Abstract
Ascites and hepatorenal syndrome (HRS) are the major and challenging complications of cirrhosis and portal hypertension that significantly affect the course of the disease. Liver insufficiency, portal hypertension, arterial vasodilatation, and systemic cardiovascular dysfunction are major pathophysiological hallmarks. Modern treatment of ascites is based on this recognition and includes modest salt restriction and stepwise diuretic therapy with spironolactone and loop diuretics. Tense and refractory ascites should be treated with a large volume paracentesis, followed by volume expansion or transjugular intrahepatic portosystemic shunt. New treatment strategies include the use of vasopressin V2-receptor antagonists and vasoconstrictors. The HRS denotes a functional and reversible impairment of renal function in patients with severe cirrhosis with a poor prognosis. Attempts of treatment should seek to improve liver function, ameliorate arterial hypotension and central hypovolemia, and reduce renal vasoconstriction. Ample treatment of ascites and HRS is important to improve the quality of life and prevent further complications, but since treatment of fluid retention does not significantly improve survival, these patients should always be considered for liver transplantation.
Keywords: Hepatic decompensation, Portal hypertension, Hyperdynamic circulation
Introduction
Ascites can be observed in various diseases, but it is most frequent due to cirrhosis with portal hypertension and peritoneal carcinomatosis. Less frequent etiologies of ascites are hepatocellular carcinoma, Budd–Chiari syndrome, congestive heart failure, pancreatitis, and tuberculosis. The clinical appearance of patients with cirrhosis as well as the course and the prognosis of the disease are characterized by its numerous complications. Development of ascites is one of the most frequent complications and occurs in more than 50% of patients within 10 years of the diagnosis of cirrhosis [1]. Ascites is defined as the presence of more than 25 ml of fluid in the peritoneal cavity. The normal hepatosplanchnic lymph production is approximately 1 ml/min. In patients with cirrhosis, this rate may increase up to 10 ml/min [2, 3]. When the production of lymphatic fluid exceeds the lymphatic transport capacity, ascites develops. According to the amount of ascites, the condition can be divided into grades I–III. Grade III represents the gross and tense ascites, and it may cause significant discomfort to the patient. However, the presence of ascites is not just a cosmetic problem since it is associated with a poor survival with 50% mortality within 3 years [4, 5]. Survival depends mainly on the degree of portal hypertension, liver insufficiency, and circulatory dysfunction. In approximately 25% of the patients, bacterial translocation leads to the development of spontaneous bacterial peritonitis (SBP), which further aggravates the prognosis [6]. A considerable number of patients with ascites and advanced cirrhosis also develop hepatic nephropathy, which bears a poor prognosis despite new treatment modalities [1, 7–9].
The treatment of hepatorenal syndrome (HRS) represents a clinical challenge, and the introduction of new approaches in this area has improved the management. These treatments comprise diuretics, use of new therapeutic principles such as aquaretics and vasoconstrictors, antibiotics, large volume paracentesis, transjugular intrahepatic portosystemic shunts (TIPS), liver supporting devices, and, at the end stage, liver transplantation. This article summarizes the most recent advances in our understanding of the formation of ascites and the development of HRS in relation to relevant pathophysiological targets for treatment.
Pathophysiology of ascites
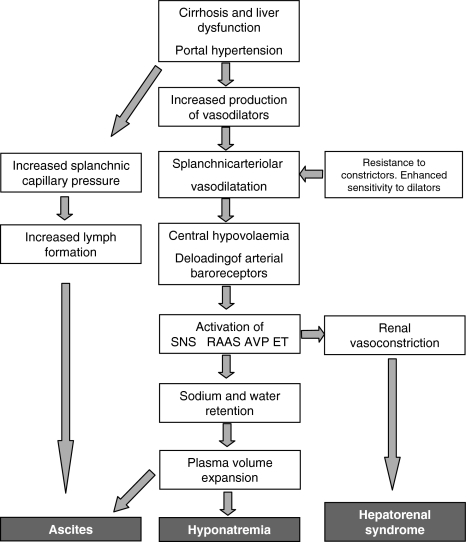
The pathophysiology behind the formation and perpetuation of ascites is complex. Three major factors are involved in the pathogenesis: portal and sinusoidal hypertension, arterial vasodilatation, and neurohumoral activation, all leading to sodium and water retention [9, 10]. Different theories have been put forward to explain the development of ascites. One theory claims primary overfilling of the circulation with a subsequent overflow of fluid into the intraperitoneal cavity, but currently, the peripheral arterial vasodilatation theory has prevailed. According to this theory, development of systemic vasodilatation results in a decrease in the effective arterial blood volume and a hyperdynamic circulation [11]. This theory has lately been modified into what has been termed “the forward theory of ascites formation” (Fig. 1), which combines arterial underfilling with a forward increase in splanchnic capillary pressure and filtration with increased lymph formation [3]. Hence, this modification takes into consideration both of the pathogenetic aspects (central and arterial underfilling and splanchnic overfilling) in the formation of ascites.
Fig. 1.
Pathophysiological mechanisms in the development of ascites, hyponatremia, and hepatorenal syndrome. The diagram is based on assumptions of the arterial vasodilatation theory and the forward theory of ascites formation. SNS, Sympathetic nervous system; RAAS, Renin–angiotensin–aldosterone system; AVP, Arginine-vasopressin; ET, Endothelin
Portal hypertension
In cirrhosis, portal sinusoidal hypertension is a prerequisite for the development of ascites. The hydrostatic pressure within the hepatic sinusoids favors transudation of fluid into the peritoneal cavity [2, 12]. However, the topographic site of the lesion is important, and patients with prehepatic portal hypertension, for example, after portal venous thrombosis, rarely develop ascites unless the serum albumin concentration becomes very low. On the contrary, patients with posthepatic portal hypertension such as cirrhosis often present with ascites, and almost all patients with the Budd–Chiari syndrome have ascites at diagnosis. The hepatic vascular resistance and portal venous inflow determine the height of the portal pressure. Factors that determine the hepatic vascular resistance include both structural and dynamic components [13]. Among the structural components are fibrosis and regeneration nodules. Dynamic structures include hepatic stellate cells, myofibroblasts, and other cells with contractile properties. A preferential sinusoidal constriction in the liver can possibly be attributed not only to a defective NO production but also to endogenous vasoconstrictors such as endothelin-1 (ET-1), angiotensin-II, catecholamines, and leukotrienes, which may increase the hepatic sinusoidal resistance [13–15]. The hemodynamic imbalance with a predominant sinusoidal constriction may significantly contribute to the development of portal hypertension and may be an important target for treatment.
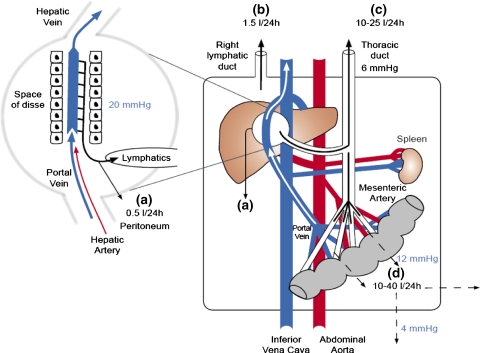
Ascites formation moreover depends on the balance between the local transvascular filtration and lymph drainage [2]. Thus, the amount of ascitic fluid produced is governed by increased transsinusoidal filtration of protein and fluid and by transperitoneal hydrostatic and oncotic dynamics. However, in contrast to earlier assumptions, the decreased oncotic pressure may be of minor importance for the generation of ascites and low plasma albumin concentrations have little influence on the rate of ascites formation (Fig. 2) [2, 16, 17]. In this context, the increased hydrostatic pressure is critical and ascites rarely develops in patients with a hepatic venous pressure gradient below 12 mmHg [18].
Fig. 2.
Hydrostatic pressures and transperitoneal fluid dynamics in cirrhosis: Increased portal and sinusoidal pressure generate increased transsinusoidal fluid filtration with an overall increased splanchnic lymph flow of 10–25 l per 24 h. Owing to the fenestrated hepatic capillaries, no oncotic counter pressure exists here. In contrast, the transcapillary pressure and filtration rate in the portal capillary area are somewhat reduced compared with the transsinusoidal filtration pressure (12 mmHg vs. 20 mmHg). As long as transsinusoidal filtration keeps pace with lymphatic drainage, no surplus protein-rich fluid will spill over into the peritoneal cavity. When the former exceeds the latter, the protein-rich fluid will accumulate in the peritoneal cavity (a). However, this spillover fraction (0.5 l per 24 h) is relatively small in comparison with the overall transsinusoidal filtration and lymphatic drainage (c). The protein-rich fluid will equilibrate according to hydrostatic and oncotic dynamics over the relatively large intestinal peritoneal surface area, and a protein-poor ascitic fluid will result. The bulk of ascitic fluid (water, proteins, and electrolytes) is returned into the circulation mainly through the right lymphatic duct (b) with a relative low flow rate (1.5 l per 24 h). In contrast, low-molecular components of the ascitic fluid (water, electrolytes, glucose, creatinine, antibiotics, etc.) are exchanged directly over the peritoneal membrane by low-pressure filtration and diffusive transport (d)
Pathophysiology of arterial vasodilatation and neurohumoral activation
The pathophysiological link between early portal hypertension and the development of a splanchnic vasodilatation and hyperdynamic syndrome is still obscure. It may be brought about either by an overproduction of circulating vasodilators induced by shear stress in the splanchnic circulation or by direct neurohumoral signals from the liver to the brain [15, 19]. Several findings indicate that the splanchnic vasodilatation precedes renal sodium and water retention [20]. In experimental as well as human portal hypertension, splanchnic arterial vasodilatation leads to reduced systemic vascular resistance, reduced arterial blood pressure, and decreased effective blood volume with activation of potent vasoconstricting systems such as the sympathetic nervous system (SNS), the renin–angiotensin–aldosterone system (RAAS), and nonosmotic release of vasopressin [3, 10, 15]. The hemodynamic consequences include the development of a hyperdynamic circulation with an increased heart rate and cardiac output. Cardiac output has previously been described as a mediator of the effective blood volume, and underfilling of the arterial circulation occurs in such patients as a result of diminished systemic vascular resistance [11]. However, at a much later stage of the disease, underfilling of the arterial circulation might occur secondary to a decrease in cardiac output as described in patients with renal failure and SBP [21].
Systemic vasodilatation may be brought about either by the presence of excess of vasodilators or by decreased sensitivity to vasoconstrictors. Among the vasodilators that have been recently implicated in the systemic vasodilatation is nitric oxide, primarily synthesized in the systemic vascular endothelium by nitric oxide synthase [22, 23]. In portal hypertension, there seems to be a diminished release of NO from sinusoidal endothelial cells in the cirrhotic liver, whereas in the systemic circulation, there is evidence for an upregulation of the NO synthesis [24]. Calcitonin gene-related peptide (CGRP) and adrenomedullin are potent vasodilatating neuropeptides, which have been found in increased concentrations in patients, especially with ascites and HRS [15, 20]. The increase in vasoactive hormones is mainly due to an increased production and, to a lesser extent, a decreased hepatic clearance [25]. It is likely that these peptides act as neurotransmitters both in the initiation of the hemodynamic changes and in the perpetuation of the hyperdynamic circulation and the formation of ascites. The systemic vasodilatation has also been related to resistance to pressor hormones such as noradrenaline, angiotensin-II, and vasopressin. An impaired response to vasoconstrictors is likely related to changes in receptor affinity, downregulation of receptors, or to postreceptor defects related to increased NO expression [15, 26, 27]. Alterations in arterial and total vascular compliance have been considered recently [28, 29].
Although the pathophysiology and the role of the arterial vasodilatation are complex, there is definite experimental and clinical evidence that it precedes the counterregulatory neurohumoral activation and the renal sodium and water retention in patients with cirrhosis. In the preascitic phase, renal sodium retention may partly be due to activation of low-pressure baroreceptors as proposed by Levy [30].
Renal dysfunction
Even in the very early phases of portal hypertension, impairment of renal function can be seen. The first renal functional abnormality is reduced renal sodium excretion in terms of a reduced natriuretic response either to an acute administration of sodium chloride or to changes in posture [31, 32]. These early events are seen before the development of ascites, but in most of the patients it represents the initiation of a more pronounced renal dysfunction. This includes progressively increased sodium and water reabsorption and decreases in renal perfusion and glomerular filtration rate often in parallel with a decrease in liver function [33]. In healthy individuals, the free water clearance approximates 10 ml/min [34]. In cirrhotic patients, the free water clearance is often reduced below 1 ml/min, which is equivalent to an intake of 1.5 l/day before fluid accumulation begins. The consequences are the development of dilutional hyponatremia (serum sodium < 130 mmol/l) [35]. At later stages, there is a progressive fall in the glomerular filtration rate (GFR) and renal blood flow (RBF), inevitably leading to the development of HRS [7]. According to the development of functional renal abnormalities, genesis of ascites has been divided into successive pathophysiological phases (Table 1). The early phase 1 is also called the preascitic phase because ascites is not present, but the renal sodium metabolism is impaired despite normal renal perfusion, GFR, and free water clearance [36, 37]. From a hemodynamic point of view, these patients often exhibit an increased plasma volume, supporting the presence of increased sodium and water retention and adaptation between the vascular capacitance and the circulating medium [36]. The second phase denotes a negative sodium balance despite decreased urinary sodium excretion, and the absence of ascites in this phase can be achieved by reducing the dietary intake of sodium. At this stage, only RAAS and SNS are activated in some patients and GFR and RBF remain normal [38]. In phase 3, sodium excretion is often below 10 mmol/day and there is immense activation of the RAAS and SNS, but still RBF and GFR are normal [39, 40]. The arterial blood pressure is often low or below normal despite activation of RAAS and SNS, and therefore these patients are very susceptible to the hypotensive effects of ACE inhibitors, angiotensin-II receptor inhibitors, and vasopressin V1-antagonist [7]. Phases 4 and 5 of the ascites denote the development of type-2 HRS and type-1 HRS, respectively.
Table 1.
Pathophysiological phases in the development of ascites and HRSa
| Phase | Presence of ascites | Sodium and water retention | Activated RAAS and SNS | Impaired RBF and GFR | Term |
|---|---|---|---|---|---|
| Phase 1 | No | No/Yes | No | No | Preascitic cirrhosis |
| Phase 2 | Yes | Yes | No/Yes | No | Mild-moderate ascites |
| Phase 3 | Yes | Yes | Yes | No/Yes | Moderate-tense ascites |
| Phase 4 | Yes | Yes | Yes | Yes | Type-2 HRS |
| Phase 5 | Yes | Yes | Yes | Yes | Type-1 HRS |
aAdapted from Arroyo and Colmenero [3]
Abbreviations: RAAS, Renin–angiotensin–aldosterone system; SNS, Sympathetic nervous system; RBF, Renal blood flow; GFR, Glomerular filtration rate; HRS, Hepatorenal syndrome
Pathophysiology of hepatorenal syndrome
Approximately 20% of cirrhotic patients with refractory ascites progress to HRS, which is defined as a functional renal failure in patients with chronic liver disease without significant morphologic changes in renal histology and with a largely normal tubular function [41, 42]. The definition and diagnostic criteria of HRS are shown in Table 2. Two types of HRS have been defined depending on the rapidness and the extent of the renal failure [42, 43]. Type-1 HRS is an acute form, with a rapid decrease in renal function and as an independent predictive factor; type-2 HRS is a chronic form, with a more stable renal dysfunction [41, 43].
Table 2.
New diagnostic criteria for hepatorenal syndrome (HRS) from the International Ascites Cluba
| Cirrhosis with ascites |
| Serum creatinine > 133 μmol/l (1.5 mg/dl) |
| No improvement in serum creatinine level (decreases to a level of <133 μmol/l) after at least 2 days with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin is 1 g/kg of body weight per day up to a maximum of 100 g/day |
| Absence of shock |
| No current treatment with nephrotoxic drugs |
| Absence of parenchymal kidney disease as indicated by proteinuria of <500 mg/day, absence of microhematuria (<50 red blood cells per high-power field), and/or a normal renal ultrasonography |
In the kidneys, a progressive afferent arterial renal vasoconstriction causes pronounced hypoperfusion with reduced GFR and increased tubular sodium and water reabsorption with severe renal failure [44, 45]. If a kidney from a patient with HRS is transplanted to a recipient without liver failure, it will function normally, which emphasizes the functional nature of the syndrome [46].
The prognosis of patients with a full-blown HRS is poor, ranging from days to weeks, and liver transplantation is the only radical treatment [41]. However, therapies that may counteract the pathophysiological process, in particular by reversing the central hypovolemia and modulating the vasoactive systems, seem promising as potential new target areas for the treatment [47–49]. The major elements in the development of HRS are the diseased liver, circulatory dysfunction, and abnormal systemic and renal neurohumoral regulation.
Liver function and hepatorenal reflex
A prerequisite for the development of HRS is a disturbed liver function and there is an overall association between the reduction of the hepatic function and the development of renal dysfunction, which is primarily seen in advanced liver diseases [50, 51]. The survival after the development of HRS is very poor, especially in type-1 HRS, which is characterized by a rapid decrease in renal function [51]. Normalization of the renal function in most of the patients with HRS after liver transplantation indicates that the liver is directly involved in the renal disturbances [52]. The existence of a hepatorenal reflex in patients with cirrhosis has been debated for years. Results of experimental and clinical studies have provided support for a direct link between the liver and the kidneys. In human cirrhosis, the presence of a hepatorenal reflex is supported by observations of reduced RBF following an increase in portal pressure and a concordant increase in the renal release of ET-1, suggesting a role of this peptide in the hepatorenal reflex [53, 54].
Arterial hypotension
A normal level of the arterial blood pressure is essential for the maintenance of an adequate renal perfusion. In cirrhosis, the arterial blood pressure is low or below normal, depending on the state of the disease, as a circulatory compromise between vasodilatating and counterregulatory vasoconstricting forces, local factors, and Starling forces [55, 56]. In healthy individuals, the renal autoregulation maintains a normal renal perfusion in spite of alterations in the arterial blood pressure, provided the level is above 70 mmHg [57]. However, below this threshold, the RBF is directly related to the renal perfusion pressure (arterial blood pressure − renal venous blood pressure) [8, 58, 59]. In patients with increased sympathetic nervous activity, the autoregulation curve may be shifted toward the right side [60]. Because of this, even minor reductions in arterial blood pressure may be harmful to renal perfusion and function in these patients [57, 61].
Previous studies have shown a relationship between the degree of arterial hypotension in cirrhosis and the severity of hepatic dysfunction, signs of decompensation, and survival [55, 62]. Among other pathophysiological mechanisms that may contribute to the circulatory and renal dysfunction are the presence of SBP, which is very frequently associated with the development of HRS and cirrhotic cardiomyopathy [63, 64]. Both of these conditions may lower the blood pressure, partly by a cardiac systolic dysfunction that may further decrease renal perfusion [63, 65]. Prevention and amelioration of the arterial hypotension represent an important target for therapy in HRS.
The GFR is reduced below 40 ml/min in patients with HRS and the sodium retention is massive owing to a combination of decreased filtered sodium and an increased sodium reabsorption mainly in the proximal tubules [51]. The amount of sodium reaching the distal nephron is therefore limited, and explains why diuretics such as spironolactone and furosemide are of only limited use in these patients. The abnormal free water clearance leads to a dilutional hyponatremia, a condition that has been successfully treated by vasopressin V2-receptor antagonists [66, 67]. Attempts with the use of vasopressin V2-receptor antagonists in the kidneys and the κ-opioid antagonist in the pituitary gland have also been suggested as targets for the improvement of free water clearance and dilutional hyponatremia [8].
Principles of ascites treatment
Diagnostic investigations
Suspicion on clinical ascites should be confirmed by abdominal ultrasonography. For the presence of ascites, diagnostic paracentesis should as a minimum include examination of the ascitic fluid for albumin or protein concentrations, a neutrophil count, and a culture on suspicion of SBP. Presence of SBP, defined as a neutrophil count of more than 250 cells/μl, is observed in approximately 15% of patients with ascites [16, 68]. Determination of the serum ascites-albumin gradient may be helpful in the differentiation of ascites due to cirrhosis, cardiac failure, and primary renal diseases from ascites due to pancreatic and malignant diseases. Thus, a serum ascites albumin gradient of more than 11 g/l favors a hepatic, cardiac, or renal etiology [17]. Ascitic fluid amylase should be measured on clinical suspicion of pancreatic disease. Cytology and, eventually, measurement of plasma LDH should be performed on suspicion of malignancy [69].
Treatment of noncomplicated ascites
Nonmedical treatment
Previous studies have shown that the supine position ameliorates RBF and GFR and improves sodium and water excretion [70]. Less activated RAAS and SNS and a more favorable diuretic response in patients resting supine position have led to the assumption that bed rest would benefit in the treatment of ascites. However, severe adverse effects due to bed rest, for example, increased risk of thromboembolic complications, decalcification of bones, and muscular atrophy, imply that bed rest in general cannot be recommended for the treatment of ascites [17].
Reduced salt intake counteracts the sodium imbalance in fluid retention and creates a negative sodium balance in a minority of patients, and therefore a dietary salt restriction is essential in the treatment of ascites. However, a rigorous salt-restrictive diet is most often unacceptable for patients, and therefore a no-added salt diet of 80–120 mmol of NaCl per day is recommended. In patients with dilutional hyponatremia, water restriction has been recommended, but the efficacy of this treatment may depend on the level of the serum sodium [17]. Moreover, the limited effect of water restriction at improving the level of serum sodium is because the daily fluid intake cannot be restricted to less than 1 l/day, which is insufficient to cause a negative fluid balance [71]. Water restriction should be reserved for only those patients who are clinically hypervolemic with severe hyponatremia and reduced free water clearance. Thus, for practical purposes, water restriction should be used only in very few (if any) patients.
Medical treatment
Diuretics have been used for the treatment of fluid retention for more than 60 years. The diuretic treatment of ascites should be initiated with an aldosterone antagonist mainly acting at the distal tubules to increase natriuresis because if only furosemide is administered with its natriuretic effects in the Henle’s loop, sodium will be reabsorbed in the distal tubules owing to an activation of aldosterone. The initial dose should be 100 mg/day, which can be gradually increased to up to 400 mg/day [3, 72]. However, it is often necessary to add a loop diuretics before the full dose of the aldosterone antagonist can be administered to avoid hyperkalemia. The full effect is normally seen after 3–5 days. Daily control of body weight and monitoring of serum sodium, potassium, and creatinine levels should follow treatment. A daily weight loss of no more than 500–800 g/day is recommended to avoid intravascular volume depletion [1]. In case of massive peripheral edema, higher weight losses can be accepted. When the weight loss is insufficient, a loop diuretic should be added. Most often furosemide is used because it may induce marked diuresis and natriuresis. The initial recommended dose is often 40 mg/day, and it can be increased to 160 mg/day with a stepwise increase every 2–3 days [72]. Additional diuretic effects can be achieved with the addition of other diuretics such as amiloride or thiazides, but adverse effects are frequent.
Treatment of refractory ascites
In the case of tense ascites, which may cause abdominal, hemodynamic, or respiratory discomfort for the patient, therapeutic paracentesis should be preferred owing to less complications and shorter hospital stay [3]. Refractory ascites is defined as diuretic-resistant ascites that cannot be mobilized by intensive diuretic therapy and salt-restricted diet (weight loss < 200 g/day during 4 days). Diuretic-intractable ascites is characterized by diuretic-induced complications such as encephalopathy and hyponatremia [72].
Ten percent of patients with ascites become refractory to medical treatment, and paracentesis and other treatment modalities become necessary [17]. After a therapeutic paracentesis, 90–95% of the patients develop recurrent ascites, and it is therefore essential to administer diuretics [73]. Therapeutic paracentesis should be combined with plasma volume support. Several randomized controlled trials of albumin vs. synthetic plasma expanders such as dextran, collagen-based colloids, and starch have shown equal effectiveness of synthetic plasma expanders and albumin in the prevention of postparacentesis-induced complications [74–77]. The intra-abdominal, right atrial, and pulmonary capillary wedge pressures decrease after a large volume paracentesis [28]. Cardiac output increases after 2–3 h and mean arterial blood pressure decreases by an average of 8–10 mmHg [78, 79]. A large volume paracentesis without adequate volume substitution may result in the development of postparacentesis-induced circulatory dysfunction (PICD) in up to 75% of the patients [80]. This condition is characterized by a pronounced activation of RAAS and SNS, which reflects central hypovolemia. It is mainly caused by a paracentesis-induced splanchnic arteriolar vasodilatation and brings about a further reduction in the systemic vascular resistance and a corresponding increase in the portal pressure [81]. After paracentesis of a large volume of ascites (>4 l), albumin seems to be more effective in the prevention of paracentesis-induced increase in RAAS and liver-related complications than polygeline [82, 83]. Intravenous albumin may therefore prevent complications caused by circulatory dysfunction such as renal failure and HRS, rapid recurrence of ascites, and shorter survival [80, 81]. However, recent studies have shown that administration of vasoconstrictors such as terlipressin or noradrenaline may also be effective either alone or in combination with albumin [48, 84, 85]. In a recent study, the vasoconstrictor midodrine was as effective as albumin to prevent PICD, but at a lower cost [86]. The PICD is an example of a condition where complications attributable to a potentially reduced effective blood volume can be prevented by a specific volume support.
In the case of recurrent ascites, insertion of a TIPS should be considered. In experienced centers, the technical success rate is usually high, about 95% [87]. Control of ascites is observed in 80–90% of patients, with complete resolution in 75%. A TIPS is considered more effective than a large volume paracentesis for the control of ascites [49, 88, 89]. A major problem with the insertion of TIPS is the relatively high frequency of hepatic encephalopathy, and although the patients often report an increase in quality of life, no significant effect on survival has been demonstrated after the insertion [90]. Improved survival after a TIPS insertion for refractory ascites has been demonstrated in only one meta-analysis [89]. Although a TIPS is more effective at removing ascites than a large volume paracentesis, it occurs at the cost of a higher frequency of hepatic encephalopathy and may not significantly affect the transplant-free survival. Therefore, a large volume paracentesis with plasma expander infusion should be first line of treatment of refractory ascites. The TIPS should be regarded as a second line of treatment for patients with preserved liver function that frequently develops into ascites [71].
Hyponatremia
Hyponatremia in cirrhosis often develops because of immense release of vasopressin. In the presence of plasma volume expansion, this is a hypervolemic or a dilutional hyponatremia [71]. Vasopressin act on G protein-coupled V2-receptors in the collecting ducts and are responsible for the vasopressin-induced water reabsorption [71]. This effect is mediated through aquaporins (AQPs), which are selective water channels, AQP2 being the most important [91]. Activation of AQPs increase water permeability, and in patients with ascites, there is evidence of reduced excretion of AQP2 [91]. The clinical use of vasopressin V2-receptor antagonists known as the vaptans may be effective in the treatment of dilutional hyponatremia, and large randomized trials are currently ongoing [67, 92].
Spontaneous bacterial peritonitis
As mentioned above, SBP is defined as a neutrophil count of more than 250 cells/μl. Culture from the ascitic fluid often displays bacterial species such as Escherichia coli and Streptococcus [68]. Antibiotic treatment of SBP significantly improves survival, and third generation cephalosporins should be considered such as cefotaxime 2 g twice a day for 2 weeks. Alternatively, amoxicillin/clavulanic acid could be considered. Fluoroquinolones have also been investigated. Other recommended antibiotics include ceftizoxime, cefonicide, ceftriaxone, and ceftazidime. In patients with ascites and a history of SBP, prophylactic treatment with, for example, ciprofloxacin 250 mg/day orally, is recommended [17, 71, 72, 93]. Aminoglycosides should not be used.
Principles of HRS treatment
Major elements for the development of HRS include the liver dysfunction and a systemic circulatory dysfunction with a preferential renal vasoconstriction [94, 95]. Hypothetically, the ideal drug would be a substance that improves liver function, reduces portal pressure, and exerts arterial volume expansion, systemic splanchnic vasoconstriction, and renal vasodilatation. Such a drug will probably never be developed, but the specific pathogenic mechanisms are each important targets for potentially combined treatment. Possible renal and splanchnic target areas for pharmacologic intervention are summarized in Fig. 3.
Fig. 3.
Potential pharmacologic targets for the treatment of ascites and hepatorenal syndrome in the nephron and splanchnic vascular territory and their pertinent receptors. α1, Alpha-adrenergic receptor; β1, Beta-adrenergic receptor; A1 and A2, Adenosine receptors 1 and 2; AldoR, Aldosterone receptor; AT1, Angiotensin-II receptor 1; ET-A, Endothelin receptor A: V1 and V2, Vasopressin receptors 1 and 2
Improvement of liver dysfunction and portal hypertension
Liver transplantation is the ultimate treatment option for HRS. Perioperatively, there may be a further deterioration of renal function, but within 1–2 months, GFR and RBF improve and hemodynamics and neurohumoral changes normalize [96] and most patients with pretransplant kidney dysfunction do not experience progression to advanced kidney disease after liver transplantation [97]. In the waiting time for a liver transplantation, the TIPS insertion has been used for portal decompression in patients with HRS [98].
Correction of circulatory dysfunction
The systemic administration of vasoconstrictors and plasma expanders in combination has shown beneficial effects on arterial vasodilatation, central hypovolemia, and renal function in patients with HRS [99, 100]. Terlipressin is a long-acting vasopressin analogue that stimulates splanchnic vasopressin V1a-receptors, and it has been shown to increase arterial blood pressure, GFR, and urine volume in patients with HRS and reversal in a considerable number of patients [101–103]. Different studies have shown that terlipressin and albumin increase arterial blood pressure, suppress vasoconstrictor systems, and improve renal function in patients with HRS [103, 104]. Despite the dramatic hemodynamic effects of terlipressin, central and arterial blood volume increases only slightly after terlipressin administration and the effect on central hypovolemia is only modest [95, 101]. In smaller studies, the combination of intravenously administered albumin and other vasoconstrictors such as ornipressin, noradrenaline, dopamine, somatostatin, and octreotide have been shown to increase GFR and normalize RAAS and SNS activity, although their effects are less potent [105–108]. However, when combined with the α-adrenergic agonist midodrine, octreotide may have a short-term effect on RBF, GFR, and sodium excretion in a few patients with HRS [95, 109]. In a recent study of 14 patients with type-1 HRS, the combination of midodrine, octreotide, and albumin significantly improved renal function [95, 110]. In a subset of the patients, TIPS insertion further improved renal function and sodium excretion for up to 12 months [110].
Recently, Ruiz-del-Arbol et al. [63] demonstrated that decompensated patients with SBP and renal failure had lower cardiac output than those without renal failure and the cardiac output further decreased in spite of antibiotic treatment. In these patients, renal failure might be precipitated as a mixed cirrhotic and septic cardiomyopathy [64, 111]. The HRS may thus develop as a combination of arterial vasodilatation, central hypovolemia, cardiac dysfunction, and renal vasoconstriction with renal hypoperfusion. Paracentesis should be considered in decompensated patients because it may improve renal perfusion by reducing the renal venous pressure [112]. However, a postparacentesis circulatory failure would have a negative effect on renal perfusion pressure because of a reduced arterial blood pressure, so a simultaneous infusion of albumin is therefore important in these patients [78, 81, 113]. Treatment should then be directed to support cardiac function and treat bacterial infections.
Support of neurohumoral regulation
Central hypovolemia and arterial hypotension lead to a volume- and baroreceptor-induced activation of RAAS and the increased plasma renin activity correlates inversely with RBF and GFR [114]. Infusion of pressor doses of angiotensin-II to decompensated patients increases renal perfusion and normalizes arterial blood pressure in some patients, but it may have no or harmful effects in others [3, 57]. Angiotensin-II mainly acts on the efferent arterioles, whereas afferent vasoconstriction is predominant in patients with HRS [114]. Low doses of the ACE inhibitor captopril induce a further reduction in GFR and filtration fraction, as well as sodium excretion [115]. Recently, infusion of the angiotensin-II receptor antagonist losartan decreased portal pressure in patients with cirrhosis, but without significant effects on renal function [116, 117]. In patients with HRS, RAAS is essential in counteracting arterial hypotension, and the administration of inhibitors of the system may have severe hypotensive action and further deteriorate renal and circulatory function.
In cirrhosis and HRS, arginine-vasopressin is increased primarily because of nonosmotic pituitary release [118]. Arginine-vasopressin induces vasoconstriction through V1-receptors and renal tubular water retention through V2-receptors in the collecting ducts [119]. In the kidneys, arginine-vasopressin acts on AQP2 in the collecting ducts and along with the secondary hyperaldosteronism contributes to the pronounced water reabsorption in patients with advanced HRS [120]. The action of arginine-vasopressin on renal vessels is limited, but systemic inhibition of V1-receptors in cirrhotic rats causes pronounced arterial hypotension [121]. Administration of the vasopressin V2-receptor antagonist VPA/985 has been found to improve dilutional hyponatremia in patients with refractory ascites [66, 67].
As this element is also present in HRS, this treatment may prove to be beneficial in improving free water clearance in these patients. Moreover, a V1-receptor agonist may improve systemic and portal circulation [122]. A progressive strategy for the treatment of sodium and fluid retention and renal complications in relation to the pathophysiological phases is shown in Table 3.
Table 3.
Suggestions for a progressive strategy in the treatment of sodium and fluid retention and renal complications in cirrhosis
| Clinical condition | Pathophysiologic phase | Treatment |
|---|---|---|
| Preascitic cirrhosis | Phase 1 | No treatment Modest salt restriction (80–120 mmol/day) |
| Mild ascites | Phase 2 | Salt restriction Stepwise spironolactone (100–400 mg/day) |
| Moderate-tense ascites | Phase 3 | Salt restriction Spironolactone Stepwise furosemide (40–160 mg/day) |
| Refractory ascites | Phase 3 | Large volume paracentesis and volume substitution and diuretics TIPS |
| Hyponatremia | Phases 3 and 4 | Serum sodium < 125 mmol/l: diuretics discontinued Serum sodium > 120 mmol/l: volume expansion Hyper/euvolemia: modest water restriction Experimental vasopressin V2-receptor antagonists |
| HRS | Phases 4 and 5 | Vasoconstrictors and albumin TIPS Liver transplantation |
Perspectives and conclusions
Ascites and its complications including HRS are conditions that are associated with poor prognoses despite treatment. However, our knowledge of the pathophysiology behind these severe complications has improved considerably, and there is now optimism with respect to novel medical treatments in combination with different pharmacological principles. The future approach will probably be to deal with different aspects in the pathophysiological process. A multitarget strategy should seek efficiently to counteract the arterial vasodilatation, central hypovolemia, and arterial hypotension by the administration of potent vasoconstrictors such as terlipressin in combination with plasma expanders such as albumin. Development of long-acting systemic vasoconstrictors should be encouraged. AQPs may have a future, especially in the treatment of dilutional hyponatremia. TIPS or β-blockers should be used to reduce portal pressure, whereas nitrates, COX-inhibitors, and nephrotoxic antibiotics should be used cautiously. Cardiac function should be supported, especially in the presence of simultaneous infections.
Abbreviations
- ET-1
Endothelin-1
- GFR
Glomerular filtration rate
- HRS
Hepatorenal syndrome
- RAAS
Renin–angiotensin–aldosterone system
- RBF
Renal blood flow
- SBP
Spontaneous bacterial peritonitis
- SNS
Sympathetic nervous system
- TIPS
Transjugular intrahepatic portosystemic shunt
References
- 1.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med 2004;350(16):1646–1654 [DOI] [PubMed]
- 2.Henriksen JH, Møller S. Alterations of hepatic and splanchnic microvascular exchange in cirrhosis: local factors in the formation of ascites. In Gines P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. Malden: Blackwell; 2005. 174–185
- 3.Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol 2003;38(Suppl 1):S69–S89 [DOI] [PubMed]
- 4.Fernandez-Esparrach G, Sanchez-Fueyo A, Gines P, Uriz J, Quinto L, Ventura PJ, et al. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol 2001;34(1):46–52 [DOI] [PubMed]
- 5.Guevara M, Cardenas A, Ginés P. Prognosis of patients with cirrhosis and ascites. In Ginés P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. Malden: Blackwell; 2005. 260–270
- 6.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005;41(3):422–433 [DOI] [PubMed]
- 7.Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol 2007;46(5):935–946 [DOI] [PubMed]
- 8.Dagher L, Moore K. The hepatorenal syndrome. Gut 2001;49(5):729–737 [DOI] [PMC free article] [PubMed]
- 9.Gentilini P, Vizzutti F, Gentilini A, Zipoli M, Foschi M, Romanelli RG. Update on ascites and hepatorenal syndrome. Dig Liver Dis 2002;34(8):592–605 [DOI] [PubMed]
- 10.Bernardi M, Caraceni P. Pathogenesis of ascites and hepatorenal syndrome: altered haemodynamics and neurohumoral systems. In Gerbes AL, Beuers U, Jungst D, Pape G, Sackmann M, Sauerbruch T, editors. Hepatology 2000. Falk Symposium 117, Munich, May 6, 2000. Dordrecht: Kluwer Academic Publishers; 2001. 185–203
- 11.Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol 2007;18(7):2028–2031 [DOI] [PubMed]
- 12.Kravetz D, Bildozola M, Argonz J, Romero G, Korula J, Munoz A, et al. Patients with ascites have higher variceal pressure and wall tension than patients without ascites. Am J Gastroenterol 2000;95(7):1770–1775 [DOI] [PubMed]
- 13.Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol 2003;38:S54–S68 [DOI] [PubMed]
- 14.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 2006;43(2 Suppl 1):S121–S131 [DOI] [PubMed]
- 15.Møller S, Henriksen JH. The systemic circulation in cirrhosis. In Gines P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. Malden: Blackwell; 2005. 139–155
- 16.Henriksen JH, Siemssen O, Krintel JJ, Malchow-Møller A, Bendtsen F, Ring-Larsen H. Dynamics of albumin in plasma and ascitic fluid in patients with cirrhosis. J Hepatol 2001;34(1):53–60 [DOI] [PubMed]
- 17.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut 2006;55(Suppl 6):vi1–vi12 [DOI] [PMC free article] [PubMed]
- 18.Casado M, Bosch J, Garciapagan JC, Bru C, Banares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114(6):1296–1303 [DOI] [PubMed]
- 19.Liu H, Schuelert N, McDougall JJ, Lee SS. Central neural activation of hyperdynamic circulation in portal hypertensive rats depends on vagal afferent nerves. Gut 2008;57:966–973 [DOI] [PubMed]
- 20.Wiest R. Splanchnic and systemic vasodilation: the experimental models. J Clin Gastroenterol 2007;41(10 Suppl 3):S272–S287 [DOI] [PubMed]
- 21.Ruiz-Del-Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–447 [DOI] [PubMed]
- 22.Ferguson JW, Dover A, Chia S, Cruden N, Hayes PC, Newby D. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut 2005;55(4):542–546 [DOI] [PMC free article] [PubMed]
- 23.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol 2007;46:927–934 [DOI] [PubMed]
- 24.Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 2002;35(2):478–491 [DOI] [PubMed]
- 25.Henriksen JH, Møller S. Hemodynamics, distribution of blood volume, and kinetics of vasoactive substances in cirrhosis. In Epstein M, editor. The Kidney in Liver Disease. Philadelphia: Hanley & Belfus; 1996. 241–258
- 26.Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver 2001;21(3):161–174 [DOI] [PubMed]
- 27.Helmy A, Newby DE, Jalan R, Johnston NR, Hayes PC, Webb DJ. Nitric oxide mediates the reduced vasoconstrictor response to angiotensin II in patients with preascitic cirrhosis. J Hepatol 2003;38(1):44–50 [DOI] [PubMed]
- 28.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut 2008;57(2):268–278 [DOI] [PubMed]
- 29.Henriksen JH, Møller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to elevated circulating calcitonin gene-related peptide (CGRP) and low adrenaline, but not to activated vasoconstrictor systems. Gut 2001;49:112–118 [DOI] [PMC free article] [PubMed]
- 30.Levy M. Pathogenesis of sodium retention in early cirrhosis of the liver: evidence for vascular overfilling. Semin Liver Dis 1994;14:4–13 [DOI] [PubMed]
- 31.La Villa G, Salmeron JM, Arroyo V, Bosch J, Gines P, Garcia-Pagan JC, et al. Mineralocorticoid escape in patients with compensated cirrhosis and portal hypertension. Gastroenterology 1992;102:2114–2119 [DOI] [PubMed]
- 32.Bernardi M, Li BS, Arienti V, de Collibus C, Scialpi C, Boriani L, et al. Systemic and regional hemodynamics in pre-ascitic cirrhosis: effects of posture. J Hepatol 2003;39(4):502–508 [DOI] [PubMed]
- 33.Wensing G, Lotterer E, Link I, Hahn EG, Fleig WE. Urinary sodium balance in patients with cirrhosis: relationship to quantitative parameters of liver function. Hepatology 1997;26:1149–1155 [DOI] [PubMed]
- 34.Møller S, Henriksen JH. Pathogenesis and pathophysiology of hepatorenal syndrome—is there scope for prevention? Aliment Pharmacol Ther 2004;20(Suppl 3):31–41 [DOI] [PubMed]
- 35.Angeli P, Wong F, Watson H, Gines P. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44(6):1535–1542 [DOI] [PubMed]
- 36.Bernardi M, Matco CD, Trevisani F, Collibus CD, Fornalé L, Baraldini M, et al. The hemodynamic status of preascitic cirrhosis: an evaluation under steady-state conditions and after postural change. Hepatology 1992;16:341–346 [DOI] [PubMed]
- 37.Sansoe G, Ferrari A, Baraldi E, Castellana CN, De Santis MC, Manenti F. Renal distal tubular handling of sodium in central fluid volume homoeostasis in preascitic cirrhosis. Gut 1999;45(5):750–755 [DOI] [PMC free article] [PubMed]
- 38.Claria J, Rodes J. Renal sodium handling in preascitic cirrhosis. Gut 2001;48(5):740–741 [DOI] [PMC free article] [PubMed]
- 39.Pozzi M, Grassi G, Redaelli E, Dell’Oro R, Ratti L, Redaelli A, et al. Patterns of regional sympathetic nerve traffic in preascitic and ascitic cirrhosis. Hepatology 2001;34(6):1113–1118 [DOI] [PubMed]
- 40.Bernardi M, Domenicali M. The renin-angiotensin-aldosterone system in cirrhosis. In Ginés P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. Malden: Blackwell; 2005. p. 43–54
- 41.Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet 2003;362(9398):1819–1827 [DOI] [PubMed]
- 42.Arroyo V, Fernandez J, Gines P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis 2008;28(1):81–95 [DOI] [PubMed]
- 43.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology 1996;23:164–176 [DOI] [PubMed]
- 44.Ring-Larsen H. Renal blood flow in cirrhosis: relation to systemic and portal haemodynamics and liver function. Scand J Clin Lab Invest 1977;37:635–642 [DOI] [PubMed]
- 45.La Villa G, Barletta G, Pantaleo P, Del Bene R, Vizzutti F, Vecchiarino S, et al. Hemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosis. Hepatology 2001;34(1):19–27 [DOI] [PubMed]
- 46.Epstein M. Renal sodium handling in liver disease. In Epstein M, editor. The Kidney in Liver Disease. Philadelphia: Hanley & Belfus; 1996. 1–31
- 47.Gerbes AL, Gulberg V, Waggershauser T, Holl J, Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology 1998;28(3):683–688 [DOI] [PubMed]
- 48.Moreau R, Asselah T, Condat B, de Kerguenec C, Pessione F, Bernard B, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut 2002;50(1):90–94 [DOI] [PMC free article] [PubMed]
- 49.Gines P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, del Arbol LR, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002;123(6):1839–1847 [DOI] [PubMed]
- 50.Laffi G, La Villa G, Gentilini P. Pathogenesis and management of the hepatorenal syndrome. Semin Liver Dis 1994;14(1):71–81 [DOI] [PubMed]
- 51.Arroyo V, Guevara M, Gines P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology 2002;122(6):1658–1676 [DOI] [PubMed]
- 52.Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transpl 2006;21(2):478–482 [DOI] [PMC free article] [PubMed]
- 53.Jalan R, Forrest EH, Redhead DN, Dillon JF, Hayes PC. Reduction in renal blood flow following acute increase in the portal pressure: evidence for the existence of a hepatorenal reflex in man? Gut 1997;40:664–670 [DOI] [PMC free article] [PubMed]
- 54.Kapoor D, Redhead DN, Hayes PC, Webb DJ, Jalan R. Systemic and regional changes in plasma endothelin following transient increase in portal pressure. Liver Transpl 2003;9(1):32–39 [DOI] [PubMed]
- 55.Møller S, Henriksen JH. Neurohumoral fluid regulation in chronic liver disease. Scand J Clin Lab Invest 1998;58(5):361–372 [DOI] [PubMed]
- 56.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral artery vasodilatation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;5:1151–1157 [DOI] [PubMed]
- 57.Henriksen JH, Ring-Larsen H. Hepatorenal disorders: role of the sympathetic nervous system. Semin Liver Dis 1994;14:35–43 [DOI] [PubMed]
- 58.Henriksen JH. Cirrhosis: ascites and hepatorenal syndrome. Recent advances in pathogenesis. J Hepatol 1995;23:25–30 [PubMed]
- 59.Wong F, Moore K, Dingemanse J, Jalan R. Lack of renal improvement with nonselective endothelin antagonism with tezosentan in type 2 hepatorenal syndrome. Hepatology 2008;47(1):160–168 [DOI] [PubMed]
- 60.Stadlbauer VP, Wright GA, Banaji M, Mukhopadhya A, Mookerjee R, Moore K, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology 2008;134(1):111–119 [DOI] [PubMed]
- 61.Dagher L, Patch D, Marley R, Moore K, Burroughs A. Review article: pharmacological treatment of the hepatorenal syndrome in cirrhotic patients. Aliment Pharmacol Ther 2000;14(5):515–521 [DOI] [PubMed]
- 62.Llach J, Ginés P, Arroyo V, Rimola A, Titó L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology 1988;94:482–487 [DOI] [PubMed]
- 63.Ruiz-Del-Arbol L, Urman J, Fernandez J, Gonzalez M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 2003;38(5):1210–1218 [DOI] [PubMed]
- 64.Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis 2008;28(1):59–69 [DOI] [PubMed]
- 65.Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac index predicts survival and renal failure in patients with ascites. Evidence of a heart-kidney axis in cirrhosis. J Hepatol 2008;48:S118 [DOI]
- 66.Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology 2003;37(1):182–191 [DOI] [PubMed]
- 67.Gerbes AL, Gulberg V, Gines P, Decaux G, Gross P, Gandjini H, et al. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology 2003;124(4):933–939 [DOI] [PubMed]
- 68.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008;28(1):26–42 [DOI] [PubMed]
- 69.Salerno F, Restelli B, Incerti P, Annoni G, Capozza L, Badalamenti S, et al. Utility of ascitic fluid analysis in patients with malignancy-related ascites. Scand J Gastroenterol 1990;25(3):251–256 [PubMed]
- 70.Ring-Larsen H, Henriksen JH, Wilken C, Clausen J, Pals H, Christensen NJ. Diuretic treatment in decompensated cirrhosis and congestive heart failure: effect of posture. BMJ 1986;292:1351–1353 [DOI] [PMC free article] [PubMed]
- 71.Gines P, Cardenas A. The management of ascites and hyponatremia in cirrhosis. Semin Liver Dis 2008;28(1):43–58 [DOI] [PubMed]
- 72.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38(1):258–266 [DOI] [PubMed]
- 73.Fernandez-Esparrach G, Guevara M, Sort P, Pardo A, Jimenez W, Gines P, et al. Diuretic requirements after therapeutic paracentesis in non-azotemic patients with cirrhosis. A randomized double-blind trial of spironolactone versus placebo. J Hepatol 1997;26(3):614–620 [DOI] [PubMed]
- 74.Salerno F, Badalamenti S, Lorenzano E, Moser P, Incerti P. Randomized comparative study of hemaccel vs. albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology 1991;13(4):707–713 [PubMed]
- 75.Fassio E, Terg R, Landeira G, Abecasis R, Salemne M, Podesta A, et al. Paracentesis with dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. J Hepatol 1992;14(2–3):310–316 [DOI] [PubMed]
- 76.Altman C, Bernard B, Roulot D, Vitte RL, Ink O. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. Eur J Gastroenterol Hepatol 1998;10(1):5–10 [DOI] [PubMed]
- 77.Planas R, Gines P, Arroyo V, Llach J, Panes J, Vargas V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Gastroenterology 1990;99:1736–1744 [DOI] [PubMed]
- 78.Cabrera J, Falcon L, Gorriz E, Pardo MD, Granados R, Quinones A, et al. Abdominal decompression plays a major role in early postparacentesis haemodynamic changes in cirrhotic patients with tense ascites. Gut 2001;48(3):384–389 [DOI] [PMC free article] [PubMed]
- 79.Pozzi M, Ratti L, Redaelli E, Guidi C, Mancia G. Cardiovascular abnormalities in special conditions of advanced cirrhosis. The circulatory adaptative changes to specific therapeutic procedures for the management of refractory ascites. Gastroenterol Hepatol 2006;29:263–272 [DOI] [PubMed]
- 80.Gines P, Guevara M, De Las HD, Arroyo V. Review article: albumin for circulatory support in patients with cirrhosis. Aliment Pharmacol Ther 2002;16(Suppl 5):24–31 [DOI] [PubMed]
- 81.Sola-Vera J, Minana J, Ricart E, Planella M, Gonzalez B, Torras X, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology 2003;37(5):1147–1153 [DOI] [PubMed]
- 82.Gines A, Fernandez-Esparrach G, Monescillo A, Vila C, Domenech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996;111:1002–1010 [DOI] [PubMed]
- 83.Moreau R, Valla DC, Durand-Zaleski I, Bronowicki JP, Durand F, Chaput JC, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trail. Liver Int 2006;26(1):46–54 [DOI] [PubMed]
- 84.Singh V, Kumar B, Nain CK, Singh B, Sharma N, Bhalla A, et al. Noradrenaline and albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized pilot study. J Intern Med 2006;260(1):62–68 [DOI] [PubMed]
- 85.Krag A, Møller S, Henriksen JH, Holstein-Rathlou NH, Larsen FS, Bendtsen F. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology 2007;46(6):1863–1871 [DOI] [PubMed]
- 86.Singh V, Dheerendra PC, Singh B, Nain CK, Chawla D, Sharma N, et al. Midodrine versus albumin in the prevention of paracentesis-induced circulatory dysfunction in cirrhotics: a randomized pilot study. Am J Gastroenterol 2008;103(6):1399–1405 [DOI] [PubMed]
- 87.D’Amico G, Luca A, Morabito A, Miraglia R, D’Amico M. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology 2005;129(4):1282–1293 [DOI] [PubMed]
- 88.Rossle M, Ochs A, Gulberg V, Siegerstetter V, Holl J, Deibert P, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med 2000;342(23):1701–1707 [DOI] [PubMed]
- 89.Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 2007;133(3):825–834 [DOI] [PubMed]
- 90.Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev 2006;(4):CD004889 [DOI] [PMC free article] [PubMed]
- 91.Esteva-Font C, Baccaro ME, Fernandez-Llama P, Sans L, Guevara M, Ars E, et al. Aquaporin-1 and aquaporin-2 urinary excretion in cirrhosis: relationship with ascites and hepatorenal syndrome. Hepatology 2006;44(6):1555–1563 [DOI] [PubMed]
- 92.Gines P. Vaptans: a promising therapy in the management of advanced cirrhosis. J Hepatol 2007;46(6):1150–1152 [DOI] [PubMed]
- 93.Gerbes AL, Gulberg V. Progress in treatment of massive ascites and hepatorenal syndrome. World J Gastroenterol 2006;12(4):516–519 [DOI] [PMC free article] [PubMed]
- 94.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis. A consensus workshop of the International Ascites Club. Gut 2007;56:1310–1318 [DOI] [PMC free article] [PubMed]
- 95.Angeli P, Merkel C. Pathogenesis and management of hepatorenal syndrome in patients with cirrhosis. J Hepatol 2008;48(Suppl 1):S93–S103 [DOI] [PubMed]
- 96.Navasa M, Feu F, Garciapagan JC, Jimenez W, Llach J, Rimola A, et al. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology 1993;17:355–360 [PubMed]
- 97.Bahirwani R, Campbell MS, Siropaides T, Markmann J, Olthoff K, Shaked A, et al. Transplantation: impact of pretransplant renal insufficiency. Liver Transpl 2008;14(5):665–671 [DOI] [PubMed]
- 98.Guevara M, Gines P, Bandi JC, Gilabert R, Sort P, Jimenez W, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology 1998;28(2):416–422 [DOI] [PubMed]
- 99.Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D, et al. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol 1998;29(4):565–570 [DOI] [PubMed]
- 100.Uriz J, Gines P, Cardenas A, Sort P, Jimenez W, Salmeron JM, et al. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol 2000;33(1):43–48 [DOI] [PubMed]
- 101.Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver 2000;20(1):51–59 [DOI] [PubMed]
- 102.Mulkay JP, Louis H, Donckier V, Bourgeois N, Adler M, Deviere J, et al. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg 2001;64(1):15–19 [PubMed]
- 103.Ortega R, Gines P, Uriz J, Cardenas A, Calahorra B, De Las HD, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology 2002;36(4 Pt 1):941–948 [DOI] [PubMed]
- 104.Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichai P, et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology 2002;122(4):923–930 [DOI] [PubMed]
- 105.Guevara M, Gines P, Fernandezesparrach G, Sort P, Salmeron JM, Jimenez W, et al. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology 1998;27:35–41 [DOI] [PubMed]
- 106.Ottesen LH, Aagaard NK, Kiszka-Kanowitz M, Rehling M, Henriksen JH, Pedersen EB, et al. Effects of a long-acting formulation of octreotide on renal function and renal sodium handling in cirrhotic patients with portal hypertension: a randomized, double-blind, controlled trial. Hepatology 2001;34(3):471–477 [DOI] [PubMed]
- 107.Duvoux C, Zanditenas D, Hezode C, Chauvat A, Monin JL, Roudot-Thoraval F, et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology 2002;36(2):374–380 [DOI] [PubMed]
- 108.Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology 2003;38(1):238–243 [DOI] [PubMed]
- 109.Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology 1999;29(6):1690–1697 [DOI] [PubMed]
- 110.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 2004;40(1):55–64 [DOI] [PubMed]
- 111.Van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol 1996;24(6):747–752 [DOI] [PubMed]
- 112.Luca A, Feu F, Garcia-Pagan JC, Jiménez W, Arroyo V, Bosch J, et al. Favorable effects of total paracentesis on splanchnic hemodynamics in cirrhotic patients with tense ascites. Hepatology 1994;20:30–33 [DOI] [PubMed]
- 113.Ruiz del Arbol L, Monescillo A, Jimenez W, Garcia-Plaza A, Arroyo V, Rodes J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology 1997;113:579–586 [DOI] [PubMed]
- 114.Bernardi M, Trevisani F, Gasbarrini A, Gasbarrini G. Hepatorenal disorders. Role of the renin-angiotensin-aldosterone system. Semin Liver Dis 1994;14:23–34 [DOI] [PubMed]
- 115.Gentilini P, Romanelli RG, La Villa G, Maggiore Q, Pesciullesi E, Cappelli G, et al. Effects of low-dose captopril on renal hemodynamics and function in patients with cirrhosis of the liver. Gastroenterology 1993;104:588–594 [DOI] [PubMed]
- 116.Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999;29(2):334–339 [DOI] [PubMed]
- 117.Garcia-Tsao G. Angiotensin II receptor antagonists in the pharmacological therapy of portal hypertension: a caution. Gastroenterology 1999;117(3):740–742 [DOI] [PubMed]
- 118.Epstein M, Weitzman RE, Preston S, Denunzio AG. Relationship between plasma arginine vasopressin and renal water handling in decompensated cirrhosis. Miner Electrolyte Metab 1984;10:155–165 [PubMed]
- 119.Guyader D, Patat A, Ellis-Grosse EJ, Orczyk GP. Pharmacodynamic effects of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology 2002;36(5):1197–1205 [DOI] [PubMed]
- 120.Fernandez-Llama P, Turner R, Dibona G, Knepper MA. Renal expression of aquaporins in liver cirrhosis induced by chronic common bile duct ligation in rats. J Am Soc Nephrol 1999;10(9):1950–1957 [DOI] [PubMed]
- 121.Claria J, Jimenez W, Arroyo V, La Villa G, Lopez C, Asbert M, et al. Effect of V-1-vasopressin receptor blockade on arterial pressure in conscious rats with cirrhosis and ascites. Gastroenterology 1991;100:494–501 [DOI] [PubMed]
- 122.Morales J, Moitinho E, Abraldes JG, Fernandez M, Bosch J. Effects of the V1a vasopressin agonist F-180 on portal hypertension-related bleeding in portal hypertensive rats. Hepatology 2003;38(6):1378–1383 [DOI] [PubMed]