Abstract
Purpose
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. HCC is notably more prevalent in males worldwide, with reported male:female ratios ranging from 2:1 to 8:1. The reasons for sex differences in the incidence of HCC are unclear. Furthermore, differences in rates of disease progression and longevity are not well studied and few series have compared the clinicopathologic characteristics of patients and their impact on survival with specific reference to gender in a large sample set.
Methods
The present study is a large single-institution study of 1138 HCC cases referred to a single individual carried out over a period of 17 years. The primary endpoint measure was over-all survival measured in months, which was defined as the time between the date of diagnosis and date of death. Differences in median survival for each subgroup analysis in survival rates were compared by log rank test.
Results
There are differences in both the distribution of evidence of disease progression at the time of diagnosis and the time for survival following diagnosis in patients with HCC between the two genders. Females had a longer survival than males in subsets matched for residual liver function and tumor extension, suggesting that the natural history of HCC is different between men and women.
Conclusion
The present study provides evidence that female gender provides a distinct survival advantage over males in unresectable HCC presenting with similar tumor characteristics, liver function, and coexisting liver disease.
Keywords: Gender, Outcome, Unresectable hepatocellular carcinoma, Survival, Female
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide with a particularly high incidence in areas where chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) are common [1]. The American Cancer Society has predicted 19,160 new HCC cases and 16,780 deaths in 2007 among U.S. residents [2]. HCC is notably more prevalent in males worldwide, with reported male:female ratios ranging from 2:1 to 8:1, being more marked in high incidence areas, but less different in low incidence regions [3]. In the United States the male:female ratio for HCC incidence is 2.4:1 [1].
Despite the extent of this evidence, the reasons for sex differences in the incidence of HCC are unclear [3, 4], although gender-based differences in carcinogen-metabolizing liver enzymes have been noted. Furthermore, it is unclear whether the differences in incidence are matched by differences in rates of disease progression and longevity. It has been reported that DNA synthesis is higher in male than in female cirrhotic livers, and a higher rate of cell turnover has been hypothesized to be a contributory factor to the gender discrepancy of HCC [5]. Despite evidence suggesting that testosterone is a growth stimulus to Transforming Growth Factor alpha (TGFα) mitogenic actions, which could stimulate hepatocarcinogenesis and hepatocyte proliferation [6]; it remains unknown whether the natural history of HCC is different between the 2 sexes. Controversial reports also exist on the contribution of sex differences to patient survival and prognosis [7–10]. However, few studies have compared the clinicopathologic characteristics of patients and their impact on survival with specific reference to gender in a large sample set.
The present study is a large single-institution study of HCC cases referred to a single individual (BIC) carried out over a period of 17 years. In the current study, we examine the hypothesis that unresectable human HCC is not only less frequent in women than men, but also has a less aggressive rate of progression to death in patients who have an equivalent extent of concurrent liver disease, residual hepatic function, and evidence of tumor burden at the time of their initial clinical appraisal.
Methods
Study population
The study population consisted of 1,138 individuals diagnosed with HCC who had unresectable tumor at the time of evaluation. The cases were accrued over a 17-year period at the Liver Cancer Center, University of Pittsburgh. At the time of diagnosis the patient’s history, physical examination, laboratory measures, and Computed Axial Tomography (CAT) scan were recorded prospectively. On initial clinical evaluation, all patients had baseline complete blood count (CBC), routine liver function tests, Alpha-fetoprotein (AFP), hepatitis serology, serum creatinine, Body Surface Area (BSA) from height and weight, physical examination, and a triphasic helical Computed Tomography (CT) scan of chest, abdomen, and pelvis. If liver biopsy diagnosis had not been conducted prior to referral, underlying random liver biopsy and directed tumor biopsy was obtained. The data and CT descriptors were recorded and entered in an HCC database designed for follow-up and analysis. Patients were followed to the time of their death either through this clinic, or through close liaison with their primary health care provider. Cases were dichotomized into categories based on abnormal biochemical indices of liver function. The “Good Liver Function” category was defined by individuals with a serum bilirubin <1.5 mg/dl, alkaline phosphatase <200 U/100 ml, albumin >3.5 g/l, and prothrombin time <13 s. “Poor Liver Function” was defined as the presence in individuals of any of the following: a serum bilirubin >1.5 mg/dl, alkaline phosphatase >200 U/100 ml, albumin <3.5 g/l, and prothrombin time >13 s. We have attempted to minimize confounding factors such as treatment in our strategy for sub group classification; all patients in the study received TACE treatment.
Statistical methods
The primary endpoint measure was over-all survival measured in months, which was defined as the time between the date of diagnosis and date of death. Follow-up data were censored at the time of last visit. Median survival was expressed in months. Data used to relate to survival time were dichotomized using the criteria defined in Table 1. Differences in median ± 95% confidence limits for each subgroup analysis in survival rates were compared by log rank test. Analyses were carried out using SAS 9.1.
Table 1.
Demographics of the population of patients with unresectable HCC and sample size of subgroups based on the stated criteria
| Females % of variable being dichotomized | Males % of variable being dichotomized | |
|---|---|---|
| Number of patients | 279 (32%) | 859 (68%) |
| Age <65 years | 57 | 61 |
| Age >65 years | 43 | 39 |
| Tumor characteristics | ||
| Number of Tumors >5 | 38 | 38 |
| Tumor size >10 cm in diameter | 28 | 27 |
| Portal vein thrombosis | 35 | 45* |
| Lymphadenopathy | 27 | 34 |
| Distant metastases | 25 | 23 |
| Ascites | 27 | 43 |
| Co-existent liver disease and lifestyle | ||
| Cirrhosis | 60 | 82.5* |
| No hepatitis | 62 | 43 |
| Hepatitis B | 8 | 18 |
| Hepatitis C | 23 | 25 |
| Hepatitis B and C | 5 | 13 |
| Alcohol | 11 | 47* |
| Smoking | 10 | 25* |
| Abnormal biochemical indices liver function | ||
| AFP >25 ng/ml | 64 | 66 |
| Bilirubin >1.5 mg/dl | 17 | 32* |
| Alkaline phosphatase >120 U/100 ml | 75 | 72 |
| Albumin <3.5 g/l | 63 | 72 |
| Prothrombin time >13 sec | 37 | 50 |
| Good liver function | 85 | 73 |
| Poor liver function | 15 | 27 |
* P < 0.05 chi-squared comparison between percentage of women versus the percentage of men with the defined clinical characteristic
Results
Over 17 years, 1,138 patients with HCC were managed by a single physician (BIC) in a single clinic. The demographic distribution of males and females with HCC for dichotomized clinical measures is presented in Table 1. Of these, 32% were female and 68% were male. The age distribution was similar in both groups. The analysis of tumor characteristics revealed a marginally higher percentage of males with portal vein thrombosis (PVT) (45% vs. 35%, P < 0.05). A higher proportion of males had cirrhosis (82.5% vs. 60%, P < 0.05) as well as alcohol (11% vs. 47%, P < 0.01) and smoking histories, and bilirubin levels higher than 1.5 mg/dl (32% vs. 17%, P < 0.05).
The influence of clinical features on HCC survival by gender is presented in Table 2. Women had a longer survival [14 months (11–18) vs. 9 months [7–10], P < 0.0001] (Table 2). In addition to the presence of PVT being more common in men, the median survival in those men with PVT was substantially less in comparison to men without PVT (5 months versus 11 months, P < 0.0001). This contrasted to a marginal change in females with PVT compared to those without PVT (10 months versus 14 months). Furthermore, in the presence of PVT, median survival in males was half that of females (5 months versus 10 months, P < 0.001). In contrast, the presence of other tumor characteristics did not significantly alter survival in either gender. The presence or absence of cirrhosis was associated with significant differences in survival in both genders.
Table 2.
The influence of various clinical features on HCC survival by gender
| Variable/ Tumor characteristic | Females, N = 279, (32%) | Males, N = 859 (68%) | ||
|---|---|---|---|---|
| Percent of variable of interest in all females | Median survival months 95% CI | Percent of variable of interest in all males | Median survival months 95% Cl | |
| Overall | 14 (11–18) | 9 (7–10)*** | ||
| Portal vein thrombosis-absent | 65 | 14 (10–18) | 55 | 11 (8–13) |
| Portal vein thrombosis-present | 35 | 10 (8–14) | 45 | 5 (4–5)**, ††† |
| Number of tumors <5 | 62 | 14 (10–18) | 62 | 7 (5–8)** |
| Number of tumors >5 | 38 | 9 (7–13) | 38 | 6 (4–7)* |
| Tumor size <10 cm | 72 | 12 (9–17) | 73 | 7 (5–8)* |
| Tumor size >10 cm | 28 | 12 (8–16) | 27 | 8 (7–12)* |
| Unilobar | 52 | 13 (9–18) | 34 | 8 (6–10)** |
| Bilobar | 48 | 11 (8–14) | 66 | 6 (5–7)* |
| Cirrhosis absent | 40 | 15 (11–19) | 17.5 | 11 (8–14) |
| Cirrhosis present | 60 | 9 (7–12)†† | 82.5 | 6 (5–7)*, ††† |
| Alcohol | 11 | 9 (7–12) | 47 | 6 (5–8) |
| Hepatitis B | 8 | 16 (10–30) | 18 | 12 (6–14) |
| Hepatitis C | 23 | 8 (3–13) | 25 | 7 (5–8) |
| Hepatitis B and C | 5 | 11 (8) | 13 | 5 (4–7) |
* Comparison of females versus males (comparison of columns within a row): * P < 0.05, ** P < 0.001, *** P < 0.0001
† = Comparison of variables (comparison of rows within a column): † P < 0.05, †† P < 0.001, ††† P < 0.0001
The analysis was further extended to evaluate the impact of baseline biochemical characteristics on gender differences in median survival (Table 3). Even though the proportion of men and women with elevated AFP was similar, males with AFP >25 ng had a reduced median survival of 5 (4–6) months as compared to either males with AFP <25 ng, (15 (13–18) months, P < 0.0001), or to females with AFP >25 ng (10 (8–14 months, P < 0.001). The presence of AFP >25 ng was not associated with a significant reduction in survival in females. Bilirubin >1.5 mg/dl was a major risk factor in both males and females (P < 0.0001), as was elevated alkaline phosphatase and prolonged prothrombin time.
Table 3.
The influence of various biochemical characteristics on HCC survival by gender
| Variable/Tumor Characteristic | Females, N = 279, (32%) | Males, N = 859, (68%) | ||
|---|---|---|---|---|
| Percent of variable of interest in all females | Median survival months 95% CI | Percent of variable of interest in all males | Median survival months 95% Cl | |
| Overall | 14 (11–18) | 9 (7–10)*** | ||
| AFP <25 ng | 36 | 14 (10–20) | 34 | 15 (13–18) |
| AFP >25 ng | 64 | 10 (8–14) | 66 | 5 (4–6)**, ††† |
| Bilirubin <1.5 mg/dl | 83 | 14 (11–18) | 68 | 10 (8–12) |
| Bilirubin >1.5 mg/dl | 17 | 3 (3–8)††† | 32 | 3 (2–4)††† |
| Alkaline Phosphatase <120 U/100 ml | 25 | 19 (18–30) | 28 | 14 (11–18) |
| Alkaline phosphatase >120 U/100 ml | 75 | 9 (7–11)††† | 72 | 5 (5–6)*, ††† |
| Albumin >3.5 g/l | 37 | 15 (11–22)† | 28 | 13 (10–15)††† |
| Albumin <3.5 g/l | 63 | 10 (8–12) | 72 | 5 (5–6)** |
| PT < 13 secs. | 63 | 16 (13–20) | 50 | 10 (8–12) |
| PT > 13 secs. | 37 | 6 (3–9)††† | 50 | 4 (4–5)††† |
* Comparison of females versus males (comparison of columns within a row): * P < 0.05, ** P < 0.001, *** P < 0.0001
† = Comparison of variables (comparison of rows within a column): †P < 0.05, ††P < 0.001, ††† P < 0.0001
The observations of significant relationships between variables with time to survival indicate that each of the clinical characteristics of tumor extension, liver function, and concomitant liver disease need to be simultaneously taken into consideration to create subgroups of comparable baseline characteristics before inferences on gender differences in the prognosis of HCC can be made. Multivariate regression analysis was not carried out as the data variables are interdependent on each other and are non-parametric. We have taken advantage of the large size of the study group to use an alternative approach by building a pathophysiological model that includes relevant variables obtained at the time of diagnosis to create mutually exclusive subgroups.
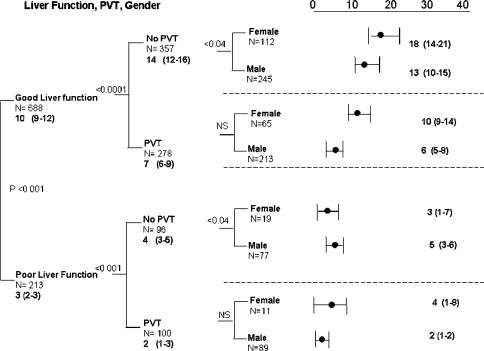
To define subsets with comparable liver function at baseline assessment, we used the previously (see methods section) defined integrated definitions of good and poor liver function. Using this definition, survival in group with poor liver function was 3 (2–3) months in the 213 patients with this trait (Fig. 1). Even in the presence of poor hepatic function, tumor characteristics offered further discrimination as a prognostic indicator with patients with PVT having a survival of 2 (1–3) months, compared to patients with no PVT, who had a median survival of 4 (3–5) months, P < 0.001. With such a short survival it is not surprising that there was no gender difference (Fig. 1).
Fig. 1.
Pathophysiological model using liver function, PVT and gender
In contrast, on comparison of survival between patients with normal liver function who were further subdivided by their tumor characteristics, sex differences were observed (Table 4, Fig. 1). In the 688 patients with normal baseline liver function, the presence of PVT was associated with a 50% reduction in median survival, 7 (6–9) months, compared to the absence of PVT, 14 (12–16) months, P < 0.0001 (Fig. 1, Table 4). Further subgroup discrimination on the basis of gender still provided substantial sample size within each subset (Fig. 1, Tier 3). In the good liver function but no PVT subgroup, median survival in females was longer than males, (18 (14–21) months vs. 13 (10–15) months, P < 0.04). In the PVT subgroup with good liver function, there was a similar trend, 10 (9–14) months, vs. 6 (5–8) months, which was not significant. The distribution of females and males in tier 3 of Fig. 1, also illustrates the predominance of female patients in the less severe categories of disease severity, either by liver function (14.5% females in the poor category, compared to 36% males), or by tumor extent, as illustrated by the presence of PVT (37% females vs. 47% males).
Table 4.
Median survival times in females and males with good liver function
| Overall | Females | Males | |||
|---|---|---|---|---|---|
| Tumor characteristic | Median survival (95% CI) | Percent of variable of interest | Median survival (95% CI) | Percent of variable of interest | Median survival (95% CI) |
| No PVT (56%) | 14 (12–16) | 63 | 18 (14–21) | 53.5 | 13 (10–15)* |
| PVT (44%) | 7 (6–9)††† | 37 | 10 (9–14)† | 46.5 | 6 (5–8)††† |
| Number of tumors <5 (62%) | 12 (10–14) | 61 | 18 (14–20) | 62.5 | 10 (7–12)** |
| Number of tumors >5 (38%) | 8 (7–10)†† | 39 | 9 (7–14)†† | 31.5 | 8 (6–11) |
| Tumor size <10 cm (68%) | 11 (9–13) | 65.5 | 15 (10–19) | 69 | 10 (8–12)* |
| Tumor size >10 cm (32%) | 10 (7–13) | 34.5 | 15 (10–19) | 31 | 7 (5–12)* |
| Unilobar (49%) | 11 (9–14) | 51 | 18 (11–21) | 48 | 10 (8–13)** |
| Bilobar (51%) | 9 (7–12)† | 49 | 12 (9–16)†† | 52 | 8 (6–11) |
* Comparison of columns within a row: * P < 0.05, ** P < 0.001, *** P < 0.0001
† Comparison of rows within a column: † P < 0.05, †† P < 0.001, ††† P < 0.0001
When the analyses were repeated using alternative variables to PVT that indicate tumor extension, similar orders of magnitude of reduction for median survival were also observed for the number of tumors, size of the largest tumor, bilobarity, and the presence of cirrhosis (Table 4). The survival data in patients with poor liver function are not presented here as median survival was already very low in this group.
Discussion
The conclusions of the present study are that there are differences in both the distribution of evidence of disease progression at the time of diagnosis and the time for survival following diagnosis in patients with HCC between the two genders. There are two possible explanations for this observation. The first being that women are diagnosed earlier in the disease but have a similar rate of disease progression to men or alternatively, the disease is different and less aggressive than in men. Our new observation is that in patients matched for tumor burden and residual liver function, the survival in women is longer than in men. This observation supports the hypothesis that there is a difference in the natural history of HCC between sexes.
The present analysis is based on a large study with extensive data on clinical covariates collected at the time of diagnosis in patients with unresectable HCC over a period of 17 years. A sample size of 279 female patients (despite the fact that they represented only one third of the study population), with adequate and long-term follow-up data has allowed us a more detailed analysis of HCC progression in the two sexes than has previously been reported [7–10]. Results of our univariate analysis showed differences in distribution of tumor burden and residual liver function between sexes at the time of presentation. We compared men and women with similar tumor number, size, lobarity, presence of environmental factors such as alcohol, HBV and HCV infection, biochemical indices, tumor extension, and underlying liver disease. Although chronic hepatitis infection is the major etiology in Western countries, in our large experience that includes resection and liver transplant (not included here) we have found that overall there is no obvious etiology in about 25% of our patients that we can discern. Furthermore there is increasing appreciation that obesity and NASH are risk factors in our Western patients and this is not included in the current analysis. These probably explain the relatively low percentage of patients with any type of hepatitis. A higher proportion of men presented with cirrhosis; when comparing men and women who both had cirrhosis, women still had a distinct survival advantage. For each of the biochemical indices evaluated except for bilirubin women did better than men, with significant differences in survival times.
To address whether there is a gender difference in rate of disease progression and hence survival, we have sought to select subgroups of patients with HCC who have equivalent clinical phenotypes at the time of their initial appraisal. In the analysis undertaken by creating a pathophysiological model based on tumor progression and loss of residual liver function, we have created discrete subgroups with a more homogeneous base of sufficient size to permit comparison of time to survival. Within this analysis, liver function (defined as a combination of abnormal biochemical indices) provided the greatest discriminator of survival. In the subset with poor liver function, creating further subsets based on tumor progression provided further marginal discrimination in survival that was not enhanced by sex. In contrast, the analysis of males and females with good liver function suggested that tumor characteristics still are important determinants of survival that need to be considered in the evaluation of a gender effect. Using this approach, females still had a longer survival than males in subsets matched for residual liver function and tumor extension. These observations support the concept that the natural history of HCC is different between men and women.
This evidence contrasts to several analyses that have failed to demonstrate gender as an independent variable for survival [11–15]. In some cases, a lead-time bias in the diagnosis of HCC in favor of female patients has been suggested to account for survival differences [5]. However, the sample sizes in most of these studies have been small, so that it has not been possible to undertake an evaluation that takes into account all the clinical, pathological, and biochemical variables that might influence the incidence and progression of HCC.
Interest in this field has led to several focused efforts to resolve the mechanism behind gender differences in HCC. These attempts have been complicated by the fact that HCC occurs more often in males with chronic liver disease, which in turn leads to a hyper estrogenic state that has been implicated in the pathogenesis of HCC [16–19]. Experimental and clinical data have shown that both estrogens and androgens have important effects in controlling the replication rate of hepatic cells [11, 20]. Both estrogens and androgens may also have an effect on inducing or at least promoting the growth of HCC. The evidence obtained from clinical trials utilizing antiandrogen and antiestrogen therapies suggests that once the tumor has developed, the therapy has no clinically significant effect on the progression of the disease [16, 19, 20].
The present study was limited by the lack of additional useful information such as age of menarche, age of menopause, number of children, contraceptive use, preexisting hormone disorders, and serial CT scan measurements. Further analysis of these factors may better address the effects of sex hormones on the progression of HCC.
In conclusion, the present study provides compelling evidence that the female gender has a distinct survival advantage over the male gender in patients with unresectable HCC that present with similar tumor characteristics, liver function, and coexisting liver disease. Although the reasons for this still remain to be elucidated, the current findings serve to emphasize the need to further study this complex biological phenomenon to better understand the pathogenesis and progression of HCC.
Abbreviations
- AFP
Alpha-fetoprotein
- BSA
Body surface area
- CAT
Computed axial tomography
- CBC
Complete blood count
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- PVT
Portal vein thrombosis
Footnotes
This study was supported by grants NIH 5RO1DK059519-O5 and NIH 1U54RR023506-01.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed]
- 2.Cancer Facts and Figures 2007, American Cancer Society, http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf.
- 3.Tangkijvanich P, Mahachai V, Suwangool P, Poovorawan Y. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J Gastroenterol 2004;10:1547–50. [DOI] [PMC free article] [PubMed]
- 4.El-Serag HB. Epidemiology of hepatocellular cancer. Clin Liver Dis 2001;5:87–107. [DOI] [PubMed]
- 5.Dohmen K, Shigematsu H, Irie K, Ishibashi H. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol 2003;18:267–72. [DOI] [PubMed]
- 6.Matsumoto T, Takagi H, Mori M. Androgen dependency of hepatocarcinogenesis in TGF alpha transgenic mice. Liver 2000;20:228–33. [DOI] [PubMed]
- 7.Chen WT, Chau GY, Lui WY, et al. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur J Surg Oncol 2004;30:414–20. [DOI] [PubMed]
- 8.Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg 2001;88:515–22. [DOI] [PubMed]
- 9.Calvet X, Bruix J, Gines P, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology 1990;12:753–60. [DOI] [PubMed]
- 10.Akashi Y, Koreeda C, Enomoto S, et al. Prognosis of unresectable hepatocellular carcinoma: an evaluation based on multivariate analysis of 90 cases. Hepatology 1991;14:262–8. [PubMed]
- 11.Shimoda M, Ghobrial M, Carmody IC, et al. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with hepatitis C. Liver Transpl 2004;10:1478–86. [DOI] [PubMed]
- 12.El-Serag HB. Hepatocellular carcinoma. J Clin Gastroenterol 2002;35:S72–8. [DOI] [PubMed]
- 13.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004;127:S27–34. [DOI] [PubMed]
- 14.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 2005;25:143–54. [DOI] [PubMed]
- 15.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol 2002;8(2):193–9. [DOI] [PMC free article] [PubMed]
- 16.Giannitrapani L, Soresi M, LaSpada E, Cervello M, D’Allessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann NY Acad Sci 2006;1089:228–36. [DOI] [PubMed]
- 17.DeMaria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol 2002;193:59–63. [DOI] [PubMed]
- 18.Lam CM, Yong JL, Chan AO, et al. Better survival in female patients with hepatocellular carcinoma oral contraceptive pills related? J Clin Gastorenterol 2005;39:533–9. [DOI] [PubMed]
- 19.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int 2003;23:63–69. [DOI] [PubMed]
- 20.Ng I, Ng M, Fan ST. Better survival in women with resected hepatocellular carcinoma is not related to tumor proliferation or expression of hormone receptors. Am J Gastroenterol 1997;92:P1355–8. [PubMed]