Abstract
Background
Radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) is a thermoablative technique to kill tumor tissue by generating areas of coagulative necrosis. Recent reports have raised concern that RFA may lead to a local recurrence of HCC with an aggressive phenotype and unfavorable prognosis, suggesting that RFA may induce further malignant transformation of HCC. However, the biological effects of RFA on HCC cells have not been directly analyzed. The aim of this study was to determine whether heat stress of the type associated with RFA induces malignant transformation of HCC.
Methods
We assessed the sensitivity of three HCC cell lines (HepG2, Alexander, and Huh7) to heat treatment for 10 min. We then determined the temperature at which a heat-resistant subline can be generated. We established and expanded sublines that survived heat treatment. And their proliferation rates, heat sensitivities, and invasive capacities were further examined.
Results
All HepG2 died after 48°C treatment, whereas 49°C treatment was required to kill all Alexander and HuH7. We generated 20 sublines for each parental cell line. A HepG2 subline, HepG2#18, proliferated 100% faster than parental HepG2. Moreover, HepG2#18 survived after 50°C treatment, whereas all parental HepG2 died after heat treatments at 48°C or higher.
Conclusion
Our results showed that even a single heat treatment could induce further transformation of an HCC cell line. Our results suggest that an insufficient treatment of HCC by RFA that enables survival of some cells might induce further malignant transformation in vivo.
Keywords: Hepatocellular Carcinoma (HCC), Radiofrequency Ablation (RFA), Malignant transformation
Introduction
Percutaneous image-guided therapy of liver tumors by local application of chemotherapeutic agents such as ethanol or by hyperthermia kills tumor tissue [1]. Radiofrequency ablation (RFA) is an electrosurgical technique using a high-frequency alternating current to heat tissues to the point of thermal coagulation within a short time [2, 3]. Due to the capacity for localized tumor necrosis, minimal damage to a functioning liver, and capacity for repeated treatments in cases of recurrence and/or new tumors, percutaneous image-guided tumor ablation has become an attractive option for the treatment of patients with HCC [2–5].
Although several clinical reports have recently described the pathological and histochemical characteristics induced by RFA in human liver tissues [6–9], how HCC cells die following RFA, or whether some cells survive is still poorly understood. Additionally, recent reports have raised a concern that RFA may predispose to a local recurrence that harbors an aggressive phenotype and unfavorable prognosis, suggesting that RFA may induce further malignant transformation of HCC [10–15].
We hypothesized that RFA may contribute to this additional malignant transformation. The aim of this study was to explore whether heat stress induces malignant transformation of HCC. Although there are reports describing the effect of hyperthermia on HCC in vitro, to our knowledge, there has been no report on the biological effects of brief heat treatments associated with RFA on tumor cells.
Methods
Cell culture
Three human hepatoma cell lines (HepG2, Alexander, and Huh7) were used in this study. These cell lines were maintained as monolayer cultures in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 × 104 U/l penicillin G, and 200 mg/l streptomycin sulfate. All of the cell lines were incubated at 37°C in a humidified incubator under 5% CO2 and 95% air.
Heat treatment
In all the experiments, cells were exposed to hyperthermic stress during the exponential phase in tissue culture plates. Approximately 1 × 103 cells were seeded into each well of the 96-well plates with 50 μl of DMEM. After 24 h of incubation, they were exposed to heat stress. Heat treatments were carried out by sealing the tops of culture plates with parafilm, and submerging the plates in a water bath set to the desired temperature for 10 min. Immediately after the hyperthermic treatment, 100 μl of fresh culture medium was added into each well and the cells were maintained in the incubator at 37°C.
Cell proliferation assay
At each time point, we determined the surviving cell number by Tetracolor One cell proliferation assay (Seikagaku Co., Tokyo, Japan). Tetracolor One assay solution contains 2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2, 4-disulfophenyl]-2 H-tetrazolium, a monosodium salt, which is converted to a formazan product through cleavage of the tetrazolium ring by the reduced form of nicotinamide adenine dinucleotide phosphate or reduced form of nicotinamide adenine dinucleotide. These reducing agents are produced by mitochondrial dehydrogenase enzymes in metabolically active cells. The formazan product can be directly measured using a spectrophotometer. After adding 50 μl of DMEM containing 10 μl of Tetracolor One assay solution to each well, the cells were incubated for 2 h at 37°C. Then optical density was measured at 450 nm using a spectrophotometer. Optimal density was converted into living cell number, using calibration curves obtained by preliminary experiments for each cell line.
Morphological observation and trypan blue exclusion test
Cells were observed under a phase contrast microscope and photographs were obtained. The trypan blue exclusion test was performed as follows. After aspirating the culture medium, 100 μl of 0.2% trypan blue solution was added gently, and 2 min later, trypan blue solution was removed before the observation.
Establishment of sublines
Temperatures for heat treatments for each HCC cells were determined on the basis of the data obtained from the cell proliferation assay. After the heat treatment of cells on 96-well plates, we continuously cultured cells. When cells in wells became confluent, we passaged them in the wells of a 6-well plate. Next, cells were passaged in a 10 cm dish, and after their passage twice in a 10 cm dish, we utilized these cells as a subline. One subline was generated from one heat treatment.
Comparison of proliferation rates
Proliferation rates were calculated by comparing cell number between 0 h and 72 h. We calculated the relative proliferation rates of sublines and compared them with the proliferation rates of their parental cell lines.
Comparison of heat sensitivities of parental HepG2 and HepG2 sublines
We selected 5 HepG2 sublines, and performed 10-min heat treatments at increasing temperatures. We compared surviving cell number among the five sublines 72 h after the heat treatments, as determined by Tetracolor One assay.
Invasion assay
To assess the invasive capacity of HepG2#18, we performed invasion assays using BD BioCoat Tumor Invasion System (BD Biosciences, MA), according to the manufacturer’s instructions. The % invasion of HepG2#18 was compared to that of parental HepG2.
Statistical analysis
All of the Tetracolor One assays were performed in octuplicate. Values on the graphs represent mean ± SD. Where indicated, data were subjected to the t-test and differences were determined to be significant when P-value was less than 0.05.
Results
Heat sensitivities differ among three HCC cell lines
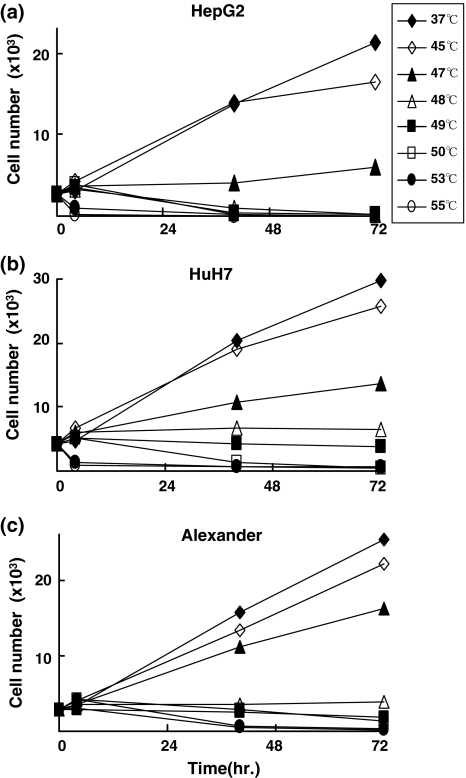
We determined the effects of 10-min heat treatment at each temperature on these three HCC cell lines. First we compared surviving cell numbers 40 and 72 h after heat treatments. The morphological effects of heat treatments and impact on cell proliferations were the same among the three HCC cell lines (Fig. 1a–c). There were at least three types of reactions of HCC cell lines after heat treatment. The first was continuous growth, which was observed after the heat treatments at relatively low temperatures (45°C and 46°C for HepG2 cells, 45–47°C for HuH7 and Alexander cells). The second type was survival for at least several hours after heat treatments, but later, cells stopped growing or died gradually. Some cells survived up to 72 h after the heat treatments, which occurred after the heat treatments at intermediate temperatures (47–50°C for HepG2 cells, 48–50°C for HuH7 cells, and 48–50°C for Alexander cells). The last type is death occurred within 40 h at relatively high temperatures (53°C and 55°C for all three cell lines). These data indicate that the reactions of cells to the short-time heat treatments are similar among these three cell lines, although the heat sensitivity of each cell line differs.
Fig. 1.
Time courses of cell numbers after heat treatment. Heat treatments were performed at temperatures from 37°C to 55°C. Cell numbers was assessed 0, 4, 40, and 72 h after the heat treatments
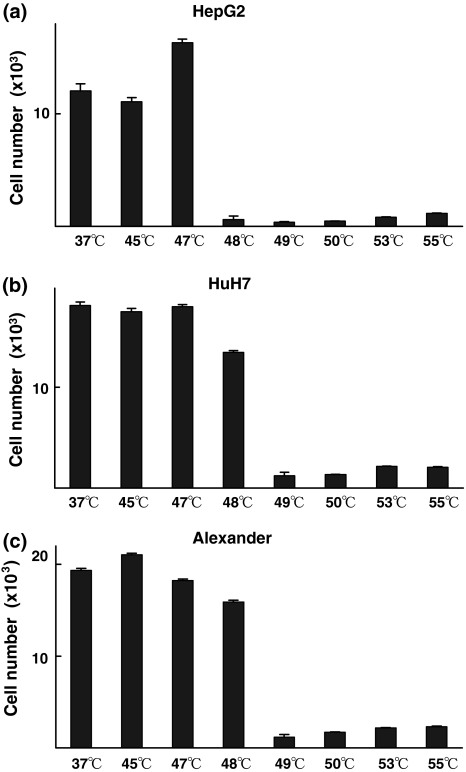
Cultures for 14 consecutive days after the heat treatments revealed that there was a clear threshold to achieve total cell killing: heat treatments between 47°C and 48°C for HepG2 cells, and between 48°C and 49°C for HuH7 and Alexander cells. These thresholds were observed at the intermediate temperatures (Fig. 2a–c).
Fig. 2.
Fourteen days of continuous culture after heat treatments revealed that there is a clear threshold at which to obtain total cell kill by a short-time heat treatment. Cells were cultured for 14 days after heat treatments, and Tetracolor One assay was then performed
Morphological changes do not predict the effect of heat treatment
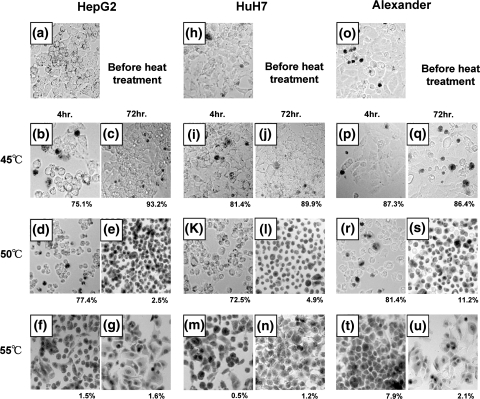
Next, we examined cell viability after heat treatments by trypan blue dye exclusion assay. The ratio of surviving and dead cells can be determined by this assay, because only the living cells can exclude trypan blue. By this method, we can functionally distinguish surviving cells from dead ones. Results of this assay confirm those of the Tetracolor One assay, by which cell viability is determined from enzymatic activity. At the same time, we examined morphological changes in cells after the heat treatments. The percentages of viable cells (Fig. 3) were almost consistent with those obtained by Tetracolor One assays (Fig. 1). This result was confirmed in three independent experiments.
Fig. 3.
Evaluation of effects of heat treatments on HCC cells by Trypan blue dye exclusion test. The ratios of alive cell numbers in total HCC cells were calculated. (a) HepG2 before heat treatment. (b, c) HepG2 4 and 72 h after 45°C treatment. (d, e) HepG2 4 and 72 h after 50°C treatment. (f, g) HepG2 4 and 72 h after 55°C treatment. (h) HuH7 before heat treatment. (i, j) HuH7 4 and 72 h after 45°C treatment. (k, l) HuH7 4 and 72 h after 50°C treatment. (m, n) HuH7 4 and 72 h after 55°C treatment. (o) Alexander before heat treatment. (p, q) Alexander 4 and 72 h after 45°C treatment. (r, s) Alexander 4 and 72 h after 50°C treatment. (t, u) Alexander 4 and 72 h after 55°C treatment
Interestingly, there were obvious differences in cell morphology among the three temperature treatment groups. In the first group, the shape of the cells did not change appreciably (Fig. 3b, c, i, j, p, q). In the second group, cells became rounded immediately after the heat treatments, but most of the cells remained viable 4 h after treatments (Fig. 3d, e, k, l, r, s). On the other hand, the viability of cells 72 h after the heat treatments varied in this group. The threshold temperature to survive heat treatment was included in this group. Finally in the third group, the cells died immediately after the heat treatments, and the morphology was not obviously different from that observed in the first group (Fig. 3f, g, m, n, t, u).
There were at least two different types of cell death induced by this short-time heat treatment. Additionally, in the second group, we were not able to identify the threshold temperature for survival on the basis of morphology, which was obviously observed 14 days after the heat treatments (Fig. 2).
A single heat treatment can induce transformation of HCC cells
We next examined whether heat treatment can affect the properties of cells. From previous experiments, we determined the temperatures of heat treatment for the HCC cell lines, at which HCC cells do not grow for about 3 days but can survive. The temperatures were 47°C for HepG2, and 48°C for HuH7 and Alexander. We chose these temperatures to maximize the effect of a single heat treatment. After the heat treatments of cells on 96-well plates, the cells were continuously cultured and expanded. Even in this condition, all the treated cells died in 10–30% of experiments. We generated 20 sublines from each HCC cell line. Each subline survived a single heat treatment.
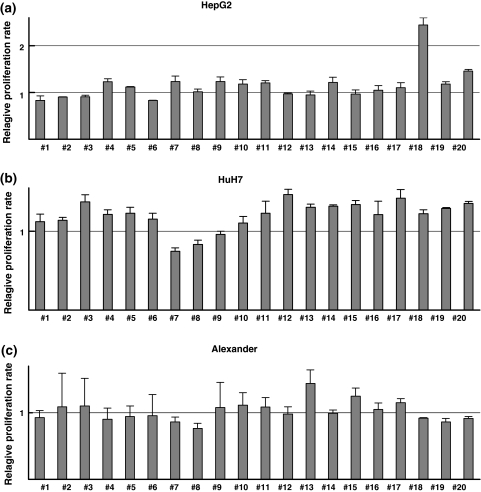
Using these sublines, we compared proliferation rates calculated from the data obtained by Tetracolor One assay. Among the 60 sublines, one of HepG2 sublines, HepG2#18, showed a more than 100% increase in proliferation rate compared with parental HepG2 cells (Fig. 4a). Other sublines showed significant increases or decreases in proliferation rate; however, the differences among them were much smaller than HepG2#18, as shown in Fig. 4. We confirmed this result in seven independent experiments.
Fig. 4.
Proliferation rates of sublines derived from (a) HepG2, (b) HuH7, and (c) Alexander. Twenty sublines were established from each of the three HCC cell lines. Proliferation rate during 3 days of culture was calculated. Relative proliferation rates of the sublines were compared with those of the parental cell lines. A HepG2 subline, HepG2#18, proliferated more than 100% faster than parental HepG2
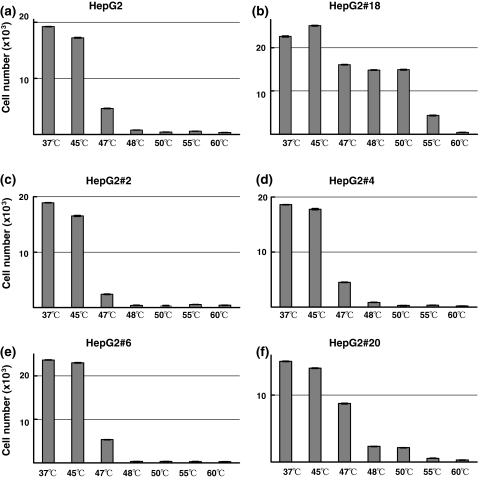
Additionally, we selected five sublines including HepG2#18, and performed heat treatments to examine whether these sublines acquired heat resistance. HepG2#18 survived 50°C treatment, whereas all parental HepG2 cells and other sublines died after heat treatments at 48°C or higher. The subline HepG2#18 showed marked heat tolerance compared with parental cells and four other sublines (Fig. 5).
Fig. 5.
Heat sensitivities of HepG2 and sublines derived from HepG2. The heat sensitivities of five HepG2 sublines were compared with that of parental HepG2. Surviving cell number was assessed 72 h after heat treatments at increasing temperature. HepG2#18 obviously showed heat resistance
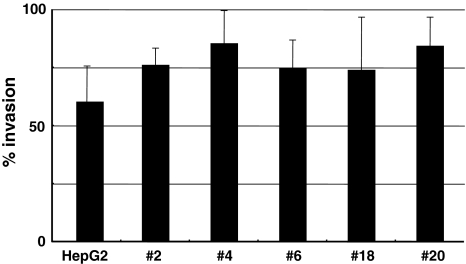
On the other hand, there were no obvious differences in invasive capacities in vitro between parental HepG2 and sublines (Fig. 6).
Fig. 6.
Invasive capacities of HepG2 and sublines derived from HepG2. The invasive capacities of sublines were compared with that of parental HepG2. There were no significant differences between HepG2 and the sublines
Discussion
Because most HCC patients are suffering from chronic liver disease, the treatment strategy for HCC must consider not only the tumor itself but also the patients’ liver function [1, 5]. As a therapeutic method for HCC, it is essential that RFA can induce tumor necrosis, and RFA is beneficial because it causes minimal damage to non-neoplastic liver tissue, and is easily repeated [5]. RFA is now an important option among the treatment strategies against HCC. On the other hand, local recurrences with rapid progression of HCC after RFA have been reported [10–15]. Patients with this type of recurrence usually have an unfavorable prognosis. Ruzzenente et al. reported this type of recurrence in about 4% of patients [15]. Some studies reported that it is accompanied by pathological changes, which is so called ‘sarcomatous change.’ Although, this pathological change is not always proven. Kojiro et al, have suggested that malignant transformation is associated with therapy used to treat HCC [16]. However, this association has not been scientifically proven, probably because experimental replication of the microenvironment of each therapeutic method is generally quite difficult in culture. Therefore, it remains unclear whether the type of therapy is directly involved in the malignant transformation of HCC cells.
We focused on RFA because it is not only an important therapeutic method, but it is also the easiest strategy in which to replicate the microenvironment in culture. Although there are many studies of the hyperthermic effect on cancer cell lines [17–19], all were carried out to define the impact of hyperthermia on cancers but not the impact of sublethal hyperthermia on surrounding cells, thereby failing to replicate the effect of RFA. As a result, the temperatures are lower and the durations of heat treatments are longer than those used in our experiments. Clinical reports and our data indicate that morphological changes in cells after heat treatments do not reflect cell viability and the potential of cells to recover [9, 20].
Cell death with negligible morphological changes, which we observed after heat treatments at 55°C, showed features typical of those following RFA [9, 20]. This cell death is characterized by the loss of enzymatic activity and the preservation of cell shape and the nucleus. We confirmed cell death biochemically by TetracolorOne assay and morphologically by trypan blue dye exclusion assay. There are clinical reports in which the pathological changes after RFA are described, and several reports in the literature describe different forms of cell death occurring after RFA [7–9]. However, the specific conditions under which those forms of cell death occur, as well as the mechanisms underlying cell death, remain poorly defined. From this point of view, we attempted to simulate at least one key aspect of an in vivo effect of RFA on cultured cells. And our experimental protocol of 10 min of heat treatment is relevant for examining the heat effect of RFA.
Our data showed differences in heat sensitivity among three HCC cell lines. It is consistent with a study describing differences in heat sensitivity of tumors and tissues in animal experiments [21]. Our finding suggests that the temperature required to kill all cells by RFA differs depending on the HCC nodule. As shown in Results, there are three groups of temperature responses, each of which induces a different response in HCC cells. After heat treatment of the second group of temperatures, cells stopped growing for at least about 3 days and died thereafter. Although we were able to obtain total cell kill in this temperature treatment group, morphological changes in the cells among those cells capable of reproliferation were the same as those of dying cells. After the heat treatment of the third temperature treatment group, cells died immediately after the heat treatment, and no cells survived. The characteristic of this type of cell death is the negligible morphological change with the loss of enzymatic activity. Our results suggest that in clinical settings, we should aim to obtain this type of death of all HCC cells by RFA.
Because all of the cell lines we used were established many years ago (HepG2 [22], Alexander [23], HuH7 [24]), further spontaneous transformation under normal culture conditions would be unlikely. Therefore, we concluded that the transformation of the subline HepG2#18 is due to the exposure to heat stress. In this study, we used three HCC cell lines and we generated 20 sublines from each cell line. Among the total of 60 sublines, only one subline displayed transformation. The probability is 5% for HepG2 cells, and 1.7% for the three cell lines. On the other hand, according to the data reported by Ruzzenente et al. [15], the frequency of the local recurrence with rapid growth is about 24% of patients, who developed local recurrences. There may be two possibilities for this discrepancy. One is that the data of Ruzzenente et al. do not reflect the average of the actual frequency observed in clinical situation. Since no other clinical studies have been published, the frequency of this type of recurrence remains to be elucidated. The other possibility relates to the mechanism of this transformation. Although we still do not know what is the mechanism of the transformation occurred in HepG2#18, we speculate that it might be related to genetic instability and/or epigenetic DNA alterations. As we mentioned above, the three HCC cell lines, which we used in our experiments established long time ago. So, it is possible that those cell lines may be genetically and/or epigenetically stable, more than HCC cells in clinical cases.
An HCC cell line can acquire heat resistance after hyperthermic treatment [25]. However, interestingly, in our experiment, rapid proliferation is also observed after a single heat treatment. On the other hand, the invasiveness of the subline HepG2#18 appeared to be the same as that of parental HepG2 and four other sublines (Fig. 6). This finding suggests the existence of factor(s) that regulate both heat resistance and rapid proliferation, which remains to be identified.
Although the precise mechanism underlying this transformation and the minimal temperature at which the same results are obtained are still unclear, our data support the idea that the physical energy derived from heat treatment can actually affect the clinical course of HCC. Our data do not diminish the importance of RFA; however, these suggest that we should aim total cell kill in one session of RFA for HCC. More importantly, there are reports showing that the initial response to percutaneous ablation predicts survival in patients with HCC [26, 27]. Our results provide an experimental basis for these clinical reports.
In conclusion, our results showed that even a single session of heat treatment could induce further transformation of an HCC cell line. While not studied here, our data would suggest that insufficient or sublethal treatment of HCC by RFA might induce further malignant transformation and lead to an unfavorable prognosis.
References
- 1.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002;122:1609–19. [DOI] [PubMed]
- 2.Buscarini L, Buscarini E. Therapy of HCC-radiofrequency ablation. Hepatogastroenterology 2001;48:15–9. [PubMed]
- 3.Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound 2001;13:159–66. [DOI] [PubMed]
- 4.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 2006;23:1535–47. [DOI] [PubMed]
- 5.Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol 2006;22:248–53. [DOI] [PubMed]
- 6.Scudamore CH, Lee SI, Patterson EJ, Buczkowski AK, July LV, Chung SW, et al. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg 1999;177:411–7. [DOI] [PubMed]
- 7.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000;88:2452–63. [DOI] [PubMed]
- 8.Itoh T, Orba Y, Takei H, Ishida Y, Saitoh M, Nakamura H, et al. Immunohistochemical detection of hepatocellular carcinoma in the setting of ongoing necrosis after radiofrequency ablation. Mod Pathol 2002;15:110–5. [DOI] [PubMed]
- 9.Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology 2002;35:1467–75. [DOI] [PubMed]
- 10.Nicoli N, Casaril A, Marchiori L, Mangiante G, Hasheminia AR. Treatment of recurrent hepatocellular carcinoma by radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg 2001;8:417–21. [DOI] [PubMed]
- 11.Seki T, Tamai T, Ikeda K, Imamura M, Nishimura A, Yamashiki N, et al. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol 2001;13:291–4. [DOI] [PubMed]
- 12.Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res 2003;27:163–7. [DOI] [PubMed]
- 13.Portolani N, Tiberio GA, Ronconi M, Coniglio A, Ghidoni S, Gaverini G, et al. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology 2003;50:2179–84. [PubMed]
- 14.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol 2003;8:332–5. [DOI] [PubMed]
- 15.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, et al. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol 2004;10:1137–40. [DOI] [PMC free article] [PubMed]
- 16.Kojiro M, Sugihara S, Kakizoe S, Nakashima O, Kiyomatsu K. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol 1989;23(Suppl):S4–8. [DOI] [PubMed]
- 17.van Rijn J, van den Berg J, Schamhart DH, van Wijk R. Morphological response and survival of hepatoma cells during fractionated hyperthermia: effect of glycerol. Radiat Res 1984;98:471–8. [DOI] [PubMed]
- 18.Schamhart DH, van Walraven HS, Wiegant FA, Linnemans WA, van Rijn J, van den Berg J, et al. Thermotolerance in cultured hepatoma cells: cell viability, cell morphology, protein synthesis, and heat-shock proteins. Radiat Res 1984;98:82–95. [DOI] [PubMed]
- 19.Wiegant FA, van Bergen en Henegouwen PM, van Dongen G, Linnemans WA. Stress-induced thermotolerance of the cytoskeleton of mouse neuroblastoma N2A cells and rat Reuber H35 hepatoma cells. Cancer Res 1987;47:1674–80. [PubMed]
- 20.Coad JE, Kosari K, Humar A, Sielaff TD. Radiofrequency ablation causes ‘thermal fixation’ of hepatocellular carcinoma: a post-liver transplant histopathologic study. Clin Transplant 2003;17:377–84. [DOI] [PubMed]
- 21.Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol 2007;18:647–54. [DOI] [PubMed]
- 22.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980;209:497–9. [DOI] [PubMed]
- 23.Alexander JJ, Bey EM, Geddes EW, Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J 1976;50:2124–8. [PubMed]
- 24.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 1982;42:3858–63. [PubMed]
- 25.Van Rijn J, Van den Berg J, Souren JE, Van Wijk R, Joenje H. Hepatoma cells adapted to proliferate under normally lethal hyperthermic stress conditions show rapid decay of thermoresistance and heat shock protein synthesis when returned to 37 degrees C. Int J Hyperthermia 1995;11:697–708. [DOI] [PubMed]
- 26.Sala M, Llovet JM, Vilana R, Bianchi L, Sole M, Ayuso C, et al. Barcelona Clinical Cancer Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004;40:1352–60. [DOI] [PubMed]
- 27.Morimoto M, Numata K, Sugimori K, Shirato K, Kokawa A, Oka H, et al. Successful initial ablation therapy contributes to survival in patients with hepatocellular carcinoma. World J Gastroenterol 2007;13:1003–9. [DOI] [PMC free article] [PubMed]