Abstract
Actinomycotic hepatic abscess was diagnosed in a 46-year-old male driver from Ukraine presenting with the symptoms of malaise, loss of appetite, upper right quadrant pain, weight loss, and night sweats which had been present for last 2 months. Computed tomography (CT) of the abdomen revealed a hypodense mass in the left liver lobe which was suspected as hepatocellular carcinoma. Histopathological examination of the CT guided biopsy specimen yielded a diagnosis of actinomycotic abscess of the liver. Treatment with intravenous penicillin for 6 weeks followed by a course of oral penicillin for 14 weeks resulted in complete cure as evidenced by clinical improvement and radiological disappearance of the lesion.
Keywords: Actinomycosis, Liver mass, Biopsy, Abscess, Penicillin
Introduction
Actinomycosis is an indolent, slowly progressive bacterial infection caused by a variety of gram-positive, non-spore-forming anaerobic or microaerophilic rods, most of which are of the genus Actinomyces [1]. Many Actinomyces species (species) are opportunistic pathogens of humans and other mammals, and constitute the commensal flora that is found in the oropharynx, gastrointestinal tract, and female genital tract [2]. While cervicofacial infection is the most frequent manifestation of the disease, gastrointestinal involvement occurs in about 13–60% of the cases, particularly in the oral cavity [2]. In rare cases, these bacteria can cause actinomycosis, a disease characterized by the formation of abscesses in the mouth, lungs, or the gastrointestinal tract [3]. Hepatic involvement is present in 15% of patients with abdominal actinomycosis and is thought to result from metastatic spread from other abdominal sites [4]. Some Actinomyces species are associated with other mixed anaerobic infections and in infections in severely compromised patients [3]. Here we describe a case of cryptogenic hepatic actinomycosis in an immunocompetent male presenting with upper right quadrant pain, constitutional symptoms, and abdominal computed tomographic (CT) images mimicking a liver tumor.
Case report
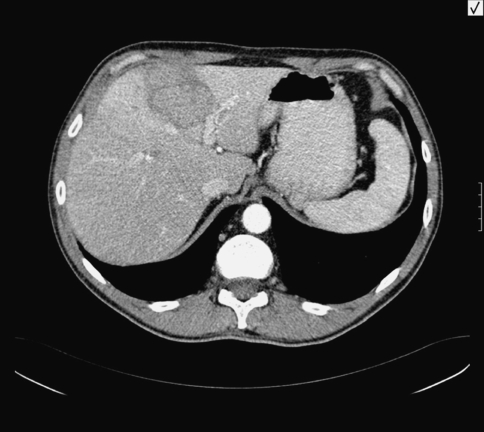
A previously healthy 46-year-old male driver from Ukraine was admitted to Acibadem Hospital on April 2005 with malaise, loss of appetite, upper right quadrant pain, weight loss, and night sweats, which had been present for the last 2 months. His initial physical examination was normal. There was no history of previous illness, smoking or alcohol intake. On laboratory investigations, his total white cell count was 10,100 cells/mm3 (neutrophils, 73%; lymphocytes, 19%; mixed [eosinophils, monocytes, and basophils], 8%), hemoglobin was 10.9 g/dl, hematocrit 32.5%, and platelet count 353,000 cells/mm3. Liver function tests were normal (AST: 18 IU/l, ALT: 19 IU/l, alkaline phosphatase: 122 IU/l, total bilirubin: 0.3 mg/dl). Erythrocyte sedimentation rate was 94 mm/h, C reactive protein was 13.75 mg/dl. Chest x-ray was normal. Abdominal ultrasound revealed a hyperechoic mass of 6 × 5 cm in the left liver lobe. A dynamic CT examination of the liver with intravenous (IV) contrast showed diffuse fatty infiltration of the liver and a solid mass of 6 × 5 cm located in the fourth segment of the left liver lobe. The lesion was slightly hypointense when compared to the liver parenchyma and was surrounded by a hypodense halo. Following IV contrast administration, the mass showed minimal homogenous enhancement in the venous phase. There was no capsular or extracapsular extension of the lesion. No perihepatic fluid was detected. The contour of the liver was smooth and the spleen size was within normal limits (Fig. 1). Preliminary diagnosis was a primary liver malignancy. α-Fetoprotein level was normal (1.44 IU/mL). Given the atypical characteristics of the mass, a CT-guided fine-needle aspiration and a tru-cut biopsy of the hepatic mass were both performed. Histopathological examination of the specimen revealed acute and chronic inflammation in continuation with liver parenchyma, granulation tissue, and gram-positive branching filamentous rods consistent with an Actinomyces species (Figs. 2 and 3). Upper GI endoscopy was normal. No pathogen was isolated from the three consecutive blood culture samples obtained. Test for HIV was negative. Owing to the typical appearance of the actinomycotic colony on histopathological examination, serological test was not done. Diagnosed as having an actinomycotic liver abscess, the patient was administered high-dose IV penicillin G (24 million units/day) as continuous infusion for 2 weeks. On the third week, the dose was reduced to 20 million units/day to be continued for another 4 weeks. The treatment was extended to 3 months by switching to oral penicillin G. The upper right quadrant pain and night sweats of the patient abated within 48 h after the start of therapy. He was symptom-free 3 months after the cessation of treatment. Control imaging confirmed the disappearance of the lesion.
Fig. 1.
A dynamic computed tomographic examination of the liver showing a solid mass of 6 × 5 cm located in the fourth segment of the left liver lobe
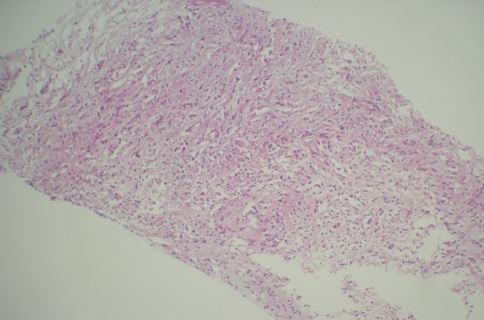
Fig. 2.
Abscess wall surrounding actınomyces colony and showing transition zone from liver tissue to abscess wall (×40 H&E)
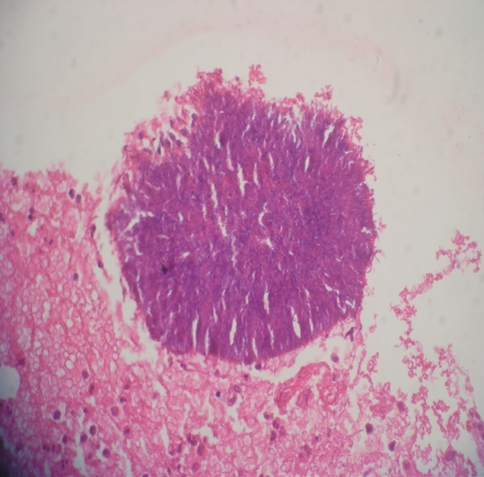
Fig. 3.
High-power photomicrograph of an intact, typical actinomyces colony pictured from the same abscess cavity. The filamentous nature of the actinomyces organisms is more discernible at this power (×400 H&E)
Discussion
Actinomycotic abscess of the liver is a rare condition [3, 4]. Actinomyces species are found as part of the commensal flora in the gastrointestinal tract. They invade the adjacent tissues and bloodstream when normal anatomical barriers are disrupted. In their review article, Sharma et al. stated that the infection was cryptogenic in about 80.7% of the patients and tended to affect predominantly males (70.2%) [5]. Among 35 previously reported isolated hepatic actinomycosis cases, predisposing factors included poor dentition in five patients, alcohol abuse in three, biliary tract disease in two, recent appendicitis in two, peptic ulcer in two, and oral neoplasm in one [6]. Hepatic actinomycotic tumor was also reported to occur with pelvic actinomycosis in women using intrauterine device [7]. Nevertheless, the majority of the cases are cryptogenic, as suggested by Sharma et al. [5, 6]. We have not found any evidence of dental abscess, gingivitis, or periodontitis in our patient. Furthermore, no other underlying source of infection could be identified. Clinical manifestations associated with actinomycotic liver abscess can be silent and tend to follow an insidious course. This was the situation in our patient.
Inflammatory masses, solitary metastatic lesions, and primary liver masses can have the same appearance on CT examination as in our case [7]. Conversely, pelvic actinomycosis in women with intrauterine devices (IUDs) may be associated with inflammatory hepatic pseudotumors [7, 8]. Considering the clinical features and the enhancement pattern of the mass at imaging studies, we were able to exclude hemangioma, focal nodular hyperplasia, and adenoma from the differential diagnosis list. Although an amoebic liver abscess was still considered as a possible diagnosis after the ultrasound examination, the lack of an enhancement at the wall and a cystic component on CT examination rendered this possibility to be less likely. Serology has not been helpful in the diagnosis of such cases [9]. Given the atypical characteristics of the mass, biopsy was the best choice to confirm the diagnosis.
In conclusion, hepatic actinomycosis should also be considered in the differential diagnosis of liver abscesses and space-occupying lesions of the liver in immunocompetent patients.
Contributor Information
Arzu Tiftikci, Email: arzutiftikci@hotmail.com.
Eser N. Vardareli, Email: evardareli@asg.com.tr
Kerim Kaban, Email: kkaban@asg.com.tr.
Onder Peker, Email: opeker@asg.com.tr.
Sertac Akansel, Email: sakansel@asg.com.tr.
Nurdan Tozun, Email: drnurdantozun@yahoo.com.
References
- 1.Russo TA. Actinomycosis. In: Fauci SA, Braunwald E, Isselbacher KJ, et al., editors. Harrison’s principles of internal medicine. 14th ed. New York: McGraw-Hill; 1998. p. 989.
- 2.Smego RA, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26(6):1255–61; quiz 1262–3. [DOI] [PubMed]
- 3.Bowden GHW. Actinomycosis. In: Baron S, Peaker RC, James DA, et al., editors Baron’s medical microbiology. 4th ed. University of Texas Medical Branch (via NCBI Bookshelf). ISBN 0-9631172-1-1.
- 4.Friedman SL, Chung RT. Liver abscess and bacterial, parasitic, fungal, and granulomatous liver disease. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran’s gastrointestinal and liver disease. 7th ed. China: Saunders; 2002. pp. 1343–63.
- 5.Sharma M, Briski LE, Khatib R. Hepatic actinomycosis: an overview of salient features and outcome of therapy. Scand J Infect Dis. 2002;34(5):386–91. [DOI] [PubMed]
- 6.Miyamoto MI, Fang FC. Pyogenic liver abscess involving actinomyces: case report and review. Clin Infect Dis. 1993;16:303–9. [DOI] [PubMed]
- 7.Kim HS, Park NH, Park KA, Kang SB. A case of pelvic actinomycosis with hepatic actinomycotic pseudotumor. Gynecol Obstet Invest. 2007;64(2):95–9. [DOI] [PubMed]
- 8.Tamsel S, Demirpolat G, Killi R, Elmas N. Primary hepatic actinomycosis: a case of inflammatory pseudotumor. Diagn. Intervent Radiol. 2004;10:154–7. [PubMed]
- 9.Lerner PI. Serologic screening for actinomycosis. In: Balows A, editor. Anaerobic bacteria: role in disease. Springfield IL: Charles C. Thomas; 1974. p. 571.