was used for real-time polymerase chain reaction (PCR).
Real-time PCR
Serial dilutions of cDNA were made to determine the linear range for amplification. Real-time PCR was performed on the ABI PRISM 7900HT Sequence Detection System using TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression Assay (Collagen α1(I), α-SMA, TGF-β, TIMP, and MMP13; Applied Biosystems) for the PCR step. A standard curve for serial dilutions of 18S rRNA was similarly generated.
In situ zymography
At the final timepoint, mice livers were removed and embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan). They were cut into 6-μm frozen sections, and put onto gelatin-coated film (MMP in situ Zymo-Film, Wako Chemical, Osaka, Japan) and incubated at 37°C for 17 h. Gelatin left undegraded on the film was stained with Biebrich Scarlet Stain Solution (Wako Chemical).
Statistical analysis
Data are expressed as mean ± SD, and the statistical significance of differences among groups was assessed by Student’s t test. P values < 0.05 were regarded as statistically significant.
Results
HGF expression in vitro
HepG2 cells (2 × 105) were infected with AAV5-LacZ (106 vector genomes) or AAV5-HGF (106 or 2 × 106 vector genomes) for 48 h. Cell lysates and culture medium were harvested at 48 h after infection, and human HGF expression was measured by ELISA. Dose-dependent expression of human HGF was detected in HepG2 cells with AAV5-HGF vector (0.10 ± 0.05 ng/ml in medium and 1.08 ± 0.12 ng/ml in cell lysates using 2 × 106 vector genomes), but no expression was observed in HepG2 cells transfected with AAV5-LacZ vector.
Human HGF expression by AAV5-HGF transduction in CCl4-treated mice
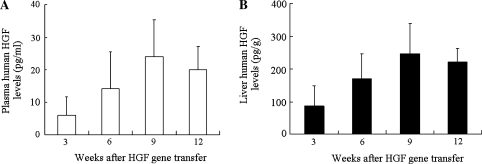
The human HGF concentrations in the plasma or livers of CCl4-treated mice were measured by ELISA up to 12 weeks after injection of AAV5-HGF vectors. Human HGF concentrations gradually increased and reached the peak values (25 ± 11 pg/ml in plasma and 250 ± 90 pg/g in the liver) 9 weeks after transduction. The level of HGF persisted for at least 12 weeks after transduction both in the serum and liver. No human HGF expression was found in mice that were transduced with AAV5-LacZ vectors (Fig. 2a and b). Endogenous HGF level was also measured by ELISA. At 12 weeks after AAV5-LacZ transduction, endogenous HGF level of AAV5-LacZ-transduced mice (n = 5) was 2.8 ± 0.29 ng/ml in plasma and 87.3 ± 13.7 ng/g in the liver. Endogenous HGF level of CCl4-treated mice without gene transduction (n = 5) was 2.3 ± 0.51 ng/ml in plasma and 79.3 ± 4.72 ng/g in the liver.
Fig. 2.
Human HGF concentrations induced by AAV5-HGF transduction in CCl4-treated mice (a: plasma, b: liver tissue). Human HGF concentrations gradually increased and reached a peak value both in the plasma and in the liver 9 weeks after transduction. Data represent the mean ± SD (n = 5)
Effects of HGF on CCl4-induced hepatic fibrosis
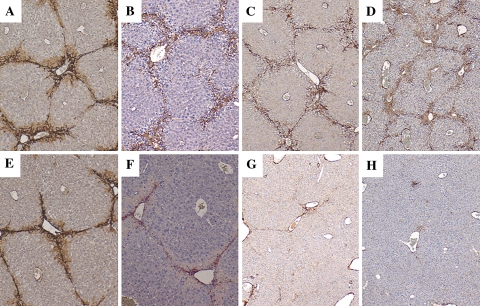
In order to assess the effect of HGF on liver fibrosis, mice received CCl4 by intragastric administration once a week for 16 weeks. Four weeks after CCl4 administration began, mice were transduced once with AAV5-HGF or AAV5-LacZ. Azan-Mallory staining demonstrated that moderate bridging fibrosis was observed in the livers of both AAV5-HGF- and AAV5-LacZ-transduced mice up to 6 weeks after transduction (Fig. 3a, b, e, f). However, AAV5-HGF markedly attenuated fibrosis at 9 or 12 weeks after transduction (Fig. 3g and h) compared with control vector (Fig. 3c and d). Quantitative analysis of the fibrosis by image analysis showed a 50% reduction in fibrosis after AAV5-HGF transduction (Fig. 3i). Immunostaining for α-SMA was performed to detect activated HSC. Expression of α-SMA in the liver was increased in AAV5-LacZ-transduced mice (Fig. 4a–d). This expression was observed up to 6 weeks after AAV5-HGF transduction (Fig. 4e and f), but was suppressed after 9 and 12 weeks of transduction (Fig. 4g and h).
Fig. 3.
a–h: Azan-Mallory staining of livers from mice treated with CCl4 after gene transduction. Upper panels, AAV5-LacZ-transduced mouse livers (a: 3 weeks, b: 6 weeks, c: 9 weeks, and d: 12 weeks after AAV-5 LacZ transduction). Lower panels, AAV5-HGF-transduced mouse livers (e: 3 weeks, f: 6 weeks, g: 9 weeks, and h: 12 weeks after AAV-5 HGF transduction). Original magnification 40×. (I): Assessment of fibrosis using image analysis techniques, calculating the ratio of connective tissue to the whole area of the liver from mice transduced with AAV5-LacZ or AAV5-HGF. Data are presented as the mean ± SD (5 fields per mouse, n = 5)
Fig. 4.
α-SMA immunostaining of livers from mice treated with CCl4 after gene transduction. Upper panels, AAV5-LacZ-transduced mouse livers (a: 3 weeks, b: 6 weeks, c: 9 weeks, and d: 12 weeks after AAV-5 LacZ transduction). Lower panels, AAV5-HGF-transduced mouse livers (e: 3 weeks, f: 6 weeks, g: 9 weeks, and h: 12 weeks after AAV-5 HGF transduction). Original magnification 40×
Effects of HGF on hepatic fibrosis induced by bile duct ligation
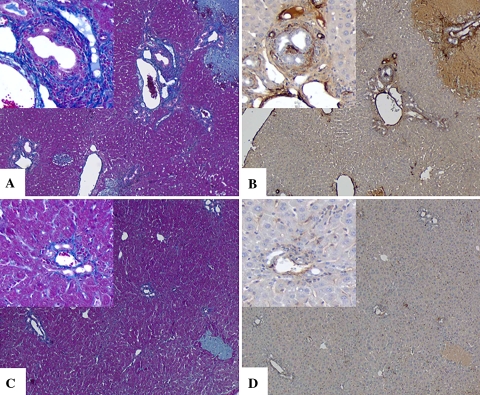
In order to assess the effect of HGF on liver fibrosis induced by BDL, mice were transduced with AAV5-HGF or AAV5-LacZ. Seven weeks after transduction, BDL was performed on all mice. Mice were killed 2 weeks after BDL to investigate the potential effect of HGF. At the time of death (9 weeks after AAV5-HGF transduction), plasma and liver human HGF concentrations were similar to those seen in the CCl4 model (16 ± 5 pg/ml in plasma and 224 ± 76 pg/g in the liver). Histologic analysis showed extensive peribiliary and interstitial collagen deposition in mice transduced with AAV5-LacZ (Fig. 5a). However, AAV5-HGF transduction largely attenuated these findings after BDL (Fig. 5c). In AAV5-LacZ-transduced mice, α-SMA-positive cells were observed in the periportal areas (Fig. 5b). The number of these cells was reduced by AAV5-HGF transduction (Fig. 5d).
Fig. 5.
Azan-Mallory staining (left panels) and α-SMA immunostaining (right panels) of BDL mouse livers after gene transduction (a, b: AAV-5 LacZ transduction; c, d: AAV-5 HGF transduction). Original magnification 40×, insets 100×
Fibrogenesis is suppressed in livers of AAV5-HGF-transduced mice
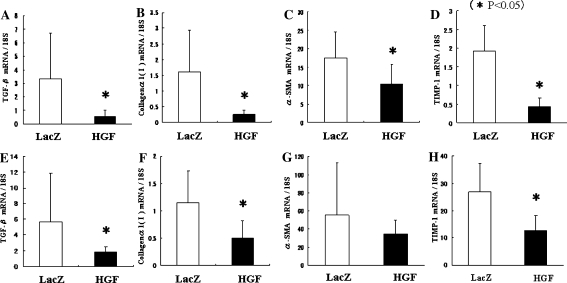
In order to investigate the role of HGF on liver fibrogenesis by CCl4 or BDL, real-time PCR was performed (Fig. 6). TGF-β is a fibrogenic cytokine that plays a central role in regulating fibrosis. Expression of TGF-β mRNA was significantly suppressed in the liver of AAV5-HGF-transduced mice treated with CCl4 (Fig. 6a) or BDL (Fig. 6e) mice. Collagen α1 (I) is the major form of collagen produced in fibrosis. The expression of Collagen α1 (I) mRNA was significantly suppressed in the liver of AAV5-HGF-transduced mice treated with CCl4 (Fig. 6b) or BDL (Fig. 6f). Expression of α-SMA mRNA was significantly suppressed in the livers of AAV5-HGF-transduced mice treated with CCl4 (Fig. 6c), although a significant difference was not seen in the livers of AAV5-HGF-transduced mice treated with BDL (Fig. 6g). Tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) inhibits collagen degradation by matrix metalloproteinase (MMP) and protects against apoptosis of HSC. The expression of TIMP-1 mRNA was significantly suppressed in the livers of AAV5-HGF-transduced mice treated with either CCl4 (Fig. 6d) or BDL (Fig. 6h). These data suggest that AAV5-HGF transduction reduces liver fibrogenesis in 2 models of hepatic fibrosis.
Fig. 6.
HSC activation was suppressed by AAV5-HGF transduction in both CCl4 mice (upper graphs) and BDL mice (lower graphs). At the final time point, total hepatic RNA was extracted. a and e: TGF-β; b and f: Collagen α1(I); c and g: α-SMA; d and h: TIMP. mRNA was quantified by real-time PCR. Data represent the mean ± SD (n = 5)
HGF transduction resolves liver fibrosis
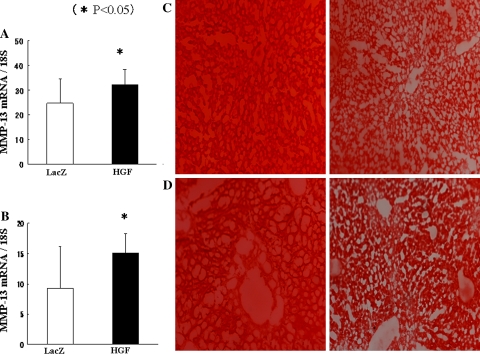
Ets-1 has been shown to modulate transcription of several MMP genes [24, 25], and recent reports have shown that HGF increases collagenase expression in hepatic stellate cells via an Ets-1 transcription factor-dependent manner [26]. The MMP-13 is the interstitial collagenase in rodents that has the capability of degrading fibrillar collagens. Real-time PCR showed an increase in MMP-13 mRNA with HGF gene transduction in both CCl4 mice (Fig. 7a) and BDL mice (Fig. 7b). In situ zymography showed extensive gelatin degradation in liver sections of HGF-transduced mice (Fig. 7c and d, right panels) compared with control mice (Fig. 7c and d, left panels). These data suggest that HGF gene transduction stimulates MMP expression followed by resolution of liver fibrosis.
Fig. 7.
HGF transduction increases resolution of liver fibrosis. Expression of MMP-13 mRNA in the livers from CCl4-treated mice (a) or BDL mice (b). Data represent the mean ± SD (n = 5). In situ zymography from mouse livers from CCl4 mice (c) or BDL mice (d). Left panels, AAV5-LacZ-transduced mouse livers. Right panels, AAV5-HGF-transduced mouse livers
Discussion
The present study demonstrates that AAV-mediated long-term HGF expression can be sustained up to 12 weeks by a single AAV5-HGF transduction, and significantly prevents liver fibrosis induced by CCl4 and BDL. Up to our knowledge, this study is the first report that demonstrates the effect of AAV-mediated HGF gene therapy on liver fibrosis. CCl4 induces acute liver injury that is attributed to inflammatory responses originating from CCl4-derived free radical formation in the liver. Sustained hepatic inflammation by repetitive CCl4 administration leads to liver fibrosis through the production of fibrogenic cytokines [27, 28]. In contrast, BDL causes biochemical stress and injury of the bile duct epithelium, which is followed by inflammation, epithelial cell proliferation, upregulation of fibrogenic cytokines that activate myofibroblasts in the periductal region, and finally leads to liver fibrosis [23]. Although the mechanisms resulting in liver fibrosis are different in these two animal models, a single round of AAV5-HGF transduction clearly suppressed liver fibrosis in both models.
With respect to the effect of HGF on fibrosis, two mechanisms are hypothesized. One is the suppression of fibrogenesis through inhibition of TGF-β gene expression. TGF-β has been implicated as a major cause of tissue fibrosis and is a potent inhibitor of hepatocyte growth. TGF-β induces the phenotypic transition of HSC into proliferating myofibroblast-like cells, which enhance production of ECM components [29]. Although the molecular mechanisms remain to be addressed, we previously demonstrated that HGF inhibits TGF-β expression [15] in dimethylnitrosamine (DMN)-treated rats. In this study, the mRNA of TGF-β was also significantly suppressed by HGF transduction in the livers of both CCl4- and BDL-treated mice. Moreover, expression of α-SMA mRNA, a marker of HSC activation, also was suppressed by HGF transduction. Immunohistochemical staining demonstrated that HGF suppressed protein expression of α-SMA.
Another HGF-mediated mechanism is the resolution of liver fibrosis by increasing collagenase expression. MMPs are a family of extracellular zinc- and calcium-dependent proteases that promote degradation of the ECM components [30]. Ozaki et al. [26] reported that HGF increases MMP-1 promoter activity through increased expression and binding activity of Ets-1 in L190 cells, a human HSC. Ets-1 has been shown to modulate transcription of several MMP genes. In the present study, real-time PCR revealed an increase in MMP-13 mRNA by HGF gene transduction, and in situ zymography showed extensive gelatin degradation in liver sections of HGF-transduced mice. These data strongly suggest that HGF gene transduction stimulated MMP expression, resulting in the resolution of liver fibrosis.
As our and other investigators have reported, HGF has powerful therapeutic effects on liver fibrosis [12, 15, 31–33]. Matsuda et al. [12] reported that the continuous recombinant HGF injection accelerates the recovery from liver cirrhosis. However, a large amount of recombinant HGF is required using this method because HGF has a very short life. Recombinant HGF is very expensive, limiting such use in patients. Gene therapy has the advantage that it could sustain gene expression compared with the administration of recombinant protein. Recent studies have established several gene delivery systems for liver fibrosis. Since adenoviral vectors are capable of powerful gene expression, several reports have used adenoviral vector-mediated HGF gene therapy for liver fibrosis [31–33]. However, because it elicits a host immune response because of the highly immunogenic nature of the virus, it could not be used for repetitive transduction, limiting its clinical use [34, 35]. Nonviral vector systems, such as HVJ-liposome or naked plasmid administration, do not induce host immune response. However, these vectors require repetitive transduction to sustain gene expression because the expression is transient. Therefore, from a clinical viewpoint, a safe gene transfer method that achieves long-term expression is necessary. AAV vectors are single-stranded DNA viruses that are derived from a replication-deficient member of the parvovirus family. AAV vectors do not contain viral coding sequences and achieve efficient gene transduction in both dividing and nondividing target cells, while eliciting little immunogenicity. Moreover, they can achieve long-term gene expression in vivo [17, 36]. Davidoff et al. recently reported that AAV5-mediated gene expression stably maintained for 2 years in nonhuman primates [37]. In fact, a human clinical trial of an AAV vector has been conducted for the treatment of hemophilia B patients [38, 39]. Therefore, the use of AAV vectors for HGF gene therapy for liver fibrosis is feasible.
The influence of HGF on tumorigenicity should be considered, because the effect of HGF on the growth of HCC is still controversial. Although tumorigenicity has been reported in transgenic mice, overexpressing HGF, HGF expression level was 5000% higher in these mice compared with normal mice [40]. On the other hand, Shiota et al. [41] reported that growth of HCC cell lines was inhibited by the addition of recombinant HGF (50–200 ng/ml). Moreover, transgenic mice that express about 200–300% more HGF than normal mice demonstrate inhibition of neoplastic tumor development [42]. In our present study, human HGF level of HGF-transduced mouse was less than 40 pg/ml in serum, and less than 400 pg/g in liver tissue. We consider that such a low-level HGF concentration may not affect the tumorigenicity of HCC.
In conclusion, we demonstrated that AAV-mediated HGF gene therapy achieved long-term HGF expression, and markedly suppressed hepatic fibrosis induced by CCl4 or BDL. These results suggest that AAV-HGF-mediated gene therapy may represent a novel strategy for the treatment of patients with progressive liver fibrosis.
Acknowledgements
This work was supported by Grant-in-aid for Scientific Research (No. 11470266) from Japan Society for the Promotion of Science and the Ministry of Health.
Abbreviations
- AAV
Adeno-associated virus
- BDL
Bile duct ligation
- CCl4
Carbon tetrachloride
- HGF
Hepatocyte growth factor
References
- 1.Pinzani M, Romanelli RG, Magli S. Progression of fibrosis in chronic liver diseases: time to tally the score. J Hepatol 2001;34:764–7. [DOI] [PubMed]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–18. [DOI] [PMC free article] [PubMed]
- 3.Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, et al. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 2001;34:395–403. [DOI] [PubMed]
- 4.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–65. [DOI] [PubMed]
- 5.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al., For the International Hepatitis Interventional Therapy Group (IHIT). Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426–32. [DOI] [PubMed]
- 6.McHutchinson JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al., For the Hepatitis Interventional Therapy Group. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998;339:1485–92. [DOI] [PubMed]
- 7.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 1984;122:1450–9. [DOI] [PubMed]
- 8.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989;342:440–3. [DOI] [PubMed]
- 9.Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60–6. [DOI] [PubMed]
- 10.Boros P, Miller CM. Hepatocyte growth factor: a multi functional cytokine. Lancet 1995;345:293–5. [DOI] [PubMed]
- 11.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, et al. HGF receptor associates with anti-apoptotic protein BAG-1 and prevents cell death. EMBO J 1996;15:6205–12. [PMC free article] [PubMed]
- 12.Matsuda Y, Matsumoto K, Yamada A, Ichida T, Asakura H, Komoriya Y, et al. Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology 1997;26:81–9. [DOI] [PubMed]
- 13.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA 1994;91:4357–61. [DOI] [PMC free article] [PubMed]
- 14.Liu KX, Kato Y, Narukawa M, Kim DC, Hanano M, Higuchi O, et al. Importance of the liver in plasma clearance of hepatocyte growth factors in rats. Am J Physiol 1992;263:G642–9. [DOI] [PubMed]
- 15.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 1999;5:226–30. [DOI] [PubMed]
- 16.Hirano T, Fujimoto J, Ueki T, Yamamoto H, Iwasaki T, Morisita R, et al. Persistent gene expression in rat liver in vivo by repetitive transfections using HVJ-liposome. Gene Ther 1998;5:459–64. [DOI] [PubMed]
- 17.Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997;16:270–6. [DOI] [PubMed]
- 18.Miao CH, Snyder RO, Schowalter DB, Patijn GA, Donahue B, Winther B, et al. The kinetics of rAAV integration in the liver. Nat Genet 1998;19:13–5. [DOI] [PubMed]
- 19.Seki T, Hagiya M, Shimonishi M, Nakamura T, Shimizu S. Organization of the human hepatocyte growth factor-encoding gene. Gene 1991;102:213–9. [DOI] [PubMed]
- 20.Chiorini JA, Kim F, Yang L, Kotin RM. Cloning and characterization of adeno-associated virus type 5. J Virol 1999;73:1309–19. [DOI] [PMC free article] [PubMed]
- 21.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther 1998;5:938–45. [DOI] [PubMed]
- 22.Paquet KJ, Kamphausen U. The carbon-tetrachloride-hepatotoxicity as a model of liver damage. First report: long-time biochemical changes. Acta Hepatogastroenterol (Stuttg) 1975;22:84–8. [PubMed]
- 23.Ezure T, Sakamoto T, Tsuji H, Lunz JG 3rd, Murase N, Fung JJ, et al. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol 2000;156:1627–39. [DOI] [PMC free article] [PubMed]
- 24.Wasylyk C, Gutman A, Nicholson R, Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO J 1991;10:1127–34. [DOI] [PMC free article] [PubMed]
- 25.Westermarck J, Seth A, Kahari VM. Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene 1997;14:2651–60. [DOI] [PubMed]
- 26.Ozaki I, Zhao G, Mizuta T, Ogawa Y, Hara T, Kajihara S, et al. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol 2002;36:169–78. [DOI] [PubMed]
- 27.Wu J, Norton PA. Animal models of liver fibrosis. Scand J Gastroenterol 1996;31:1137–43. [DOI] [PubMed]
- 28.Britton RS, Bacon BR. Role of free radicals in liver diseases and hepatic fibrosis. Hepatogastroenterology 1994;41:343–8. [PubMed]
- 29.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994;331:1286–92. [DOI] [PubMed]
- 30.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–4. [DOI] [PubMed]
- 31.Ozawa S, Uchiyama K, Nakamori M, Ueda K, Iwahashi M, Ueno H, et al. Combination gene therapy of HGF and truncated type II TGF-beta receptor for rat liver cirrhosis after partial hepatectomy. Surgery 2006;139:563–73. [DOI] [PubMed]
- 32.Lin Y, Xie WF, Chen YX, Zhang X, Zeng X, Qiang H, et al. Treatment of experimental hepatic fibrosis by combinational delivery of urokinase-type plasminogen activator and hepatocyte growth factor genes. Liver Int 2005;25:796–807. [DOI] [PubMed]
- 33.Oe H, Kaido T, Furuyama H, Mori A, Imamura M. Simultaneous transfer of vascular endothelial growth factor and hepatocyte growth factor genes effectively promotes liver regeneration after hepatectomy in cirrhotic rats. Hepatogastroenterology 2004;51:1641–7. [PubMed]
- 34.Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther 1993;4:403–9. [DOI] [PubMed]
- 35.Marshall E. Gene therapy death prompts review of adenovirus vector. Science 1999;286:2244–5. [DOI] [PubMed]
- 36.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood 2002;100:1662–9. [DOI] [PubMed]
- 37.Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther 2005;11(6):875–88. [DOI] [PubMed]
- 38.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000;24:257–61. [DOI] [PubMed]
- 39.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003;101:2963–72. [DOI] [PubMed]
- 40.Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 1997;94:701–6. [DOI] [PMC free article] [PubMed]
- 41.Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci USA 1992;89:373–7. [DOI] [PMC free article] [PubMed]
- 42.Santoni-Rugiu E, Preisegger KH, Kiss A, Audolfsson T, Shiota G, Schmidt EV, et al. Inhibition of neoplastic development in the liver by hepatocyte growth factor in a transgenic mouse model. Proc Natl Acad Sci USA 1996;93:9577–82. [DOI] [PMC free article] [PubMed]