Abstract
Chronic hepatitis B constitutes a significant health and economic burden to Asian countries. Six medications are now approved for the treatment of chronic hepatitis B, but there is still significant uncertainty with regards to treatment outcomes, cost impact, and benefits in view of the absence of long-term outcomes data. Cost-effectiveness Markov modeling thus allows us to project and estimate long-term outcomes based on current data and compare the cost–benefit between different treatment options. However, there are limitations to these reported studies. Cost-utility indices such as cost/quality–adjusted life years (cost/QALY) may not be intuitive to clinicians and patients. These studies are also usually based on first-world economies, using a benchmark of US$50,000/QALY, and cannot be extrapolated directly to Asia-Pacific countries. Cost-effectiveness of various treatment strategies using a combination of cost-effectiveness indices may provide a more complete picture. These include cost/HBeAg seroconversion for HBeAg-positive patients (range: US$19,400–30,800) and cost/HBV DNA negative (PCR assay) for HBeAg-negative patients (range: US$14,400–32,000) over 5-year time horizon; cost per cirrhosis prevented (range: US$326,000–686,000) and cost per HCC prevented (range: US$654,000–1,380,000) over 10-year horizon using data from REVEAL study, cost per end point complication prevented in cirrhotics (US$9,630/year), and cost per HCC prevented in cirrhotics (US$ 27,600/year) over a 32-month horizon, using data from Asia Lamivudine Cirrhosis Study. More potent antivirals with low resistance appear to have lower cost/clinical end point averted. Published reports of cost-utility analysis comparing treatment using conventional cost/QALY show that all treatment modalities fall below the first-world benchmark of US$50,000/QALY but vary in modeling assumptions and in quality, making comparisons difficult. Reimbursement policies affect out-of-pocket expenses to the patient, and increases the proportion of patients who can afford therapy, but generally do not affect cost-effectiveness. In conclusion, cost-effectiveness analysis is an important tool for health care administrators, clinicians, and patients to decide on the optimal therapy for chronic hepatitis B, but the methodology permits considerable leeway for interpretation of results, thus a combination of cost-effective indices may be needed to paint a more complete picture.
Keywords: Hepatitis B treatment, Cost-effectiveness analysis
Introduction
Hepatitis B is the 10th leading cause of death worldwide with up to 1.2 million deaths per year. An estimated 350 million people in the world are chronically infected with chronic hepatitis B (CHB) and more than 75% of these CHB come from endemic areas in the Asia-Pacific region [1, 2]. Of these chronic carriers, approximately 15–40% of them will go on to develop cirrhosis, liver failure, or hepatocellular carcinoma (HCC) [3].
This constitutes a significant health and economic burden to Asian countries where prevalence of CHB can be as high as 20% [2]. Mortality and morbidity of the disease with the attendant loss of economically productive life years, cost of treatment for established complications all add up to significant cost for the individual and society. In high endemic countries like South Korea, a study in 1997 estimated that the total annual societal cost was US$957 million with direct cost of US$696 million being equivalent to 3.2% of the national health care expenditure for 1997 [4].
Six medications have now been licensed by the Food and Drug Agency, USA for the treatment of CHB and have shown efficacy in viral suppression, reducing elevated transaminases, improving histology, as well as improving outcomes in decompensated liver diseases [5]. These include lamivudine, adefovir, entecavir, telbivudine, regular interferon, and pegylated interferon. However, many of these drugs may not be affordable to the average CHB patients, especially in developing Asian countries. The relative potency, resistance profile, as well as their costs also varies significantly. These differences are suspected to result in differences in outcome in the long term, but there is no such data currently. Consequently, there still remain significant uncertainties with regards to treatment outcomes, cost impact, and benefits. How does treatment of CHB alter the costs of the disease burden on the country? Would treatment results in eventual savings from the disease complications that would be avoided? What would be the most cost-effective medication or strategy? Yet, there are hardly any long-term actual prospective studies that provide direct answers. Short of actual long-term evidence-based data, cost-analysis studies using models that attempt to predict the cost and outcome of various treatments, gives us the best available information to understand this complex issue of cost-effectiveness of treating CHB.
Effectiveness of treating chronic hepatitis B
Clinicians are well aware of the costs of treatment particularly in countries where there is no reimbursement policy and all expenses are borne by the patient. In prescribing antiviral therapies, are clinicians aware of the effectiveness of their choice? This would be easy if there were clinical trials that demonstrated clearly that treatment reduced complications and improved survival. There is only one randomized controlled study to have demonstrated a reduction in complications, the Asian lamivudine cirrhotic study [6], but the entry criteria makes this study only applicable to cirrhotics, a much smaller subpopulation of chronic hepatitis B patients. The majority of treated patients have low or minimal fibrosis but have liver inflammation. To perform a study in such patients involving real clinical end points would not be practical due to the long natural history of the disease and large study size required. Early in HIV therapy, HIV viral load was validated as a good surrogate end point that had a strong correlation with disease progression as HIV is a characteristically progressive disease if untreated [7]. Chronic hepatitis B, however, has many different clinical phases, sometimes with remitting and relapsing patterns [5] that make surrogate end points difficult to validate. A one-time measurement of viral load is only partly useful as shown by the REVEAL study [8, 9], where patients with an initial high HBV DNA that then fell to low levels showed a decrease in relative risk of HCC. The only clear surrogate end point of therapy is HBsAg seroconversion, but this is such a remote possibility during therapy that it can be discounted. HBeAg seroconversion, on the other hand, is certainly achievable, and is cumulative, making long-term antiviral therapy more attractive. Cohort studies have shown that patients who undergo HBeAg seroconversion do have subsequent benefits with regards to reduction in development of cirrhosis and HCC as well as survival, but these studies have been usually with limited duration interferon therapy [10, 11]. Another attractive reason for this end point is that treatment can be stopped in the majority of patients, permitting calculation of finite costs related to therapy.
What is cost-effectiveness?
In simple terms, cost-effectiveness analyzes how much health benefit do we get for the dollar. One way of examining this is to account the costs of paying now for hepatitis B treatment as well as the savings from avoiding complications of liver disease (hospitalizations and treatment) and express these in terms of cost per clinical end point avoided. By comparing different strategies, we can estimate the relative efficacy in preventing cirrhosis, delaying decompensation, reducing HCC and transplant, and determine which strategy gives us the best value for money.
In the absence of real long-term data of treatment impact on outcomes, cost-effectiveness studies have used modeling to overcome this problem and provide estimates of such cost measures. The classic model is that of Markov modeling [12], a complex analysis which provides for all the complications and rescue strategies cycled each successive year with incremental costs for each strategy thus allowing for the costs of different strategies to be compared. At each year cycle, each individual can be channelled to an exclusive disease state such as inactive carrier, active CHB, compensated cirrhosis, decompensated cirrhosis, and death using a predefined transition risk probability. All patients in the cohort are cycled repeatedly until the end of lifespan to capture all hepatitis B-related events. At the end of the lifespan, the cost of treatment associated with each disease state and the benefit with each cycle is accumulated. This is extremely useful as this allows us to take into account resistance rate, incidence of complications, the cost of treating each complication, and the liver deaths avoided. Consequently, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) has set guidelines for cost-effectiveness analyses (CEA) and reporting [13].
ISPOR have suggested that cost-effectiveness analysis should be reported as cost-utility studies using quality-adjusted life year (QALY) saved, and the incremental cost-effective ratio (ICER), a measure of cost-effectiveness between strategies. The problem with these measures are that while they are extremely useful as a standardized index for comparison from health policy point of view, they are rather conceptual, nonintuitive, and of less direct relevance to clinicians and patients trying to understand the cost–benefit of each strategy. In addition, the lack of long-term data also makes life cycle projection difficult, as interpretation is dependant on the assumptions built into the model and is open to error and manipulation.
Cost-effectiveness indices such as cost per clinical end point averted are more intuitive and can provide clearer estimates of cost of risk reduction linked to specific complications such as HCC or cirrhosis, a concept easily understood by clinicians. The problem with this is that such data are usually extracted from clinical trials where the defined risk reduction is known precisely only for the defined duration and conditions of the trial. Although both the regular interferon cohort studies [10] and the Asian Lamivudine Cirrhotic study [6] have reported improvements in clinical outcomes in treated patients, the lack of similar data with other treatment modalities makes it difficult to compare relative efficacies. For the purpose of comparison, we have to extrapolate from the next best risk estimate that of cohort studies such as REVEAL [8, 9]. Such “cost per complication averted” indices have an advantage over classical cost-utility indices as they are based on actual reported data. However, reporting of cost-effectiveness of achieving a surrogate end point, or of preventing a complication of liver disease, only account for direct costs and do not factor in indirect costs, and only factors in fixed short-term effects based on clinical trial data, unlike Markov modeling which factors in life-cycle effects.
While an analysis of CHB cost-effectiveness within the defined limitations of reported studies and extending them in lifelong cost-utility models is critical to understanding the full cost dynamics of various treatment strategies, we believe that a variety of methods (Markov modeling, surrogate end points, and preventing liver complications) to report cost-effectiveness provide a more complete picture.
Antiviral treatment strategies
Which is the best treatment strategy for CHB? We could choose a strategy that is the most effective, such as combination therapy from the start which would result in maintained viral suppression in the long term, but this would likely be expensive and also a strategy that is unlikely to be realistic as a practical or practicing strategy for most clinicians. The most common strategies are the use of monotherapy with lamivudine followed by addition of adefovir with the development of resistance [14]. Adefovir monotherapy with addition of lamivudine is less common with little data to support such a strategy but could be modeled based on anticipated resistance rates and type of resistant mutations, all of which could theoretically be rescued with the addition of lamivudine. Entecavir monotherapy appears to be an alternative strategy since it was licensed and its appeal is its reported low resistance rates [15]. Rescue is theoretical as there are very few cases of entecavir resistance in naïve patients, and little experience with rescue therapy, but again could be modeled based on anticipated resistance rates and types of mutations. For pegylated interferon, treatment is of fixed duration hence the fixed costs are easy to calculate but the benefits in the long term are less clear. In addition, many of these patients are quite likely to need rescue with oral antiviral therapy after completing their course of pegylated interferon therapy, and this needs to be factored into a therapeutic strategy. Based on these scenarios, we can then work out the costs of achieving surrogate end points such as HBeAg seroconversion and undetectable HBV DNA (PCR) comparing the cost to society as well as from patients’ perspective. Since most studies of therapy have not gone beyond 5 years, we used this as short-term time frame to determine the cost-effectiveness of various treatment strategies. For those with HBeAg-negative disease, the only possible end point after 5 years is persistent viral suppression, as HBsAg seroconversion is such a remote possibility as discussed previously. This end point mainly evaluates strategies that reduce viral resistance, and is a primer for long-term therapy. While debate over duration of antiviral therapy is still ongoing, matters may be taken out of the hands of clinicians. Patients who started antiviral therapy after lamivudine was licensed have now had 7–8 years of therapy, many of whom have had rescue therapy with adefovir either as sequential therapy or in combination, but stopping therapy in many such patients are associated with viral rebound, and even ALT flares, leaving clinicians no choice but to continue treatment.
Similarly, for the clinical end points of HCC and cirrhosis, using the lamivudine cirrhosis study [6] (3-year time frame) and REVEAL [8, 9] (10-year time frame), we can work out the number needed to treat and costs of preventing HCC, cirrhosis, and clinical end points. Then looking at reported cost-utility analysis, we can then have a thorough understanding of the full life cycle cost-effectiveness in terms of cost per QALY saved.
Proposed cost-effectiveness of treating CHB to achieve surrogate end points
The cost of various drugs and subsidy policies varies significantly between countries and impact on the total costs to the society, health payer as well to the individual patient. To have an idea of the relative cost of CHB treatment in Asian countries with respect to the surrogate end points [HBeAg seroconversion for HBeAg-positive patients, undetectable HBV DNA (PCR) for HBeAg-negative patients], as well as reduction in incidence of cirrhosis and hepatocellular carcinoma, we built a Markov model [16] comparing the following strategies: lamivudine followed by lamivudine + adefovir combination rescue (LMV/ADV), adefovir followed by adefovir + lamivudine combination rescue (ADV/LMV), entecavir monotherapy (ETV), and pegylated interferon treatment (PEG). Costs, including additional cost incurred for progression to cirrhosis and death, durable seroconversion, resistance, and disease progression rates, were obtained from several surveyed Asia-Pacific countries (Table 1) and took into account the cost of drug as well as reimbursement policies. We assume in these models that the cost of various drugs remained the same over the time horizon that we analyzed in our models. For long-term cirrhosis and HCC prevention, we assume that HBV DNA level is correlated linearly with risk of cirrhosis and HCC and that undetectable HBV DNA (PCR) provide the best protection. We also assume that incidence of efficacy and resistance follows expected trends based on observation and this allows for mathematical projection. Pegylated interferon is assumed to be superior and no worse than regular interferon.
Table 1.
Estimated annual cost of drugs, reimbursement policies and cost of treatment in Asian countries in USDa
| LMV | ADV | ETV | PEG | TBV | Public reimbursement policy | Cirrhosis costa | HCC costa | Liver transplanta | |
|---|---|---|---|---|---|---|---|---|---|
| South Korea | 1314 | 3285 | 2847 | 9,600 | 50% LMV (lifelong), ETV (1 year) | 1419 | 3,044 | 67,155 | |
| PEG (0.5 year HBe+ , 1 year HBe−) | |||||||||
| ADV or ETV for LAM-R (50% 2.5 years) | |||||||||
| Malaysia | 1044 | 1460 | 2084 | 10,992 | No | ||||
| Singapore | 1570 | 1971 | 2738 | 13,440 | 2299 | LMV (50% lifelong), ADV (50% lifelong) | 8794 | 7,036 | 49,353 |
| Indonesia | 1935 | 2168 | 2398 | 11,040 | No | ||||
| Thailand | 913 | 1898 | 2774 | 16,464 | 1898 | LMV 100%, others only on application | |||
| Bangladesh | 183 | 183 | 365 | 14,400 | No | ||||
| Taiwan | 1095 | 2190 | 2665 | 5,760 | LMV (100% 1.5 years) | 1560 | 1,690 | 2,779 | |
| PEG (100% 0.5 year for HBeAg pos, 1 year for HBeAg neg) | |||||||||
| LMV-R, ADV switch only 2 years | |||||||||
| Hong Kong | 1278 | 3431 | 3869 | 11,280 | LMV and ADV for LMV-R | 7490 | 15,618 | 65,961 | |
| China | 704 | 803 | 1935 | 7,968 | 1168 | Variable in different provinces | 1702 | 4740 | NA |
| India | 76 | 178 | 1707 | No reimbursement. LMV and ADV are generics | |||||
| Philippines | 2058 | 2306 | 2555 | 13,713 | 1324 | No | |||
| Australia | 1618 | 6775 | 4162 | 14,627 | Co-payment USD28 ADV switch for LAM-R | ||||
| Japan | 2097 | 4220 | 3474 | – | – | 70% for Peg IFN 0.5 year and lifelong for oral antiviral. LAM/ADV for LAM-R |
HBeAg-positive CHB
Using HBeAg seroconversion as a treatment target end point [5] in a time horizon of 5 years, we calculated the cost per HBeAg seroconversion event for each of the therapeutic strategies discussed previously (Table 2). LMV/ADV was the cheapest strategy from patients’ perspective in countries where lamivudine and adefovir were fully reimbursed, or in countries where generic options were available (Table 1). PEG (given for 48 weeks) was the most expensive with average total cost of US$13,440 over 5 years. Over 5 years, ETV and LMV/ADV were in the same ballpark range of median US$20,000 per seroconversion. This is not surprising given their relatively similar efficacy in seroconversion, and the benefit of cheaper lamivudine in the early years is offset by the more expensive rescue with combination therapy over time. We need to keep in mind that although 70% of patients in LMV/ADV arm would be lamivudine resistant and 29% in ADV arm would be adefovir resistant by year 5, the efficacy parameters would be similar to those of ETV, assuming that rescue therapy is 100% effective. Consequently, in the cost-effectiveness for HBeAg seroconversion, we factored the cumulative resistance and assumed that all patients with resistance would have addition of therapy as rescue. On the other hand, the HBV DNA suppression profile during treatment may be very different across the various treatment arms and the significance of these differences in the long term is not known. This highlights the importance of factors such as time horizon and relevant outcome measures when comparing cost of different treatment options.
Table 2.
Cost analysis of treating HBeAg-positive patients up to 5 years using HBeAg seroconversion as an outcome measure
| Cost/dose (USD)a | Cost year 1 (USD) | Cost year 5 (USD) | Year 1 SCb | Year 5 durable SCc | Cost/SC Year 5 | |
|---|---|---|---|---|---|---|
| No treatment | 0 | 0 | 0 | 6 | 30 | 0 |
| LMV/ADV | 4.30/5.40 | 1,115 | 10,500 | 17 | 54 | 19,400 |
| ADV/LMV | 5.40 | 1,932 | 10,200 | 12 | 44 | 23,300 |
| ETV | 7.50 | 2,408 | 11,700 | 21 | 58 | 20,400 |
| PEG | 280 | 13,440 | 13,440 | 27 | 55 | 24,436 |
Note: Lamivudine had the lowest initial outlay at US$1115/year, but became more expensive than adefovir monotherapy at 5 years due to resistant patients requiring combination therapy. LMV/ADV, ADV/LMV, and ETV had comparable cost per seroconversion. Although entecavir is the most expensive oral antiviral, it is notable that by 5 years 70% of patients on lamivudine would be resistant and would need rescue with adefovir, and similarly 29% in the adefovir group would be adefovir resistant and need rescue with lamivudine, hence contributing to additional costs. LMV = lamivudine, ADV = adefovir, ETV = entecavir, PEG = pegylated interferon, SC = HBeAg seroconversion, USD = United States dollars, ICER = incremental cost-effectiveness ratio
aEstimated from average retail cost 2007 and converted to USD
bYear 1 seroconversion rates [31–35]
cYear 5 durable seroconversion rates. Estimated for entecavir based on projections using 96 weeks data and assumed not worse than lamivudine. Estimated for pegylated-interferon. Durability estimated as 70% for lamivudine, 91% for adefovir, 82% for entecavir and 95% for PEG-interferon
HBeAg-negative CHB
HBeAg-negative CHB usually requires lifelong therapy [5]. We made the same assumptions as in the treatment of HBeAg-positive CHB, but this time using undetectable HBV DNA at 5 years as the end point (Table 3). In our analysis using a 5-year horizon, and undetectable HBV DNA (PCR) as a surrogate end point, entecavir had the lowest cost/undetectable HBV DNA (PCR) (median US$14,400) compared to US$25,600 and US$20,500 for adefovir and LMV/ADV, respectively. Despite its more expensive cost per dose of treatment, ETV appeared to have the lowest cost/undetectable HBV DNA (PCR) due to its higher proportion of patients who achieved a negative PCR than the other therapeutic strategies. LMV/ADV and ADV/LMV are seemingly less potent with lower proportion of patients achieving undetectable HBV DNA (PCR assay). This, together with the added cost of combination therapy when resistance develops, results in a higher cost/undetectable HBV DNA (PCR). Pegylated interferon, despite using an extremely generous estimate of 35% undetectable HBV DNA at 5 years, had the highest cost/undetectable HBV DNA (PCR) at US$38,200. What is not known, however, is whether undetectable HBV DNA by PCR assays is the ideal aim of treatment or whether a threshold of <10 copies/ml [4] would be sufficient in significantly reducing end point complication as suggested by the REVEAL studies [8, 9].
Table 3.
Cost analysis of treating HBeAg negative patients up to 5 years using undetectable HBV DNA (PCR assay) as an outcome measure
| Cost/dose (USD)a | Cost year 1 (USD) | Cost year 5 (USD) | Year 1 DNAb Neg (PCR) | Year 5 DNAc Neg (PCR) | Cost/DNA Neg (PCR) Year 5 | |
|---|---|---|---|---|---|---|
| No treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| LMV/ADV | 4.30/5.40 | 1,115 | 13,794 | 72 | 67 | 20,500 |
| ADV/LMV | 5.40 | 1,932 | 13,580 | 51 | 53 | 25,600 |
| ETV | 7.50 | 2,408 | 13,686 | 90 | 95 | 14,400 |
| PEG | 280 | 13,400 | 13,400 | 19 | 35 | 38,200 |
Note: Entecavir being the most potent antiviral, is the cheapest drug per patient with undetectable HBV DNA (PCR). The proportion of patients achieving HBV DNA negativity (PCR assay) is in part related to the potency of the drug. LMV = lamivudine, ADV = adefovir, ETV = entecavir, PEG = pegylated interferon, SC = HBeAg seroconversion, USD = United States dollars, ICER = Incremental cost-effectiveness ratio, Neg = negative, PCR = polymerase chain reaction
aEstimated from average retail cost 2007 and converted to USD (1 Singapore dollar = 0.625USD)
bYear 1 undetectable DNA (PCR) rates [33, 36–38]. Assays with different lower limit of detectability were used in various trials
cYear 5 undetectable DNA (PCR) rates [34, 38–42]. Estimated for entecavir based on projections using 144 weeks data [42] and assumed not worse than lamivudine. Estimated for PEG-interferon
Proposed cost-effectiveness of treatment to prevent clinical end points
Reducing cirrhosis and HCC in CHB patients
The REVEAL study has shown that subjects with persistently high HBV DNA (>100,000 copies/ml) had the highest risk of HCC. In their subgroup analysis of patients who had paired HBV DNA levels over 10 years, the risk of HCC was highest in those who maintained high HBV DNA while those who had reduction of HBV DNA to undetectable levels over 10 years had significant decrease in risk [8]. We thus modeled a 10-year simulation using the relative risk data from REVEAL study assuming that (i) the HBV DNA reported at baseline and at 10-year follow-up was representative of the absolute trend for these patients, (ii) risk reduction of HBV DNA with treatment would be similar to patients who develop spontaneous HBV DNA reduction to undetectable levels by PCR (Cobas Amplicor, Roche Diagnostics, Indianapolis, IN), and (iii) the magnitude of risk reduction for cirrhosis prevention is similar to that of HCC. By grouping the patients who had drop of the HBV DNA level to undetectable HBV DNA (PCR) at 10-year follow-up, we were able to work out the relative and absolute risk reduction from the reported HCC rates as well as estimate the reduction in progression to cirrhosis rate with successful reduction of HBV DNA to undetectable levels by PCR. Using reported HBV DNA-negative (PCR) rates for each treatment, we were then able to calculate that the number needed to treat (NNT) to prevent one case of cirrhosis was 30, 20, 18, and 50 for LMV/ADV, ADV/LMV, ETV, and PEG, respectively (Table 4). Conversely, the number needed to treat to prevent one case of HCC was 58, 41, 36, and 100 for LMV/ADV, ADV/LMV, ETV, and PEG, respectively. Using cost to society (total retail cost) as comparison without taking into account reimbursement policies, ETV had the lowest median cost/cirrhosis prevented (US$500,000) and cost/HCC prevented (US$1.0 million) (Figs. 1 and 2). However, using the patient’s perspective in countries where there is partial reimbursement, and using the patients’ median cost (what patient has to pay) for each treatment strategy in Asian countries, we calculated the cost per clinical end point averted. The lowest cost to prevent one case of cirrhosis (see Fig. 1) was a median of US$326,000, using ADV/LMV, and the highest was for PEG with median user cost of US$686,000. To prevent one case of HCC (see Fig. 2), the cost from patient’s perspective ranged from US$654,000 (ADV/LMV) to US$1.38 million (PEG). In countries where lamivudine and adefovir are fully reimbursed, the cost to patients to prevent a cirrhosis or HCC is virtually zero. This approach allows us to use a common denominator (HBV DNA reduction) to compare the cost-efficacy of various treatment modalities. However, the actual reduction of clinical outcomes may not be dependant on HBV DNA alone. In fact, data from the regular interferon follow-up study in the HBeAg-positive cohort (beyond 6 years) [10] suggest that reduction of cirrhosis and HCC (7.6 and 4.8%, respectively) may be higher than what is extrapolated from the REVEAL study and this may potentially bias against interferon-based regimes which perform less well for surrogate markers such as negative HBV DNA by PCR assays.
Table 4.
Risk reduction in CHB patients with various treatments and cirrhotic patients with LMV
| HCC | Cirrhosis | |||||
|---|---|---|---|---|---|---|
| RRR | ARR | NNT | RRR | ARR | NNT | |
| REVEAL Study [8, 9] | ||||||
| LMV/ADV | 0.38 | 0.02 | 58 | 0.38 | 0.03 | 29 |
| ADV/LMV | 0.51 | 0.02 | 44 | 0.51 | 0.05 | 22 |
| ETV | 0.61 | 0.03 | 37 | 0.61 | 0.05 | 18 |
| PEG | 0.22 | 0.01 | 100 | 0.22 | 0.02 | 50 |
| RRR | ARR | NNT | Median total cost of LMV | |||
|---|---|---|---|---|---|---|
| Asian Lamivudine Cirrhotic Study [6] | ||||||
| End point reductiona | 0.559322 | 0.099 | 10.10101 | US$26,100 | ||
| HCC | 0.472973 | 0.035 | 28.57143 | US$74,567 | ||
Note: RRR = relative risk reduction, ARR = absolute risk reduction, NNT = Number needed to treat, LMV = lamivudine, ADV = adefovir, ETV = entecavir, PEG = pegylated interferon
aDefined as increase in Child-Pugh score of 2 or spontaneous bacterial peritonitis with proven sepsis, renal insufficiency, bleeding gastric or esophageal varices, the development of hepatocellular carcinoma, or death related to liver disease
Fig. 1.
Median cost per cirrhosis prevented over 10 years in Asian countries (USD in thousands). For full-paying patients, ETV had the lowest cost per cirrhosis saved at US$500,000. In Asian countries where there is partial reimbursement, ADV/LMV is the cheapest cost per cirrhosis (median) saved from patient’s perspective. For countries where there is full reimbursement, the cost per cirrhosis or HCC would be identical to full-paying patients, except that society pays for it. Median cost taken from survey on various Asian countries (Table 1). Subsidized cost = cost to patient after reimbursement, ETV = entecavir, ADV = adefovir, LMV = lamivudine, USD = United States dollars
Fig. 2.
Median cost per HCC prevented over 10 years in Asian countries (USD in thousands). ETV had the lowest cost per HCC saved for full paying patients (US$1 million). However, there is no reimbursement for ETV in most Asian countries, consequently ADV/LMV and LMV/ADV strategies are cheaper from patients’ perspective after reimbursement, as patients would have to pay the full cost for ETV. Median cost taken from survey on various Asian countries (Table 1). Subsidized cost = cost to patient after reimbursement, ETV = entecavir, ADV = adefovir, LMV = lamivudine, USD = United States dollars
Reducing disease progression in cirrhotic patients
The Asian Lamivudine Cirrhosis study showed that the incidence of disease progression defined by increase in Child-Pugh score ≥2, or spontaneous bacterial peritonitis with proven sepsis, renal insufficiency, bleeding gastric or esophageal varices, or the development of HCC were reduced by lamivudine treatment over a median of 32 months [6]. The number needed to treat to prevent disease progression and incidence of HCC was 10 and 28.5, respectively. Extrapolating the median cost of lamivudine treatment in Asia, this works out to US$9,630/year to prevent one disease progression (composite end point) in cirrhotic patients and US$27,600/year to prevent one HCC over 32.4 months (Table 4). In perspective, the estimated cost of treatment of decompensated cirrhosis per year ranged from US$1,419 to US$8,794 and that of HCC from US$1,700 to US$15,000 (Table 1). However, we need to remember that although the cost of prevention appears to be higher than the cost of treating complications of liver disease, the gain in lifespan and quality of life cannot be quantified in dollars alone. This is the weakness of an analysis focused on the cost of drug and prevention of an end point, which full life cycle modeling attempts to rectify by considering all possible factors.
Cost-effectiveness analysis of CHB
The limitations of short-term 5-year analysis, albeit based on actual resistance emergence, outcomes, and costs, fail to take into account long-term efficacies such as reduction in incidence of HCC and decompensated liver cirrhosis as well as the accrual of life years saved by treatment. Consequently, ISPOR has proposed guidelines for cost-effectiveness studies [13] (see later). Several cost-effectiveness studies have been performed to try to estimate the long-term costs and benefits of CHB treatment, and we have reviewed their results.
A review of cost-effectiveness studies in CHB (Table 5)
Table 5.
Reported cost-effectiveness analysis of chronic hepatitis B
| Treatment | Year of costing | Incremental cost-effectiveness ratio |
|---|---|---|
| Compared to no treatment | ||
| IFN | 1995 [17, 18, 43, 44] | Cost saving—USD 16,000/QALY |
| LMV | 2002 [17, 44] | Cost-saving—USD7125 |
| Compared to interferon, LMV, ADV mono therapy | ||
| LMV followed by ADV | 2005 [19] | US$8,446/QALY |
| Compared to regular interferon | ||
| PEG | 2007 [21] | US$32,554/QALY |
| Compared to LMV ± salvage | ||
| ENT | 2007 [20] | US$7,600/QALY |
| PEG | 2007 [22] | US$20,945–35,245/QALY |
| PEG | 2007 [23] | US$11,500/QALY |
| PEG + LMV | 2007 [21] | US$28,200/QALY |
| PEG + LMV + ADV rescue | 2007 [21] | US$44,300/QALY |
| Compared to no treatment in cirrhotic population | ||
| ADV | 2006 [24] | US$19,731 |
| Compared to ADV in cirrhotics | ||
| ETV | 2006 [24] | US$25,626/QALY |
Note: IFN = interferon, LMV = lamivudine, PEG = pegylated interferon, ADV = adefovir, ETV = entecavir, QALY = quality-adjusted life years, LY = life year
There have been many studies of chronic hepatitis B cost-effectiveness analysis that have been published. However, the quality of these studies varies significantly. The differences in countries in which the study was conducted in, the year horizon, cost, benefit and transition estimates, as well as the Markov model states make comparison between various studies impossible. We searched PUBMED for published articles using the search term “Hepatitis B, cost-effectiveness and economic analysis” and graded them arbitrarily using ISPOR guidelines for cost-effectiveness analysis reporting [13] as follows:
- Level I Evidence: High quality CEA: (satisfying all criteria)
- Broad perspective including societal or health payer’s perspective.
- All treatment strategies relevant to real-life scenarios are studied.
- Baseline groups, for example, HBeAg-positive versus HBeAg-negative patients versus decompensated cirrhotics, are clearly defined.
- Costs and benefits with discounts clearly defined.
- Outcome indices for both short-term cycles and full life cycle reported.
- Sensitivity analysis performed.
- Level II Evidence: Moderate quality CEA report
- Fulfils four or more criteria.
- Level III Evidence: Suboptimal Quality
- Fulfils less than four criteria.
Treating CHB with interferon and lamivudine is consistently cost-effective compared to no treatment as determined by several studies [17, 18] (Level II). Both regular interferon and lamivudine saves lives and the incremental cost-effectiveness ratio range from “cost saving” (cheaper to treat than not to treat) to US$22,000 per life year saved.
In comparing various treatment strategies, Kanwal et al. [19] compared regular interferon, lamivudine monotherapy, adefovir monotherapy, and lamivudine with adefovir rescue therapy. In his analysis, lamivudine followed by adefovir rescue had the lowest ICER with an incremental US$8,446 per QALY gained. Both lamivudine monotherapy and adefovir monotherapy were dominated (more expensive and yet save less QALY) (Level 1). More recent analysis comparing newer agents reports that entecavir has an ICER of US$7,600/QALY compared to LAM/ADV salvage over a 2-year treatment [20] (Level II) and pegylated interferon cost an additional £16,166 (US$32,554)/QALY compared to regular interferon, and an additional £10,400–17,500 (US$20,945–35,245)/QALY when compared to lamivudine (calculated) [21, 22] (Level II). Even when pegylated interferon was combined with lamivudine for nonresponders and followed up with adefovir rescue, it had an ICER of £14,000 (US$28,200)/QALY and £22,000 (US$44,300)/QALY, respectively (Level II). A Taiwanese study comparing pegylated interferon with lamivudine also reported an additional cost of NTD380,619 (US$11,500) per QALY [23], compared to lamivudine (Level III). However in this latter study, the lamivudine was limited to only 2 years and the implication of continuing lamivudine for longer period was not assessed. In the cirrhotic population, adefovir cost an incremental US$19,731/QALY compared to no treatment. Entecavir had an ICER of US$25,626 per QALY gained compared to adefovir [24] (Level I). Sensitivity analyses in most studies have suggested that cost of medication and efficacy of drug (incorporating resistance) are the main drivers in cost-effectiveness analysis.
It is important to note that in all these studies, claims that one therapeutic strategy was cost-effective against standard therapy (usually lamivudine) or no therapy merely mean that the additional costs over standard therapy or no treatment are within the US$50,000/QALY benchmark considered cost-effective for first-world countries.
Cost-effectiveness of telbivudine
Telbivudine is the latest antiviral drug to be approved by FDA for the treatment of CHB. The approximate retail cost in Asia-Pacific countries range from US$3.20 to US$6.32. Up to 104 weeks, it appears to be more potent compared to lamivudine with higher number of patients with negative HBV DNA (PCR) after 104 weeks and a resistance rate of 8.6% among HBeAg-negative patients and 21.6% among HBeAg-positive patients [25]. It has been reported to be more cost-effective compared to lamivudine [26]. We have, however, not used telbivudine in our cost-effectiveness analysis comparison as the availability of only 2-year data does not allow trend analysis and makes it difficult to project meaningfully the efficacy and resistance over time. However, just for perspective comparison, we assumed arbitrarily on best-case scenario that telbivudine resistance tailed off and peaked at 30% at 10 years and efficacy of HBV DNA suppression (PCR) reaches 70% at 10 years. Using the same model based on REVEAL data and the strategy of ADV add-on for telbivudine resistance, this strategy yields a median cost-effectiveness of US$503,000 per cirrhosis prevented and US$1.22 million per HCC prevented. This is comparable to ADV/LMV strategy for full-paying patients but becomes inferior to ADV/LMV from patient’s perspective if ADV/LMV is reimbursed and TBV is not, as the case with ETV.
Issues with cost-effectiveness
Who pays and who benefits?
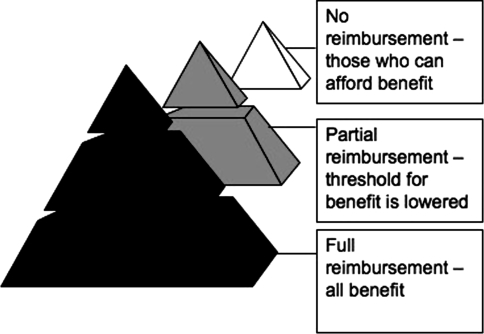
The perspective of cost-effectiveness analysis is important as costs and benefits to the government, the patient, society, and third party health payer can be very different [27]. Especially in Asia, the cost of therapy and cost-effectiveness analyses from the patient’s perspective is heavily influenced by reimbursement policies of countries. There are three basic types of policies—full reimbursement where the patient pays no direct costs as the costs are borne by the taxpayer (Australia, Japan, and New Zealand), partial reimbursement where only part of the costs are reimbursed and the balance is paid directly by the patient (Singapore, Thailand, Hong Kong, Korea, and Taiwan), and no reimbursement where the patient pays for everything (Indochina, India, Bangladesh, Pakistan, Malaysia, and Indonesia). We can see that cost-effectiveness studies are somewhat skewed by the differential costs of therapy of complications of liver disease, while there is less differential in the cost of antiviral drugs (see Table 1). For societies where there is full reimbursement, patients from all social strata benefit in reduction of cirrhosis and HCC because the patient bears no out-of-pocket expenses. In societies where there is no reimbursement, only those who can afford antiviral therapy benefit, while in societies where there is partial reimbursement, the threshold for those who can afford therapy is lowered but does not cover all potential patients. We can see a schematic representation of this in Fig. 3.
Fig. 3.
Pyramid representation of proportion of people benefiting from CHB treatment
Cost-effectiveness analysis provides the best alternative evidence in predicting the best value-for-money treatment strategy. Yet, inherent within cost-effectiveness analysis, especially in the Asian context, lay several caveats that need close attention.
Variables for transition probabilities are derived from short-term or retrospective analyses. The natural history of progression from one disease state to another are at best estimates based on wide unproven assumptions. Using these estimates as linear constant transition probabilities over repeated cycles may result in gross magnification of errors. For example, it is unclear if the HBeAg seroconversion achieved during treatment either with oral antiviral or interferon-based regimens is durable over time. If reactivation occurs and the return of HBV DNA result in resumption of cirrhosis progression and HCC occurrence, the benefit of treatment in the HBeAg-positive cohort would be greatly exaggerated. The surrogate end points used in conventional treatment guidelines are not validated by prospective trials.
Many of the treatment outcome indices are derived from Western data. In the Asian setting where CHB is usually acquired from vertical transmission, it is still a point of contention whether drugs such as interferon still carry the same efficacy in Asian population as the western population. It may well be that long-term viral suppression, as suggested by REVEAL data, holds the key to improving clinical outcome in Asian CHB patients.
Benchmarks for cost-effectiveness across countries
How much is society willing to pay for the added benefit of a superior health care strategy? While newer antivirals with low resistance have made long-term viral suppression possible, they can still be prohibitively expensive. The US$50,000/QALY willingness-to-pay threshold is an arbitrary value that is determined by societies with first-world economies. The WHO has suggested rationalizing the threshold to the countries’ gross national income (GNI) per capita to better reflect the spending power of the society [28]. To put the issue in perspective, the GNI per capita of United States in 2006 was US$32,476 compared to China’s US$854 and that of Vietnam’s US$384 [29]. In reality, for many Asian countries with low GNI, ICER may be meaningless compared to total cost needed to treat each CHB patient and the cost savings, if any. Different countries thus have to apply cost-effectiveness analyses outcomes in the specific context of that country, taking into account cost of the drug, incidence of disease, and the availability of treatment options for complications such as liver surgery and transplant. What should the willingness-to-pay threshold be for Asian countries? The traditional benchmark of cost of renal dialysis or HIV treatment provides a useful benchmark to gauge the willingness of society to pay for the added benefit of a quality-adjusted life (year). Countries that have state-funded renal dialysis programs should, by similar principle, consider funding hepatitis B treatment that have equal incremental cost-effectiveness ratios. Countries with low GNI, by analogy, would likely not invest heavily in health care and have limited reimbursement, if at all, for renal dialysis, treatment of CHB, HCC, and complications of liver disease.
As a result of cost of drug, lamivudine is thus still widely used in Asia as it is perceived to be the cheapest drug available with the lowest total cost impact to society and individual. The cost of each drug varies across countries in Asia and depends on pricing by pharmaceutical companies as well as the availability of generic forms of drugs. In addition, state reimbursements, either total or partial reimbursements, change significantly the dynamics of cost-effectiveness and makes therapy significantly cheaper and more affordable from the patient perspective.
Conclusion
In an ideal setting, every chronic hepatitis B patient should receive the most potent treatment to suppress hepatitis B viral replication. In a real world, modeling allows us to take into account various complex factors such as resistance, side effects, cost, natural history and affordability, and help health policy makers understand the impact of various treatment options and plan public health care strategies. Caution is needed, however, in extrapolating cost-effectiveness findings, especially those derived in developed countries, to other countries in Asia. Validity of transition probabilities, time horizon of analysis, appropriateness of end point measures, and finally the unique conditions of the country such as cost budget ceiling are critical factors that need close scrutiny. Long-term data on natural history of CHB acquired during perinatal transmission are needed to make this mathematical guesswork less arbitrary.
Summary statement
Guidelines for cost-effectiveness cannot be universal as this varies from country to country, the data are extrapolated based on modeling rather than direct, and the results can vary considerably based on the assumptions built into the model.
Published cost-effectiveness data are based currently on first-world economies and cannot be extrapolated directly to Asia-Pacific countries.
Cost-effectiveness is typically benchmarked on quality-adjusted life years (QALY) saved, but concepts more intuitive to clinicians may be cost of achieving surrogate end points such as HBeAg seroconversion or undetectable HBV DNA at 5 years, or cost of preventing one case of cirrhosis or HCC.
In comparison of treatment, intervention B being cost-effective compared to intervention A does not mean that intervention B is cheaper than intervention A. In essence, it merely states that the additional cost of intervention B to achieve one additional end point gain/averted as compared to intervention A is within the willingness-to-pay threshold of the country in which the modeling was based. Most cost-effective analyses have used US$50,000/QALY as the benchmark of additional cost to be considered cost-effective.
- Conclusions from cost-effectiveness analysis are not facts nor claim of future predictions but a synthesis of projections from the best evidence available and the most reasonable assumptions for the disease, to allow understanding and insight into the implications of cost impact and outcome benefits [13]. Published analysis currently shows that based on first-world economies,
- Regular interferon and lamivudine range from cost-saving to an additional USD 2,035-16,206/QALY and cost-saving to USD 7,125-/QALY respectively compared to no treatment (Level II)
- Adefovir costs an additional US$31,223/QALY compared to lamivudine (Level II)
- Sequential lamivudine with adefovir rescue is cost saving compared to no treatment and cost an additional US$8,446/QALY compared to no treatment (Level II)
- Pegylated-interferon costs an additional US$11,641–28,793/QALY compared to lamivudine or an additional US$33,012/QALY compared to regular interferon (Level II, III)
- Pegylated-interferon followed by lamivudine costs an additional US$28,794/QALY compared to lamivudine and pegylated-interferon followed by lamivudine with adefovir rescue costs additional US$45,938/QALY compared to no adefovir rescue (Level II)
Reviews of cost-effectiveness are heavily dependent on published literature, which can lead to potential publication bias. In this regard, studies comparing multiple treatment options using the same model are more likely to present a balanced comparison rather than two-arm studies showing one treatment is cost-effective compared to another.
Cost-effectiveness analysis needs to be taken in the proper context. Cost-utility analyses from societal perspective are more relevant to health policy makers. Cost-effectiveness indices based on clinical outcomes and from user’s perspective may be more useful for doctors and patients making decision choices.
As there are currently no universal threshold for cost-effectiveness, benchmarking cost-effectiveness of treatment of chronic hepatitis B, for example, to cost and availability of public-funded renal dialysis programs (the benchmark in first-world economies) in Asian countries, allows us to evaluate the relative cost-effectiveness threshold across countries.
Reimbursement policies generally do not affect cost-effectiveness, they however will decide who pays for the treatment, and whether all or only some patients benefit.
Acknowledgments
The authors thank Deepak Amarapurkar, Henry Chan, Ed Gane, Jia Jidong, Jia-Horng Kao, Mobin Khan, Laurentius Lesmana, Rosmawati Mohammed, Teerha Piravisuth, Jose Sollarno, Seung Kew Yoon, Masao Omata, and Joe Sasadeusz for providing information about reimbursement policies in their countries.
Declarations This study was commissioned by the APASL Hepatitis B Guideline Committee. No funding was received from any agency for this study. Yock Young, Dan is a full time employee of the National University of Singapore and has no declarations. Myat Oo, Aung is a full time employee of the Agency for Science, Technology and Research, Singapore and has no declarations. Seng Gee, Lim is an employee of the National University of Singapore and Agency for Science, Technology and Research. He has been an advisory board member for Novartis Pharmaceuticals, Idenix Pharmaceuticals, Triangle Pharmaceuticals, Valeant Pharmaceuticals, Bristol Myers Squibb Pharmaceuticals, and Schering Plough Pharmaceuticals. He is on the Speaker's Bureau for GlaxoSmithKline Pharmaceuticals and Novartis Pharmaceuticals. He is a consultant for CombintoRx. He does not own patents or stocks related to any of these companies.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11:97–107. [DOI] [PubMed]
- 2.Lesmana LALN-Y, Mahachai V, Phiet PH, Suh DJ, Yao GB. Hepatitis B: overview of the burden of disease in the Asia-Pacific region. Liver Int 2006;26:3–10. 17051681 [DOI]
- 3.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005;9:191–211, v. [DOI] [PubMed]
- 4.Yang BM, Paik SW, Hahn OS, Yi DH, Choi MS, Payne S. Economic evaluation of the societal costs of hepatitis B in South Korea. J Gastroenterol Hepatol 2001;16:301–8. [DOI] [PubMed]
- 5.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology (Baltimore, MD) 2007;45:507–39. [DOI] [PubMed]
- 6.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31. [DOI] [PubMed]
- 7.Holodniy M. HIV-1 load quantitation: a 17-year perspective. J Infect Dis 2006;194 Suppl 1:S38–44. [DOI] [PubMed]
- 8.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73. [DOI] [PubMed]
- 9.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678–86. [DOI] [PubMed]
- 10.Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45–52. [DOI] [PubMed]
- 11.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology (Baltimore, MD) 1999;29:971–5. [DOI] [PubMed]
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322–38. [DOI] [PubMed]
- 13.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health 2003;6:9–17. [DOI] [PubMed]
- 14.Lampertico P, Vigano M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology (Baltimore, MD) 2005;42:1414–9. [DOI] [PubMed]
- 15.Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology (Baltimore, MD) 2006;44:1656–65. [DOI] [PubMed]
- 16.Dan YY, Lim SG. Cost effectiveness of chronic Hepatitis B (Abstract). Hepatology 2006;44(S1):562A.
- 17.Crowley S, Tognarini D, Desmond P, Lees M, Saal G. Introduction of lamivudine for the treatment of chronic hepatitis B: expected clinical and economic outcomes based on 4-year clinical trial data. J Gastroenterol Hepatol 2002;17:153–64. [DOI] [PubMed]
- 18.Wong JB, Koff RS, Tine F, Pauker SG. Cost-effectiveness of interferon-alpha 2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med 1995;122:664–75. [DOI] [PubMed]
- 19.Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Intern Med 2005;142:821–31. [DOI] [PubMed]
- 20.Veenstra DL, Sullivan SD, Clarke L, et al. Cost effectiveness of entecavir versus lamivudine with adefovir salvage in HBeAg-positive chronic hepatitis B. PharmacoEconomics 2007;25:963–77. [DOI] [PubMed]
- 21.Takeda A, Jones J, Shepherd J, Davidson P, Price A. A systematic review and economic evaluation of adefovir dipivoxil and pegylated interferon-alpha-2a for the treatment of chronic hepatitis B. J Viral Hepat 2007;14:75–88. [DOI] [PubMed]
- 22.Veenstra DL, Sullivan SD, Dusheiko GM, et al. Cost-effectiveness of peginterferon alpha-2a compared with lamivudine treatment in patients with HBe-antigen-positive chronic hepatitis B in the United Kingdom. Eur J Gastroenterol Hepatol 2007;19:631–8. [DOI] [PubMed]
- 23.Sullivan SD, Veenstra DL, Chen P-J, Chang T-T, Chuang W-L, Tsai C, et al. Cost-effectiveness of peginterferon alfa-2a compared to lamivudine treatment in patients with HBe-antigen positive chronic hepatitis B in Taiwan. J Gastroenterol Hepatol 2007;22:1494–9. [DOI] [PubMed]
- 24.Kanwal F, Farid M, Martin P, et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am J Gastroenterol 2006;101:2076–89. [DOI] [PubMed]
- 25.Lai CLGE, Hsu CW. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LdT) vs lamivudine (Abstract). Hepatology (Baltimore, MD) 2006;44:222A.
- 26.Wong J, Stephen P. Cost-effectiveness of telbivudine versus lamivudine for chronic Hepatitis B (Abstract). J Hepatol 2007;46(S1):S199.
- 27.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med 1998;13:716–7. [DOI] [PMC free article] [PubMed]
- 28.WHO. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health: Executive Summary. World Health Organisation. 2001.
- 29.Bank W. GNI 2006 Atlas Method: World Development Indicators database. 2007.
- 30.Hsieh CR, Kuo CW. Cost of chronic hepatitis B virus infection in Taiwan. J Clin Gastroenterol 2004;38:S148–52. [DOI] [PubMed]
- 31.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–10. [DOI] [PubMed]
- 32.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999;341:1256–63. [DOI] [PubMed]
- 33.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med 1998;339:61–8. [DOI] [PubMed]
- 34.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95. [DOI] [PubMed]
- 35.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808–16. [DOI] [PubMed]
- 36.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800–7. [DOI] [PubMed]
- 37.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20. [DOI] [PubMed]
- 38.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17. [DOI] [PubMed]
- 39.Guan R, Lai CL, Liaw YF, et al. Efficacy and safety of 5 years lamivudine treatment of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 2001;16 Suppl 1:A60 (Abstract).
- 40.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006;131:1743–51. [DOI] [PubMed]
- 41.Marcellin PCT, Lim SG, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B (CHB) patients in Study GS-98-437. 57th Annual Meeting of the American Association for the Study of Liver Disease. Boston, MA. 2006.
- 42. Chang TC, Kaymakoglu S. Entecavir maintained virologic suppression through 3 years of treatment in antiviral-naive HBeAg(+) patients (ETV 022/901). 57th Annual Meeting of the American Association for the Study of Liver Disease. Boston, MA. 2006.
- 43.Dusheiko GM, Roberts JA. Treatment of chronic type B and C hepatitis with interferon alfa: an economic appraisal. Hepatology (Baltimore, MD) 1995;22:1863–73. [PubMed]
- 44.Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation. Health Technol Assess (Winchester, England) 2006;10:iii–iv, xi–xiv, 1–183. [DOI] [PubMed]