Abstract
Background
Liver stiffness measurement (LSM) using transient elastography (FibroScan®) reflects the degree of hepatic fibrosis. This prospective study investigated how well LSM predicts the development of hepatic insufficiency after curative liver resection surgery for hepatocellular carcinoma.
Methods
The study enrolled 72 consecutive patients who underwent a preoperative LSM to assess the degree of liver fibrosis followed by curative liver resection surgery for hepatocellular carcinoma between July 2006 and December 2007. The primary end point was the development of hepatic insufficiency.
Results
The mean age of the patients was 54.9 years. Twenty patients (27.7%) had chronic hepatitis and 52 (72.3%) had cirrhosis (44 and 8 patients showed Child-Pugh class A and B, respectively). The mean LSM was 17.1 kPa. Twelve patients (16.6%) had segmentectomy only, 16 patients (22.2%) had bisegmentectomy, and 44 patients (61.2%) had lobectomy. Nine patients (12.5%) had stage I tumor, 56 (77.7%) had stage II, and 7 (9.8%) had stage III. Univariate and subsequent multivariate analyses revealed that preoperative LSM was the only independent risk factor for predicting the development of postoperative hepatic insufficiency (cutoff, 25.6 kPa; P = 0.001; relative risk, 19.14; 95% confidence interval, 2.71–135.36).
Conclusions
LSM is potentially useful to predict the development of postoperative hepatic insufficiency in patients with hepatocellular carcinoma undergoing curative liver resection surgery.
Keywords: Liver stiffness measurement, Indocyanine green, Hepatic insufficiency, Hepatectomy
Introduction
With considerable improvements in perioperative intensive care and refinements in surgical technique, the rates of death and complications after major liver resection surgery have decreased significantly [1–4]. Nevertheless, because many patients still have liver cirrhosis or other chronic liver disease, death and complications may follow liver resection surgery. Therefore, it is important to investigate the functional liver reserve before liver resection surgery [5–7]. The Child-Pugh scoring system is widely used to determine the hepatic functional status, although its ability to predict mortality after liver resection surgery is inconsistent. Consequently, various laboratory and imaging techniques have been used to complement the Child-Pugh scoring system in order to predict the development of postoperative hepatic insufficiency. For example, the serum hyaluronic acid (HA) level, liver volumetry measured using computed tomographic (CT) scan, hepatic uptake ratio of technetium-99m-diethylene triaminepentaacetic acid galactosyl-human serum albumin at 15 min (LHL 15), indocyanine green retention rate at 15 min (ICG R15), and hepatic venous pressure gradient are usually performed [8–10]. These preoperative tests are important because they allow physicians to decide the extent of liver resection [11]. Such careful preoperative evaluation of liver function together with the refined operating techniques has significantly reduced the incidence of postoperative hepatic insufficiency [12–14].
Of these preoperative functional tests, hepatic venous pressure gradient is a widely used preoperative test to estimate the degree of hepatic fibrosis and liver reserve in Western countries, whereas ICG R15 is used in Eastern countries including Korea and Japan. However, ICG R15 remains imperfect because of its dependency on both the hepatic flow and the functional capacity of the liver [15]. Recently, liver stiffness measurement (LSM) using FibroScan® was reported to reflect the degree of hepatic fibrosis, which is an important factor determining the functional liver reserve [16]. Therefore, we hypothesized that LSM can predict the hepatic functional reserve.
This prospective study investigated the usefulness of LSM as a predictor of the liver reserve.
Patients and methods
Patients
In this pilot study, 91 consecutive patients who were eligible for curative liver resection surgery for hepatocellular carcinoma between July 2006 and December 2007 at Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea, were enrolled prospectively. Patients who underwent surgery because of causes other than hepatocellular carcinoma or had Child-Pugh class C liver function were excluded.
Among all enrolled patients, 19 patients who underwent liver transplantation were also excluded. Written informed consent was obtained from all patients. This study was approved by the Institutional Review Board of Severance Hospital.
The primary end point was the development of postoperative hepatic insufficiency. Hepatic insufficiency was defined as persistent hyperbilirubinemia (total bilirubin level >5 mg/dl) for more than 5 days after surgery or postoperative death without other causes [17, 18].
Liver stiffness measurement
On the same day that the ICG R15 test was performed, liver stiffness in the right lobe of the liver was measured, using FibroScan®, through the intercostal spaces with the patient lying in the dorsal decubitus position and with the right arm in maximal abduction. The tip of the transducer probe was covered with coupling gel and placed on the skin between the ribs at the level of the right lobe of the liver. Before performing FibroScan® in all patients, sonographic evaluation was used to target nontumor liver parenchyma. The operator, assisted by real time ultrasound, located a liver portion that was at least 6-cm thick and free of large vascular structures, and then pressed the probe button to commence the measurements. Ten validated measurements were performed on each patient. The success rate was calculated as the number of validated measurements divided by the total number of measurements. The results were expressed in kilopascals (kPa). The median value was considered as representative of the elastic modulus of the liver. Only procedures with ten validated measurements and a success rate of at least 60% were considered reliable.
ICG R15 evaluation
After an overnight fast, 0.5 mg/kg of ICG was administered intravenously. Blood samples were drawn at 5, 10, and 15 min and the plasma ICG concentration was measured spectrophotometrically (710 nm). The plasma retention rate at 15 min (ICG R15, %) and the plasma disappearance rate (ICG-k, min−1) were calculated.
Liver resection surgery
All the patients were examined to confirm the number, size, location, and extent of the tumor and the existence of distant metastases by abdominal ultrasonography, CT scan, magnetic resonance imaging, hepatic angiography, and positron emission tomography. In addition to preoperative routine laboratory examinations and physical examination for determining Child-Pugh classification, ICG R15 was performed to determine the optimal treatment strategy. Anatomical resection was performed according to tumor size, location, and liver reserve function. Indications for hepatic resection and the types of operative procedures were mainly determined on the basis of the criteria of Makuuchi, i.e., the presence or absence of ascites, the serum total bilirubin level, and ICG R15 [19]. All liver resection surgeries were performed by two surgeons (J.S. Choi and K.S. Kim) and followed the anatomic definitions of segments and lobes of Couinaud [20]. Patients routinely underwent intraoperative ultrasonography to determine tumor localization and extent and to exclude the presence of additional lesions in the residual liver.
Statistical analysis
Patient characteristics are given as the mean ± SD or median (range). Continuous variables were compared using an independent t-test and categorical variables were compared using χ2-test. A two-sided P-value < 0.05 was considered significant.
Variables associated with the development of postoperative hepatic insufficiency were first assessed using a univariate analysis. Then, the variables that were significant (P < 0.1) were subjected to multivariate logistic regression analysis to identify the independent predictors for the development of postoperative hepatic insufficiency.
The optimal cutoff value for liver stiffness was set as the value maximizing the sum of sensitivity and specificity. The predictive ability of LSM and ICG R15 was assessed by the receiver operating characteristic (ROC) curve and corresponding area under the ROC (AUROC) curve for each. All statistical analyses were performed with SPSS 12.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
The baseline characteristics of the patients are summarized in Table 1. The mean age of the 72 patients (56 men and 16 women) was 54.9 years. The surgical specimens of the 72 hepatocellular carcinomas revealed a background of chronic hepatitis in 20 cases (27.7%) and cirrhosis in 52 cases (72.3%). Forty-four patients had Child-Pugh class A and eight had class B. The mean liver stiffness value was 17.1 kPa and the mean ICG R15 was 11.8%. There were no dropouts due to LSM failure.
Table 1.
Patient baseline characteristics (n = 72)
| Variables | n (%), mean ± SD, or median (range) |
|---|---|
| Male | 56 (72.2%) |
| Age (years) | 54.9 ± 10.6 |
| Background liver diseasea | |
| CHB/child A cirrhosis/child B cirrhosis | 20 (27.7%)/44 (61.1%)/8 (11.2%) |
| Body mass index (kg/m2) | 24.0 ± 2.8 |
| White blood cell count (103/μl) | 5,740 (2,420–26,930) |
| Hemoglobin (g/dl) | 14.1 ± 1.5 |
| Platelet count (109/l) | 154.2 ± 72.8 |
| Total protein (mg/dl) | 7.0 ± 1.1 |
| Albumin (mg/dl) | 4.1 ± 0.7 |
| Total bilirubin (mg/dl) | 1.2 ± 0.6 |
| AST (IU/l) | 48.5 ± 49.3 |
| ALT (IU/l) | 49.3 ± 40.9 |
| Cholesterol (mg/dl) | 150.2 ± 32.2 |
| Gamma glutamyltranspeptidase (IU/l) | 63.4 ± 70.5 |
| Alkaline phosphatase (IU/l) | 95.6 ± 45.2 |
| Prothrombin time (%) | 90.0 ± 9.5 |
| Alpha-feto protein (ng/ml) | 15.5 (2.6–39,879.7) |
| <20 | 41 (56.9%) |
| <400 and ≥20 | 17 (23.6%) |
| ≥400 | 14 (19.5%) |
| Spleen size (cm) | 10.8 ± 2.29 |
| ICG R15 (%) | 11.8 ± 7.1 |
| Liver stiffness/IQR (kPa)/SR | 17.1 ± 11.2/1.7 ± 0.8/88.8% |
CHB, chronic hepatitis B; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ICG R15, indocyanine green retention at 15 min; IQR, interquartile range; SR, success rate
aBackground liver disease was defined after surgery
Operation and tumor characteristics
Twelve patients (16.6%) had segmentectomy only, 16 patients (22.2%) had bisegmentectomy, and 44 patients (61.2%) had lobectomy. According to the Tumor-Node-Metastasis stage of the modified Union Internationale Contre le Cancer (UICC) staging system, 9 patients (12.5%) had stage I tumor, 56 patients (77.7%) had stage II tumor, and 7 patients (9.8%) had stage III tumor (Table 2).
Table 2.
Operation and tumor characteristics (n = 72)
| Variables | n (%), mean ± SD, or median (range) |
|---|---|
| Etiology | |
| HBV/HCV/non-B and non-C | 60 (83.3%)/9 (12.5%)/3 (4.2%) |
| Operation method | |
| Segmentectomy | 12 (16.6%) |
| Bisegmentectomy | 16 (22.2%) |
| Trisegmentectomy | 0 (0.0%) |
| Lobectomy | 44 (61.2%) |
| Right/left/central | 28/12/4 |
| Blood loss (cc) | 800 (10–8,100) |
| Operation time (min) | 341.4 ± 147.9 |
| Tumor size (cm) | 3.32 ± 1.82 |
| Tumor number | |
| One | 65 (90.2%) |
| Two | 7 (9.8%) |
| Tumor site | |
| Right | 37 (51.4%) |
| Left | 33 (45.8%) |
| Both | 2 (2.8%) |
| Tumor stagea | |
| Stage I | 9 (12.5%) |
| Stage II | 56 (77.7%) |
| Stage III | 7 (9.8%) |
HBV, hepatitis B-virus; HCV, hepatitis C-virus
aTumor stage is expressed according to the modified UICC staging system
Comparison between patients with and without postoperative hepatic insufficiency
Seven patients had hepatic insufficiency postoperatively, and there was no mortality associated with liver resection surgery. Surgical specimens of those with postoperative hepatic insufficiency revealed that all of them had liver cirrhosis. The seven patients who developed postoperative hepatic insufficiency after curative liver resection surgery had a significantly higher mean LSM before surgery (26.8 ± 9.5 kPa) than those without postoperative hepatic insufficiency (15.1 ± 10.5, P = 0.010). The other tested variables affecting liver function did not differ statistically between the groups (Table 3).
Table 3.
Comparison between patients without and with postoperative hepatic insufficiency
| Variables | Patients without hepatic insufficiency (n = 65) | Patients with hepatic insufficiency (n = 7) | P-value |
|---|---|---|---|
| Age (years) | 55.3 ± 10.3 | 52.9 ± 12.6 | 0.580 |
| Liver stiffness (kPa) | 15.1 ± 10.5 | 26.8 ± 9.5 | 0.010 |
| AST (IU/l) | 51.1 ± 53.6 | 35.4 ± 10.5 | 0.448 |
| ALT (IU/l) | 51.9 ± 44.0 | 36.1 ± 15.6 | 0.358 |
| Albumin (mg/dl) | 4.15 ± 0.68 | 4.13 ± 0.90 | 0.229 |
| Total bilirubin (mg/dl) | 1.19 ± 0.59 | 1.34 ± 0.98 | 0.292 |
| Prothrombin time (%) | 90.1 ± 9.7 | 89.4 ± 9.2 | 0.858 |
| Platelet (109/L) | 159.4 ± 66.1 | 128.6 ± 102.7 | 0.313 |
| ICG R15 (%) | 11.3 ± 7.0 | 14.2 ± 7.5 | 0.317 |
| Blood loss (cc) | 1,222.1 ± 1,418.3 | 2,028.6 ± 2,751.2 | 0.258 |
| Operation time (min) | 322.6 ± 125.2 | 435.7 ± 219.5 | 0.229 |
| Tumor size (cm) | 3.42 ± 1.86 | 2.84 ± 1.63 | 0.453 |
| Tumor stage I/II/III (n) | 8/51/6 | 1/5/1 | 0.170 |
| <lobectomy vs. ≥lobectomy (n) | 27/38 | 1/6 | 0.010 |
ALT, alanine aminotransferase; ICG R15, indocyanine green retention at 15 min
a25.6 kPa of liver stiffness and 12.0% of ICG R15 are the cutoff of best accuracy
All patients who developed hepatic insufficiency postoperatively decompensated with ascites.
Prediction accuracy of LSM and ICG R15
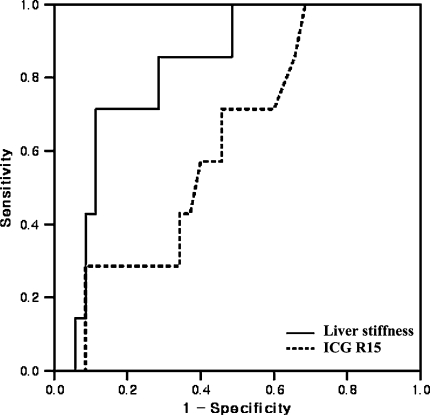
In order to compare the predictive value of LSM and ICG R15, we first analyzed ROC curve of LSM and R15 (Fig. 1). Corresponding AUROC curve was 0.824 (95% confidence interval, 0.682–0.967; P = 0.007) for LSM and 0.620 (95% confidence interval, 0.422–0.819; P = 0.319) for the ICG R15, respectively. The cutoff of LSM was set at 25.6 kPa, which gave the best statistical accuracy (sensitivity, 71.4%; specificity, 88.6%; positive predictive value, 55.6%; and negative predictive value, 93.9%).
Fig. 1.
The ROC curves of LSM and ICG R15 for predicting postoperative hepatic insufficiency (0.824 and 0.620, respectively)
Analyses for identifying the risk factors predicting development of postoperative hepatic insufficiency
Table 4 lists the results of the univariate and subsequent multivariate logistic regression analyses for identifying the various clinicopathologic factors associated with postoperative hepatic insufficiency.
Table 4.
Logistic regression analysis of risk factors associated with postoperative hepatic insufficiency after hepatectomy
| Variables | P-value | Odds ratio | |
|---|---|---|---|
| Univariate | Multivariate | ||
| Gender | |||
| Male vs. female | 0.247 | – | |
| Age (years) | |||
| <55 vs. ≥55 | 0.885 | – | |
| Background liver disease | |||
| Noncirrhosis vs. cirrhosis | 0.099 | 0.922 | 1.214 |
| Body mass index (kg/m2) | |||
| <30 vs. ≥30 | 0.308 | – | |
| Child-Pugh class | |||
| A vs. B | 0.434 | – | |
| Total bilirubin (mg/dl) | |||
| <1.2 vs. ≥1.2 | 0.337 | – | |
| Albumin (mg/dl) | |||
| <4.0 vs. ≥4.0 | 1.000 | – | |
| ALT (IU/l) | |||
| <50 vs. ≥50 | 0.308 | – | |
| Cholesterol (mg/dl) | |||
| <150 vs. ≥150 | 0.415 | – | |
| Prothrombin time (%) | |||
| <90 vs. ≥90 | 0.836 | – | |
| Tumor stage | |||
| Stage I vs. stages II and III | 0.836 | – | |
| Stage I and II vs. stage III | 0.434 | – | |
| Type of resection | |||
| <Lobectomy vs. ≥lobectomy | 0.142 | – | |
| Operative bleeding (ml) | |||
| <1,350 vs. ≥1,350 | 0.604 | – | |
| Operative time (min) | |||
| <340 vs. ≥340 | 0.783 | – | |
| Blood transfusion | |||
| Yes vs. no | 0.675 | – | |
| Liver stiffness (kPa) | |||
| <25.6 vs. ≥25.6a | <0.001 | 0.001 | 19.140 |
| ICG R15 (%) | |||
| <10.0 vs. ≥10.0 | 0.415 | – | |
| <12.0 vs. ≥12.0a | 0.675 | – | |
| <15.0 vs. ≥15.0 | 0.753 | – | |
Multivariate analysis identified LSM as the only significant predictor of postoperative hepatic insufficiency (cutoff 25.6 kPa, P = 0.001; relative risk, 19.14; 95% confidence interval, 2.71–135.36).
Discussion
Liver transplantation, liver resection, and local ablation therapy are curative treatments for hepatocellular carcinoma. Among them, liver transplantation is the best option because it is the only treatment that offers a chance of cure for hepatocellular carcinoma and the underlying cirrhosis by complete extirpation of both. However, the limitation of organ supply remains unresolved. Therefore, liver resection surgery for curative goal is widely performed instead of liver transplantation regardless of restriction of its application to a liver with limited functional reserve and high chance of recurrence in the liver remnant [21].
Because surgery removes parts of the functioning liver, the volume of the remnant liver determines the risk of postoperative hepatic insufficiency, which is the major cause of mortality and morbidity after liver resection surgery, especially in the cirrhotic liver.
The lack of well-designed, randomized, controlled trials, the use of different staging systems, and the different definitions of postoperative hepatic insufficiency have led to the confusion in the analysis of postoperative outcomes for liver resection surgery [22]. Careful preoperative evaluation of the functional liver reserve is necessary to minimize the postoperative morbidity and mortality in cirrhotic and noncirrhotic patients [12, 13]. Several preoperative tests are available for such purposes, including the serum HA assay, liver volumetry using CT scan, LHL 15, ICG R15, and hepatic venous pressure gradient [8–10]. Of these, the ICG R15 is the most reliable and widely available test to determine the extent of liver resection and liver reserve function in Eastern countries [12]. Although, the ICG R15 has some limitations because it depends on both the hepatic flow and the functional capacity of the liver, there is general agreement concerning the retention value [15].
Poon et al. [23] assessed the patient suitability for liver resection surgery by evaluating the Child-Pugh score combined with ICG R15 measurements; in their study, the occurrence of hepatic failure was 1%. Torzilli et al. [4] reported a preoperative evaluation pattern for liver resection surgery, which included the presence of ascites, serum bilirubin levels, and estimation of the ICG R15, and reported no mortality after liver resection in 107 patients.
Recently, LSM has been shown to reflect the degree of hepatic fibrosis, which is an important determinant of the development of postoperative hepatic insufficiency [16]. Therefore, we postulated that LSM could be used to predict postoperative hepatic insufficiency before liver resection surgery. In order to test this hypothesis, we compared the abilities of LSM and ICG R15 in predicting the development of hepatic insufficiency after curative liver resection surgery. Although several studies have already reported the correlation between intraoperative liver consistency using specific probes and postoperative outcome [24–26], to the best of our knowledge, no other study has investigated LSM as a preoperative evaluation for predicting the development of postoperative hepatic insufficiency after liver resection surgery, compared with the relationship for ICG R15.
In this study, the cutoff liver stiffness value was set at 25.6 kPa, which gave the best accuracy. Multivariate logistic regression analysis revealed that LSM was the only independent predictor of the development of postoperative hepatic insufficiency. In terms of the AUROC for predicting hepatic insufficiency, the value for LSM was greater than that for the ICG R15. Therefore, in our study population, LSM was better than the ICG R15 in predicting the development of postoperative hepatic insufficiency.
We are aware of several limitations of our study. First, the multivariate logistic regression analysis did not include other variables that can affect the outcome of surgery, such as the serum HA level, which is closely correlated with the functional liver reserve and is a useful predictor of liver regeneration [27], or the total or resected liver volume measured using CT scan. Second, there were some factors differently represented between who showed hepatic insufficiency and who did not, such as total bilirubin level, bleeding amount, operation time, and the portion of undergoing lobectomy, although there were no statistical differences between the two groups. These points might influence the final results. Finally, because all enrolled patients showed chronic liver disease status, the results of this study are not applicable to patients without chronic liver disease. In order to overcome these limitations, a well-designed, well-controlled, randomized study of a large population is required.
In conclusion, our study showed that preoperative LSM was significantly higher in patients who developed postoperative hepatic insufficiency than in those patients who did not. Therefore, our results suggest that the preoperative liver stiffness is a potentially useful predictor of the development of postoperative hepatic insufficiency in patients with hepatocellular carcinoma undergoing liver resection surgery.
Acknowledgments
The authors thank Eun Hee Choi (Department of Biostatistics, Yonsei University College of Medicine, Seoul, Republic of Korea) for critical comments on statistics and Hyo Jin Yang for FibroScan® examination. This study was supported by the grant of the Good Health R&D Project from the Ministry of Health and Welfare, Republic of Korea (A050021), and in part by the grant from Brain Korea 21 Project for Medical Science.
Footnotes
S. U. Kim and S. H. Ahn equally contributed to this work.
References
- 1.Lee JG, Kang CM, Park JS, Kim KS, Yoon DS, Choi JS, et al. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J 2006;47:140–143 [DOI] [PMC free article] [PubMed]
- 2.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995;221:291–298. doi:10.1097/00000658-199503000-00012 [DOI] [PMC free article] [PubMed]
- 3.Shuto T, Hirohashi K, Kubo S, Tanaka H, Tsukamoto T, Yamamoto T, et al. Changes and results of surgical strategies for hepatocellular carcinoma: results of a 15-year study on 452 consecutive patients. Surg Today 1998;28:1124–1129. doi:10.1007/s005950050299 [DOI] [PubMed]
- 4.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg 1999;134:984–992. doi:10.1001/archsurg.134.9.984 [DOI] [PubMed]
- 5.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon’s role in long-term survival. Arch Surg 1999;134:1124–1130. doi:10.1001/archsurg.134.10.1124 [DOI] [PubMed]
- 6.Furuhama K, Yabe K. Application of hepatic tolerance tests to the functional reserve assessment in rat models of fatty liver. J Vet Med Sci 1998;60:635–637. doi:10.1292/jvms.60.635 [DOI] [PubMed]
- 7.Asano M, Ozawa K, Tobe T. Postoperative prognosis as related to blood ketone body ratios in hepatectomized patients. Eur Surg Res 1983;15:302–311. doi:10.1159/000128373 [DOI] [PubMed]
- 8.Nanashima A, Yamaguchi H, Shibasaki S, Sawai T, Yamaguchi E, Yasutake T, et al. Measurement of serum hyaluronic acid level during the perioperative period of liver resection for evaluation of functional liver reserve. J Gastroenterol Hepatol 2001;16:1158–1163. doi:10.1046/j.1440-1746.2001.02599.x [DOI] [PubMed]
- 9.Hwang EH, Taki J, Shuke N, Nakajima K, Kinuya S, Konishi S, et al. Preoperative assessment of residual hepatic functional reserve using 99mTc-DTPA-galactosyl-human serum albumin dynamic SPECT. J Nucl Med 1999;40:1644–1651 [PubMed]
- 10.Ribero D, Abdalla EK, Thomas MB, Vauthey JN. Liver resection in the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther 2006;6:567–579. doi:10.1586/14737140.6.4.567 [DOI] [PubMed]
- 11.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176–1181 [DOI] [PubMed]
- 12.Lau H, Fan ST, Ng IO, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer 1998;83:2302–2311. doi: 10.1002/(SICI)1097-0142(19981201)83:11<2302::AID-CNCR9>3.0.CO;2-1 [DOI] [PubMed]
- 13.Noguchi T, Imai T, Mizumoto R. Preoperative estimation of surgical risk of hepatectomy in cirrhotic patients. Hepatogastroenterology 1990;37:165–171 [PubMed]
- 14.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–862. doi:10.1016/j.jamcollsurg.2006.12.032 [DOI] [PubMed]
- 15.Schneider PD. Preoperative assessment of liver function. Surg Clin North Am 2004;84:355–373. doi:10.1016/S0039-6109(03)00224-X [DOI] [PubMed]
- 16.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41:48–54. doi:10.1002/hep.20506 [DOI] [PubMed]
- 17.Nanashima A, Yamaguchi H, Tanaka K, Shibasaki S, Tsuji T, Ide N, et al. Preoperative serum hyaluronic acid level as a good predictor of posthepatectomy complications. Surg Today 2004;34:913–919. doi:10.1007/s00595-004-2845-y [DOI] [PubMed]
- 18.Ohwada S, Kawate S, Hamada K, Yamada T, Sunose Y, Tsutsumi H, et al. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg 2006;93:339–346. doi:10.1002/bjs.5258 [DOI] [PubMed]
- 19.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298–304. doi:10.1002/ssu.2980090404 [DOI] [PubMed]
- 20.Couinaud C. Le foie, In Masson, editor. Etudes anatomiques et chirurgicales. Paris; 1957. 469–479
- 21.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology 2004;127 Suppl 1:S248–S260. doi:10.1053/j.gastro.2004.09.039 [DOI] [PubMed]
- 22.Wildi S, Pestalozzi BC, McCormack L, Clavien PA. Critical evaluation of the different staging systems for hepatocellular carcinoma. Br J Surg 2004;91:400–408. doi:10.1002/bjs.4554 [DOI] [PubMed]
- 23.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg 2002;236:602–611. doi:10.1097/00000658-200211000-00010 [DOI] [PMC free article] [PubMed]
- 24.Yamanaka N, Okamoto E, Toyosaka A, Ohashi S, Tanaka N. Consistency of human liver. J Surg Res 1985;39:192–198. doi:10.1016/0022-4804(85)90142-8 [DOI] [PubMed]
- 25.Nishizaki T, Matsumata T, Kamakura T, Adachi E, Sugimachi K. Significance of intraoperative measurement of liver consistency prior to hepatic resection. Hepatogastroenterology 1995;42:5–8 [PubMed]
- 26.Kusaka K, Harihara Y, Torzilli G, Kubota K, Takayama T, Makuuchi M, et al. Objective evaluation of liver consistency to estimate hepatic fibrosis and functional reserve for hepatectomy. J Am Coll Surg 2000;191:47–53. doi:10.1016/S1072-7515(00)00309-4 [DOI] [PubMed]
- 27.Kanematsu T, Takenaka K, Matsumata T, Furuta T, Sugimachi K, Inokuchi K. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg 1984;199:51–56. doi:10.1097/00000658-198401000-00009 [DOI] [PMC free article] [PubMed]