Abstract
A small-cell carcinoma of the extrahepatic bile duct in a 69-year-old woman is herein reported. A tumor measuring approximately 3 cm in diameter was located at the confluence of the common bile duct, cystic duct, and common hepatic duct. Histopathologically, the tumor was small-cell neuroendocrine carcinoma without any gland formation or differentiation to squamous cell carcinoma. Tumor cells were immunoreactive for epithelial markers such as epithelial membrane antigen and cytokeratin and for the neuroendocrine markers such as neuron-specific enolase, chromogranin A, and synaptophysin. Although the carcinomas in more than half of the reported cases have been reported to be associated with well-to-moderately differentiated squamous or glandular components, seven cases, including our case, showed the carcinomas without squamous or glandular components. According to the review of 16 previously reported cases and our case of small-cell carcinoma of the extrahepatic bile ducts, there is no significant difference in the clinicopathological findings, namely, age, sex, site of carcinoma, and prognosis between the cases with or without squamous or glandular components. No CD34-positive multipotent adult progenitor cells, which might be the origin of the small-cell carcinoma, were detected in the bile duct epithelium in our case.
Keywords: Small-cell carcinoma, Extrahepatic bile duct, Neuroendocrine carcinoma, Immunohistochemistry
Introduction
Small-cell carcinomas arising in organs other than the lung account for about 4% of all small-cell carcinomas. Recently, a growing number of reports have described small-cell carcinomas arising in the gastrointestinal organs, including the esophagus, colon, and pancreas. However, small-cell carcinomas originating in the biliary tract are extremely rare, with most occurring in the gallbladder. We report an autopsy case of small-cell carcinoma of the extrahepatic bile duct.
Case report
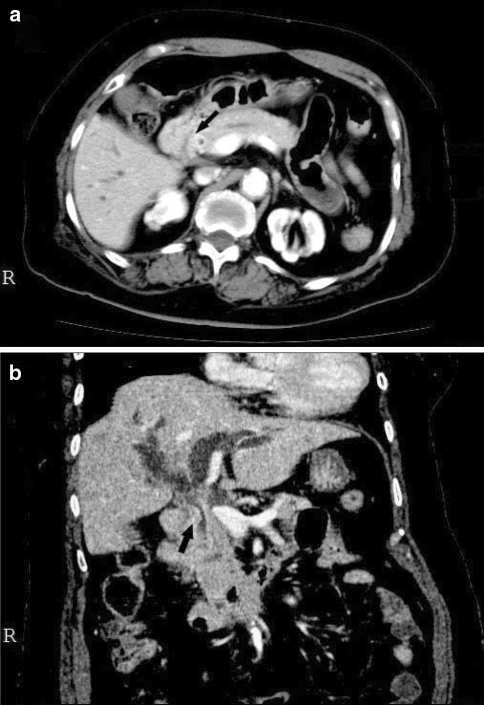
A 69-year-old woman was admitted to our hospital because of jaundice. The laboratory findings on admission were as follows: total bilirubin, 6.4 mg/dl; aspartate aminotransferase, 258 U/l; alanine aminotransferase, 396 U/l; alkaline phosphatase, 1058 U/l; γ-glutamyl transpeptidase, 1033 U/l; amylase, 78 U/l; carcinoembryonic antigen, 170 ng/ml; and CA19-9, 352 U/ml. The chest X-ray findings were normal. The patient had diabetes mellitus and a history of cerebral infarction. She had no family history of cancer and had never smoked or drunk alcohol. Endoscopic retrograde cholangiography revealed an irregular luminal narrowing situated at the confluence of the common bile duct, cystic duct, and common hepatic duct, suggesting an extrahepatic carcinoma of the bile ducts. Computed tomographic scanning showed the partially and heterogeneously enhanced and poorly demarcated tumor (Fig. 1a and b) narrowing the lumen of the extrahepatic bile duct, marked dilatation of the intrahepatic bile ducts (Fig. 1b), and nodules with low attenuation in the liver, thus suggesting liver metastasis. Since the patient was in poor general condition, she received symptomatic treatment by endoscopic retrograde biliary drainage (ERBD) to reduce jaundice. Although the jaundice improved after ERBD, she died about 2 months after the onset of jaundice because of a worsening general condition.
Fig. 1.
An enhanced abdominal computed tomography scan (horizontal sectional view, a; frontal sectional view, b). The lumen of extrahepatic bile duct is narrowed by the partially and heterogeneously enhanced and poorly demarcated tumor (arrows). Intrahepatic bile ducts are markedly dilated
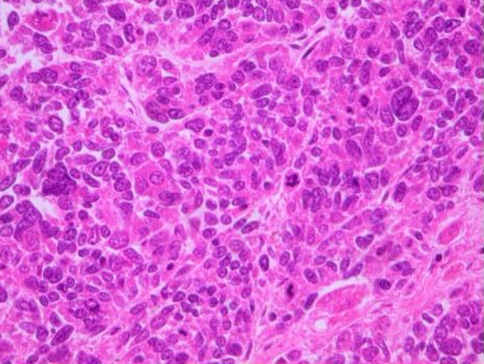
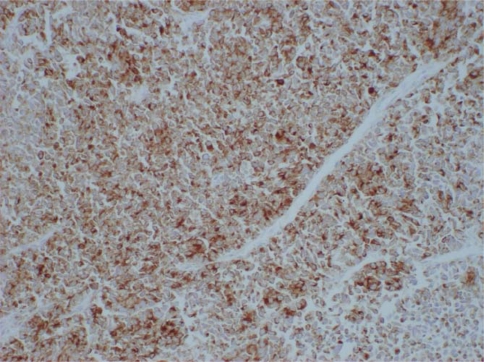
An autopsy revealed tumor-induced occlusion of the biliary ducts, extending for about 3 cm at the confluence of the common bile duct, cystic duct, and common hepatic duct. The tumor invaded the cystic duct. Multiple masses were detected in the liver. A histological examination showed a dense proliferation of small tumor cells with hyperchromatic oval nuclei and scant cytoplasm (Fig. 2). There was a marked trend toward overall necrosis in the tumor. There was also no evidence of gland formation or of differentiation to squamous cell carcinoma. The tumor cells invaded the portal venous system of the liver and pancreas. On immunohistochemical staining, the tumor cells were focally immunoreactive to epithelial membrane antigen as epithelial markers, and were diffusely positive for a broad-spectrum cytokeratin (AE1/AE3). On the other hand, the tumor cells were diffusely positive for all applied neuroendocrine markers such as neuron-specific enolase (NSE), Grimelius, chromogranin A (Fig. 3), and synaptophysin, except for negative immunoreactivity to S-100. These findings closely agreed with primary small-cell carcinoma of the extrahepatic bile ducts.
Fig. 2.
Small-sized, round oval carcinoma cells with hyperchromatic oval nuclei and scant cytoplasm proliferated, and they were arranged in either solid sheets or cords (H&E, 200×)
Fig. 3.
Immunohistochemical staining of the tumor. The tumor cells in small-cell carcinoma were diffusely immunoreactive to chromogranin A (chromogranin A, 200×)
Discussion
To our knowledge, only 16 cases of primary small-cell carcinoma of the extrahepatic bile ducts have been previously reported (Table 1) [1–6]. Histopathologically, small-cell carcinoma of the extrahepatic bile duct is characterized by a diffuse proliferation of atypical small round cells with hyperchromatic nuclei and scant cytoplasm, which are also characteristics shared by small-cell lung or gastrointestinal carcinoma [7]. While the carcinomas in more than half of the above reported cases were associated with well-to-moderately differentiated squamous or glandular components, seven cases, including our case, showed carcinomas without any squamous or glandular components [1–6]. Immunohistochemically, most small-cell carcinomas are immunoreactive for neuroendocrine markers such as NSE, chromogranin, synaptophysin, and Leu-7 [8]. The tumor cells also expressed some epithelial markers. The squamous or glandular components usually lack endocrine differentiation.
Table 1.
Patients with small-cell carcinoma of the extrahepatic bile duct
| Number | Age | Gender | Location | Symptom | Histology | Positive immunostaining |
|---|---|---|---|---|---|---|
| 1 | 67 | M | Bi | Jaundice, diarrhea | Small-cell neuroendocrine carcinoma | NSE |
| 2 | 55 | M | Bm | Upper abdominal pain, jaundice | Composite small- and intermediate cell neuroendocrine carcinoma and well-differentiated adenocarcinoma | NSE, Cytokeratin, EMA |
| 3 | 67 | M | Bm | Jaundice | Composite anaplastic small-cell carcinoma and moderately differentiated adenocarcinoma | EMA |
| 4 | 71 | M | Bm | Jaundice | Composite small-cell neuroendocrine carcinoma and adenocarcinoma | Grimelius, EMA, NSE, Leu-7, EGC |
| 5 | 75 | F | Bi | Jaundice | Composite small-cell neuroendocrine carcinoma and tubular adenocarcinoma | Grimelius, Chromogranin A |
| 6 | 64 | M | Bm | Jaundice, appetite loss, body weight loss | Composite small-cell neuroendocrine carcinoma and intestinal-type adenocarcinoma | AE1/AE3, CEA, CA19-9, CAM 5.2, NSE, Chromogranin A, Serotonin, Leu-7 |
| 7 | 75 | M | Bi | Jaundice | Small-cell neuroendocrine carcinoma | Keratin, EMA, NSE, Chromogranin A, CA19-9 |
| 8 | 65 | M | Bm | Jaundice | Composite small-cell neuroendocrine carcinoma and well-differentiated adenocarcinoma | EMA, Chromogranin A, Serotonin, Ki-67 |
| 9 | 65 | M | Bi | Jaundice | Small-cell neuroendocrine carcinoma | NSE, CA19-9 |
| 10 | 85 | F | Bi | Jaundice | Small-cell neuroendocrine carcinoma | NSE, Chromogranin A |
| 11 | 64 | M | Bm | Jaundice, right upper abdominal pain | Composite small-cell neuroendocrine carcinoma and well-differentiated adenocarcinoma | NSE, Chromogranin A, CEA |
| 12 | 75 | M | Bi | Jaundice | Small-cell neuroendocrine carcinoma | NSE, Chromogranin A, Synaptophysin, CD56, Leu-7, S-100, CEA, EMA, Cytokeratin 7, Cytokeratin 20, Ki-67, p16 |
| 13 | 60 | M | Bi | Jaundice | Intermediate-type small-cell neuroendocrine carcinoma | Keratin, NSE, Chromogranin A, Synaptophysin |
| 14 | 66 | F | Bm | Epigastralgia, back pain, body weight loss | Composite small-cell neuroendocrine carcinoma and well-differentiated papillary adenocarcinoma | Synaptophysin, Chromogranin A |
| 15 | 54 | F | Bi | Anorexia, pain in the right hypochondrium, jaundice | Composite small-cell neuroendocrine carcinoma and squamous cell carcinoma | Ki-67, NSE, Leu-7 |
| 16 | 57 | M | NA | NA | NA | NA |
| Our case | 69 | F | Bi | Jaundice | Small-cell neuroendocrine carcinoma | EMA, AE1/AE3, NSE, Chromogranin A, Synaptophysin, Grimelius |
Bi, lower portion of bile duct carcinoma; Bm, middle portion of bile duct carcinoma; CA19-9, carbohydrate antigen 19-9; CD56, cluster of differentiation 56; CEA, carcinoembryonic antigen; EGC, endocrine granule constituent; EMA, epithelial membrane antigen; NA, not applicable; NSE, neurone-specific enolase
According to a review of the 16 previously reported cases [1–6] and our case of small-cell carcinoma of the extrahepatic bile ducts, elder age, men, a distal site of the extrahepatic bile duct, all tend to be predominant clinicopathological features. Most of the cases showed a poor prognosis, as is the case with the small-cell carcinoma arising in the other organs. In addition, there is no significant difference in the clinicopathological findings, namely, age, sex, site of carcinoma and prognosis between the cases with or without squamous or glandular components. There were no specific features of underlying bile duct findings such as those seen in primary sclerosing cholangitis in reported cases, including our case.
The existence of a common precursor cell for endocrine cells and nonendocrine epithelial cells in the bile duct mucosa has been proposed regarding the histogenesis of small-cell carcinoma [9]. In composite tumors, two components may originate in a common precursor cell, and thereafter, the tumor simultaneously develops in two different directions [1]. CD34-positive multipotent adult progenitor cells have been known to be derived from adult bone marrow and then differentiate to hematopoietic lineage, in addition to the epithelium of the liver, lung and gut [10], thus suggesting that they also might be the origin of small-cell carcinoma. In the present case, there were the endocrine cells immunoreactive to neuroendocrine markers such as NSE, chromogranin A, and synaptophysin in the tumor cells, thus suggesting that the tumor cells might be derived from them. No precursor cells positive to CD34 were found in the bile duct epithelium in our case. Due to the limited number of reported cases, further studies are therefore needed to clarify the mechanisms underlying the histogenesis of small-cell carcinoma of the extrahepatic bile duct.
References
- 1.Nishihara K, Tsuneyoshi M, Niiyama H, et al. Composite glandular-endocrine cell carcinoma of the extrahepatic bile duct: immunohistochemical study. Pathology 1993;25:90–4 [DOI] [PubMed]
- 2.Sabanathan S, Hashimi H, Nicholson G, et al. Primary oat cell carcinoma of the common bile duct. JR Coll Surg Edinb 1988;33:285–6 [PubMed]
- 3.Van der Wal AC, Van Leeuwen DJ, Walford N. Small cell neuroendocrine (oat cell) tumour of the common bile duct. Histopathology 1990;16:398–400 [DOI] [PubMed]
- 4.Miyashita T, Konishi K, Noto M, et al. A case of small cell carcinoma of the common bile duct. Nippon Shokakibyou Gakkai Zasshi (Jpn J Gastroenterol) 2001;98:1195–8 (In Japanese) [PubMed]
- 5.Kuraoka K, Taniyam K, Fujitaka T, et al. Small cell carcinoma of the extrahepatic bile duct: case report and immunohistochemical analysis. Pathol Int 2003;53:887–91 [DOI] [PubMed]
- 6.Kaiho T, Tanaka T, Tsuchiya S, et al. A case of small cell carcinoma of the common bile duct. Hepatogastroenterology 2005;52:363–7 [PubMed]
- 7.Albores-Saavedra J, Molberg K, Henson DE. Unusual malignant epithelial tumors of the gallbladder. Semin Diagn Pathol 1996;13:326–38 [PubMed]
- 8.Nishihara K, Tsuneyoshi M. Small cell carcinoma of the gallbladder. A clinicopathological immunohistochemical and flow cytometrical study of 15 cases. Int J Oncol 1993;3:901–8 [DOI] [PubMed]
- 9.Fish DE, Al-lzzi M, George PP, et al. Combined endocrine cell carcinoma and adenocarcinoma of the gallbladder. Histopathology 1990;17:471–2 [DOI] [PubMed]
- 10.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–9 [DOI] [PubMed]