Abstract
A novel microstirring strategy is applied to accelerate the digestion rate of the substrate Nα-benzoyl-L-arginine-4-nitroanilide (L-BAPA) catalyzed by sol-gel encapsulated trypsin. We use an ac nonlinear electrokinetic vortex flow to stir the solution in a microfluidic reaction chamber to reduce the diffusion length between the immobilized enzyme and substrate in the solution. High-intensity nonlinear electroosmotic microvortices, with angular speeds in excess of 1 cm∕s, are generated around a small (∼1.2 mm) conductive ion exchange granule when ac electric fields (133 V∕cm) are applied across a miniature chamber smaller than 10 μl. Coupling between these microvortices and the on-and-off electrophoretic motion of the granule in low frequency (0.1 Hz) ac fields produces chaotic stream lines to stir substrate molecules sufficiently. We demonstrate that, within a 5-min digestion period, the catalytic reaction rate of immobilized trypsin increases almost 30-fold with adequate reproducibility (15%) due to sufficient stirring action through the introduction of the nonlinear electrokinetic vortices. In contrast, low-frequency ac electroosmotic flow without the granule, provides limited stirring action and increases the reaction rate approximately ninefold with barely acceptable reproducibility (30%). Dye molecules are used to characterize the increases in solute diffusivity in the reaction reservoir in which sol-gel particles are placed, with and without the presence of granule, and compared with the static case. The solute diffusivity enhancement data show respective increases of ∼30 and ∼8 times, with and without the presence of granule. These numbers are consistent with the ratios of the enhanced reaction rate.
INTRODUCTION
Despite the small dimensions of microfluidic devices, reactant transport by diffusion still requires an unacceptably long time, especially when one of the reactants is immobilized. For moderately slow analyte diffusion coefficients of D=5×10−6 cm2∕s (organic molecules or short peptides), the docking time t=l2∕D within an l=1 mm, the characteristic length of a microreaction chamber, is approximately 40 min. This long transport time problem is a bottleneck to develop high throughput screening protocols when one of the reactants is static. For instance sol-gel immobilized enzymes, which remain static at the bottom of a reaction chamber, catalyze cleavage of substrates. Immobilized enzymes are often used to lower their rate of denaturation or inactivation, to avoid secondary reactions like autoproteolysis, and to allow their reuse. However, it takes unacceptably long time for the substrates to access the immobilized enzymes at the reaction chamber bottom by diffusion even when the chamber size has been miniaturized.
There have been many recent efforts to fabricate microreaction chambers to overcome the diffusion limitation. Unlike analytical-scale reactors of several miniliters, small Reynolds numbers (Re<0.1) of most microfluidic flows implies that fluid stirring is difficult. Hence, either turbulent or vortex flows cannot be easily utilized to enhance reaction in microfluidic devices. Typical static mixers employ a flow splitting (multilamina) technique,1, 2 to shorten the diffusion length l or use obliquely oriented grooves to speed up transverse components in flows. However, reduction of l below 100 microns requires expensive fabrication of microsplitters and microbaffles in a long mixing channel. Such baffles are also problematic in chips that employ electroosmosis or electrophoresis because the high field near sharp corners and the dull regions within the baffle often cause protein precipitation and colloid aggregation.3 Microstirrers and other mixers with moving parts suffer from fabrication difficulties. Long-term reliability of using microstirring devices is also questionable. For electrokinetic microdevices, several simple acceleration strategies have appeared. An apparent electrokinetic mixing instability has been observed in a high-field (103 V∕cm) and low frequency (20 Hz) ac field.4 Although electrokinetic mixing devices are straightforward to fabricate, the mixing intensity of these electrokinetic mechanisms, when measured by the vortex velocity, is only at the same order of the electroosmotic velocity at the same applied field.4 With typical fields of 100 V∕cm in practical electrokinetic devices, the electroosmotic velocity is less than 1 mm∕s. As a result, effective stirring action is not expected.
We have developed a microstirring scheme using microvortices generated via nonlinear electrokinetics in a 10 μl reaction reservoir.5 The transport time of this method has been reported to be reduced by two orders, as compared with the static transport time. When one conducting and ion-selective granule is placed in the uniform field, the electric field lines are attracted into the charged granule and counterions carrying the current migrate toward the granule but co-ions are screened. This depletion zone of co-ions breaks the alignment between the streamline and the electric field. Furthermore, the depletion of co-ions creates an overpotential across the polarized zone on the granule surface. Nonlinear Smoluchowski slip therefore takes place. This mechanism of electroosmotic flow around a granule is first proposed by Dukhin,6 and is promising for creating microvortices in miniaturized devices.5 Such a mechanism has also been confirmed with visualization and theoretical studies.7, 8, 9
Recently, ac field has been used in our research group to induce rattling motions of granule inside the reaction reservoir electrophoretically.5 The rattling motion creates another flow to break the closed stream lines of granule vortices. The solute transport is therefore significantly accelerated. Having tuned the ac frequency properly, we have optimized the transport acceleration to almost 100 folds.
In this report, we use ac nonlinear electrokinetics vortex flows to stir enzyme-entrapped sol-gel particles to reduce the diffusion length between substrate and enzyme. The sol-gel particles are placed in the reaction reservoir (10 μl) filled with a substrate solution. Once the granule overcomes the friction of sol-gel particles to start rattling in the reservoir, effective stirring is achieved to circulate the substrate molecules in the reactor. As a result, the length of transporting the substrate to react with the immobilized enzyme is significantly reduced. The enzymatic reaction rates therefore increase to almost 30-fold. Diffusivity characterizations of dye molecules show that as compared with the static diffusivity the transport enhancement in the vortex flow has increased to 32-fold. This enhancement is consistent with the increase of catalytic reaction rate of sol-gel encapsulated trypsin assay accelerated by vortex flows.
EXPERIMENTAL SECTION
Materials
Sol-gel precursor, tetramethoxysilane (TMOS), was obtained from Fluka (Switzerland). Trypsin enzyme (1800 U∕mg) was purchased from Sigma (St. Louis, USA). The digestion substrate Nα-benzoyl-L-arginine-4-nitroanilide (L-BAPA) and product standard p-nitroaniline (p-NA) were bought from Fluka and Sigma respectively. Phosphate buffer was prepared with sodium phosphate monobasic monohydrate (Tedia, OH, USA). Hydrochloric acid was from Riedel-de Haën Fine Chemicals (Germany). Copolyester plastic sheets were obtained from DSM Engineering Plastic Products (Sheffield, MA, USA).
Preparation of trypsin-entrapped sol-gel particles
The sol-gel reaction basically followed the procedures developed by Ellerby et al.10 Briefly, a typical silica sol was prepared by sonicating TMOS (2 g), water (0.44 mL), and HCl (0.0285 mL, 0.04 M) in an ice bath for 30 min to hydrolyze the monomer. A 62.5 μl portion of the resulting sol was mixed with a trypsin solution (50 μl, 5.75×10−3 mg∕μl) and a phosphate buffer solution (62.5 μl, 0.01 M, pH 7) in an ice bath until gelation took place. The gel was then aged for 24 h under ambient conditions and was then freeze-dried to powders.
Microfluidic reaction device
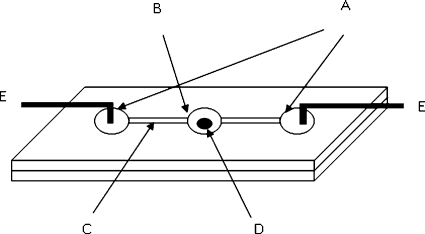
The fabrication procedures of our microfluidic reaction device are the same as those in our previous report.5 In order to sufficiently release the bubbles generated at the electrodes, the anode at the left and the cathode at the right were housed in two side reservoirs as shown in Fig. 1. These side reservoirs were connected by two straight channels to a central mixing reservoir between them. All three reservoirs were 3 mm in diameter and were drilled on one thermoplastic slide (20×40 mm2; 1 mm in thickness). The connecting channels were 1.0 mm in width, slightly narrower than the 1.2 mm granule, and each segment between two reservoirs was 12 mm long. The holes and grooves were machined on one plastic slide with conventional drilling and milling tools. The substrate slide was then thermally bonded to the drilled one at 80 to 100 °C. Pressure was briefly applied using binder clips when the slides were glued together. Electrodes of platinum wires were placed in the two side-reservoirs with their tips bent to dip into the reservoirs to contact the liquids and the remaining part taped on the slides.
Figure 1.
Schematic illustration of microfluidic mixing device using chaotic vortex flows. A. Side reservoirs; B. Reaction reservoir; C. Connection channels; D. Conductive cation exchange granule; E. Electrodes.
One granule (approximately 1.2 mm in diameter) was placed inside the mixing reservoir prior to the experiment. The power supply circuitry connecting the electrodes then applied an electric field across the mixing reservoir during the period of enzymatic digestion as described in the next section.
Diffusivity characterizations and ac frequency optimization in microfluidic device
One cation exchange granule (1.2 mm i.d.) and sol-gel powders (1 mg) were placed in the reaction reservoir. Two drops of glycerin solution stained with or without food-color dyes were held in pipette tips to add in the reservoir when the tips were dipped. When the external fields of ±133 V∕cm square waveform under the ac frequencies 0.1, 1, 10, and 100 Hz (50% duty cycle) were activated, the images of mixing process were recorded with a video camera respectively. The images were digitized and transferred into a personal computer as black-and-white pictures to score gray scales at dark regions. Comparison experiments were accomplished without a granule in the center reservoir and with an electric field. Gray scale score statistics across the middle of center reservoir along the transverse direction were used to indicate the degree of color heterogeneity. To avoid counting the shadows at the edge, we only used twenty pixels in total inside the reservoir, which cover more than 90% of the diameter. We computed the gray scale standard deviation of these pixels. The standard deviations decreased as the mixing was getting progressed. The ac frequency resulting in the most intense mixing was used to perform accelerated enzymatic digestion experiments in Sec. 2E.
Enzymatic digestion
Sol-gel powders (1 mg, entrapping 1×10−9 moles trypsin) were placed in the mixing reservoir, which was filled with 40 μl Tris buffer (1.0 mM, pH 8), and allowed to soak for 5 min. An aliquot of 10 μl Nα-benzoyl-L-arginine-4-nitroanilide (L-BAPA) substrate solution (6.0 mM) was then added into the reservoir. Subsequently, the ac external field of square waveform at the optimum frequency obtained in Sec. 2D was turned on to stir the solution in the reservoir for 5 min with or without the cation exchange granule. After filtering out the sol-gel particles and diluting the filtrate 10-fold, the concentration of the product, p-nitroaniline (p-NA), was monitored at 405 nm by UV-vis absorption spectroscopy. To validate this accelerated digestion scheme, a calibration curve was prepared using standard solutions of p-NA from 3.0 to 12 mM.
Results and discussion
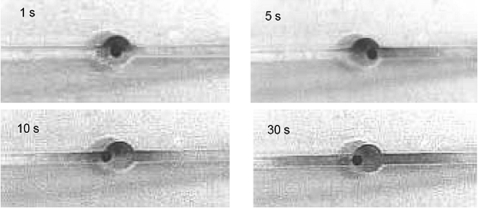
Figure 2 shows the mixing action of dyes in the reaction reservoir via granule mixing when sol-gel powders are also present. The granule rattles from time to time and mostly it does not travel through the whole reservoir within one cycle. The dye concentration becomes almost uniform in about 30 s.
Figure 2.
Mixing action using chaotic vortex flow produced by applying a square waveform ac field (0.1 Hz; −133 to 133 V∕cm; duty cycle 50%) across one cation exchange granule. Two segregated glycerin drops stained with and without food-color dyes in glycerin solution are mixed homogenously within 30 s.
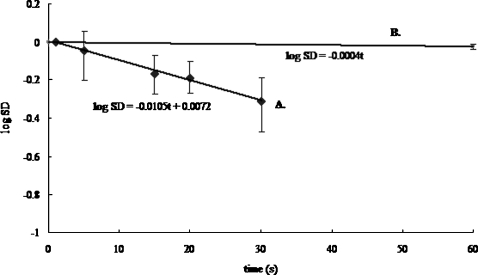
Since the uniformity of the concentration image can be quantified by determining the standard deviation of the pixel intensity values, the curve of gray scale standard deviation versus time measures the mixing action when an electric field ±133 V∕cm is applied across the reservoir with a cation exchange granule present in the reservoir. The normalized concentration within the reservoir is expected to approach a homogeneous field exponentially if only diffusion is at play. The exponent is −Dt∕l2 multiplied by a unit-order factor, where l=3 mm is the diameter of the reservoir. Hence, a measure of our mixing efficiency can be obtained by assuming a purely diffusive mechanism. As such, the mixing diffusivity can be derived via the linear regression of the logarithm value of standard deviation against time (Fig. 3). The slope of this curve scales linearly with respect to the diffusivity coefficient.
Figure 3.
Mixing action quantification is obtained from the global temporal change of standard deviation of gray scale scores of camera pixels imaging mixing action inside the reservoir. The mixing diffusivity (curve A) for a cation exchange granule in square waveform ac field (0.1 Hz; −133 to 133 V∕cm; duty cycle 50%) is compared to that without a field (curve B).
As such, we use the slope of the fieldless data in Fig. 3 as reference points (D0) and then estimate Deff∕D0 by taking the ratio of similar slope, with an applied field to this slope and without an applied field. Data in Fig. 3 fall on a straight line, indicating that the vortex mixing in the reservoir can be accurately represented by a diffusing mixing model with an effective mixing diffusivity Deff. The normalized mixing diffusivity hence represents the effects of electrokinetic flow on mixing relative to the case without flow. Similarly, mixing diffusivity coefficient is obtained in comparison experiment without the presence of granule.
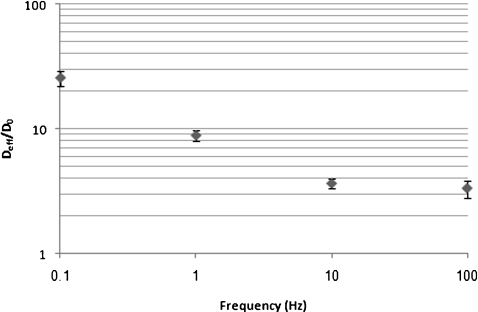
Figure 4 shows the frequency effects on the normalized mixing diffusivity. Similar to the previously reported results, the normalized mixing diffusivity decreases when the ac frequency increases to gradually damp the granule rattling motion. The optimum waveform frequency to achieve the largest mixing diffusivity is at 0.1 Hz, close to the optimum frequency reported in the literature when sol-gel particles are not present in the reservoir. This frequency is used to perform accelerated enzymatic digestion experiments.
Figure 4.
Normalized mixing diffusivity dependence on ac frequency of applied electric field at 133 V∕cm.
Having been incubated in the miniaturized reactor for five minutes, L-BAPA is cleaved by sol-gel encapsulated trypsin to p-NA, which has absorbance at 405 nm. Using Beer’s law, the concentration change (ΔC) of p-NA is determined to obtain the initial digestion rate (V0).
| (1) |
where Δt is five minutes. When no external field is applied across the channel, the mass transport is only driven by diffusion. In this case, the initial rate obtained is 1.0±0.3×10−7 M∕min, at an L-BAPA concentration of 6.0 mM. The rate constant under the diffusion-driven conditions is about 2.0×10−5 min−1.
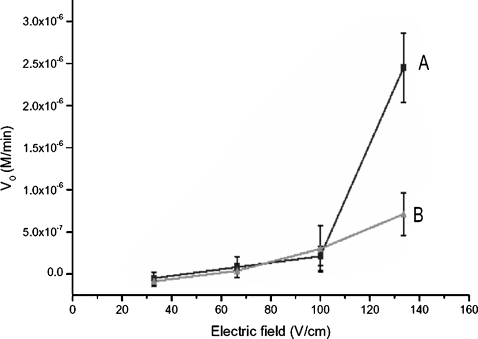
Curve A of Fig. 5 shows the dependence of V0 value on the ac field strength with the presence of one cation exchange granule. When the field strength is less than 100 V∕cm, the initial rate slowly increases as the field becomes higher. At this regime, we notice that the granule is trapped in the sol-gel powders without the rattling motions driven by electrophoresis. The microvortices generated around the granule therefore remain closed without coupling to other secondary flows. Electrokinetic flows under this field strength are localized, which pump solute molecules only within the vortices. As a result, substrate transport to the vortices still relies on diffusion. The initial rate (V0) only increases from 2×10−7 to 4×10−7 M∕min. As the field strength reaches 133 V∕cm, the granule starts rattling, although only from time to time, across the reaction reservoir. Chaotic flows due to the coupling between microvortices and granule’s electrophoretic motions are then able to sufficiently stir the substrate molecules so that they circulate throughout the whole reactor. The V0 value at this field strength jumps to 2.8±0.4×10−6 M∕min, which is 28-fold faster than the V0 at the static condition. The rate constant promoted by the mixing action is about 6.0×10−4 min−1.
Figure 5.
The dependence of enzymatic reaction rate on field strength in the microfluidic device. Curve A, with the presence of cation exchange granule in the mixing reservoir; Curve B, in the absence of the granule.
In contrast, when the granule is not present in the reactor, the dependence of V0 value on field strength is shown in curve B of Fig. 5. When the field strength varies from 33 to 100 V∕cm, V0 monotonically increases from 1×10−7 to 5×10−7 M∕min. When no microvortices exist, the mass transport in the reservoir is mainly driven by electroosmotic flows, which are linearly dependent on field strength and arise due to the charged carboxylate groups on the reservoir and channel walls. These carboxylate functionalities are hydrolyzed from copolyester plastics. The flow speed under normal field strength (33–133 V∕cm) is not fast enough to provide sufficient stirring. The V0 value in linear electroosmotic flows is only 9.5±2.6×10−7 M∕min at the field strength 133 V∕cm. This value is ninefold greater than that obtained at static conditions, but is only one-third of that obtained with chaotic vortex flows.
Sufficient solution stirring takes place when microvortices couple with the granule’s electrophoretic rattling to create chaotic vortex flows. Due to the reduction of transport length by stirring, the enzymatic reaction rate significantly increases as chaotic flows arise. In contrast, when this chaotic condition does not exist, stirring is either constrained locally or limited by the speed of linear electroosmotic flows. The reaction rate enhancement is therefore not as pronounced as that with chaotic flows.
As shown in Fig. 5, the reproducibility (relative standard deviation; RSD) of five replicate measurements using granule stirring is about 15%. In contrast, the reproducibility without granule stirring is 28%, which is greater than the required precision of most assays for bioanalytical determinations.
Table 1 illustrates the enhancement ratios of solute diffusivity and reaction rate, as compared with static case, when the field of 133 V∕cm is applied across the reaction reservoir with and without the presence of granule. These two sets of enhancement ratio data show good consistency. This indicates that the reaction rate enhancement is indeed due to the diffusivity intensification using nonlinear vortex flows. Due to the friction by sol-gel particles in the reservoir, the granule rattling motion is only on-and-off and the granule does not move through the whole reservoir. In contrast, when the granule is continuously rattling in the whole reservoir, a 100-fold diffusivity enhancement is reported.5 In the presence of sol-gel powders, although using a higher electric field would further accelerate the rattling motion, the Joule heating generated in the presence of high fields usually dries up the buffer solution in the reaction reservoir.
Table 1.
As compared with static case, the enhancement ratios of solute diffusivity and digestion reaction rate in linear electroosmotic flows and chaotic vortex flow using mini-enzymatic assays with trypsin-encapsulated sol-gel powders
| Linear electroosmotic flow | Chaotic vortex flow | |
|---|---|---|
| Solute diffusivity enhancement ratio | 7.6±2.7 | 32±11 |
| Digestion reaction rate enhancement ratio | 9.5±3.8 | 28±9.4 |
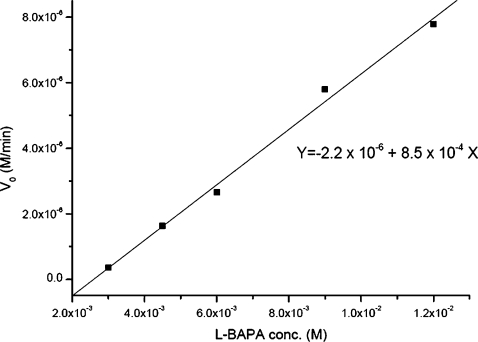
Figure 6 shows the dependence of initial rate V0 value on the L-BAPA concentration when the external field is 133 V∕cm. In the substrate concentration range of 3.0 to 12 mM, the V0 value increases linearly (R=0.997), indicating that the quantity of products that are generated by this accelerated digestion method is directly proportional to the amount of substrate fed in the chip. Furthermore, the maximum reaction rate has not been reached under these conditions since the response would otherwise level off at high substrate concentrations.
Figure 6.
The dependence of the initial enzymatic digestion rate V0 on L-BAPA concentration. Concentration range of L-BAPA: 3.0×10−3 to 1.2×10−2 M; correlation coefficient R=0.997. The external field strength is 133 V∕cm.
CONCLUSIONS
We have successfully developed a fast enzymatic digestion assay by stirring L-BAPA substrate molecules using chaotic vortex flows to efficiently reduce their contact distances with trypsin-entrapped sol-gel particles in microfluidic devices. These chaotic vortex flows promoting the stirring of fluids in the entire reaction reservoir are produced via the coupling between microvortices around a conducting cation exchange granule and the flow due to granule’s electrophoretic rattling when a periodic ac electric field is applied across the mixing reservoir. When substrate molecules are sufficiently stirred in the whole reservoir, the digestion rate is 28 times faster than that in static conditions. In nonchaotic conditions without the coupling between two flows, the digestion acceleration is only ninefold in the case of using pure ac electroosmotic flows. The additional mixing enhancement in microfluidic devices arises only when chaotic conditions exist. In the reaction reservoir as compared with the static case, the diffusivity enhancement ratios of dye molecules are 32 and 8 using chaotic vortex flows and linear electroosmotic flows respectively. These consistent increases in ratios between diffusivity and reaction rate enhancement indicate that the acceleration of enzyme digestion is promoted by chaotic vortex flows. The preliminary results we have obtained are very encouraging since they open new avenues for the design and construction of high throughput enzymatic microreactors.
ACKNOWLEDGMENTS
The authors thank the National Science Council of Taiwan for financial support under contracts NSC 92-2113-M-194-022, NSC 89-2323-13-006-008, and NSC 93-2120-M-194-005. Helpful discussion with Prof. H.-C. Chang at the Department of Chemical and Biomolecular Engineering, University of Notre Dame, is acknowledged.
References
- Jacobson S. C., Mcknight T. E., and Ramsey J. M., Anal. Chem. 10.1021/ac990576a 71, 4455 (1999). [DOI] [Google Scholar]
- Schwesinger N., Frank T., and Wurmus H., J. Micromech. Microeng. 10.1088/0960-1317/6/1/023 6, 99 (1996). [DOI] [Google Scholar]
- Thamida S. and Chang H. -C., Phys. Fluids 10.1063/1.1519530 14, 4315 (2002). [DOI] [Google Scholar]
- Oddy M. H., Santiago J. G., and Mikkelsen J. C., Anal. Chem. 10.1021/ac0155411 73, 5822 (2001). [DOI] [PubMed] [Google Scholar]
- Wang S. C., Lai Y. W., Ben Y., and Chang H. -C., Ind. Eng. Chem. Res. 10.1021/ie030689r 43, 2902 (2004). [DOI] [Google Scholar]
- Dukhin S. S., Adv. Colloid Interface Sci. 10.1016/0001-8686(91)80022-C 35, 173 (1991). [DOI] [PubMed] [Google Scholar]
- Ben Y. and Chang J., J. Fluid Mech. 10.1017/S0022112002008662 461, 229 (2002). [DOI] [Google Scholar]
- Ben Y., Demekhin E. A., Takhisov P. V., and Chang H. -C., J. Chin. Inst. Chem. Eng. 33, 15 (2002). [Google Scholar]
- Mishchuk N. A. and Takhistov P. V., Colloids Surf., A 10.1016/0927-7757(94)02988-5 95, 119 (1995). [DOI] [Google Scholar]
- Ellerby L. M., Nishida C. R., Nishida F., Yamanka S. A., Dunn B., Valentine J. S., and Zink J. I., Science 10.1126/science.1312257 255, 1113 (1992). [DOI] [PubMed] [Google Scholar]