Abstract
We demonstrate here the discovery of a unique and direct three-dimensional biomicrofabrication concept possessing the ability to revolutionize the jet-based fabrication arena. Previous work carried out on similar jet-based approaches have been successful in fabricating only vertical wall∕pillar-structures by the controlled deposition of stacked droplets. However, these advanced jet-techniques have not been able to directly fabricate self-supporting arches∕links (without molds or reaction methods) between adjacent structures (walls or pillars). Our work reported here gives birth to a unique type of jet determined by high intensity electric fields, which is derived from a specially formulated siloxane sol. The sol studied here has been chosen for its attractive properties (such as an excellent cross-linking nature as well as the ability to polymerize via polycondensation on deposition to its biocompatability), which promotes direct forming of biostructures with nanometer (<50 nm) sized droplets in three dimensions. We foresee that this direct three-dimensional biomicrofabrication jet technique coupled with a variety of formulated sols having focused and enhanced functionality will be explored throughout the physical and life sciences.
INTRODUCTION
Solid freeform fabrication is the concept where material is added by way of, point, line, or planer delivery to form a desired architecture. This fabrication concept has two avenues, namely, the jet- and non-jet-based methodologies. Non-jet-based approaches have made great progress, which has to a large extent provided the required motivation for the jet community to pursue its capabilities in this endeavor. Photolithography, dip-pen to soft lithography are some of the techniques for fabricating complex constructs having three-dimensional components in the micro and nano range.1, 2, 3, 4 Although these approaches have shown great promise for patterning architectures in both two and three dimensions in the nanoscale over large areas, the processing methodologies have associated high instrumentation cost and, when applied to biological-based applications, are cell specific, which is viewed as a limitation. Nevertheless the jet community has learned much from these advanced processing sciences, which has assisted and helped focus on jet-processing approaches that will eventually have an influence in overcoming the obstacles in this area of research.
The principal jetting route is the well-established ink-jet printing (IJP) technique.5, 6, 7, 8 In summary, this jetting or drop and place methodology is driven either by way of peizoelectricity or via a controlled thermal explosion within a needle.9 These routes essentially draw out a measured quantity of media from a reservoir through a needle and propel a droplet to a desired location on a substrate. IJP has been combined with three-axes movement to successfully develop a novel jet-based solid free-forming route. Although IJP has been developed by a global community in a wide range of research fields spanning the physical and life sciences,10, 11 the jetting technology has limitations, which hold back the resolution of the residues once deposited. This obstacle stems from the drop and place technique itself; essentially, the generated droplets in IJP are double the size of the needle used for forming these droplets. Generally, IJP uses needles sized in the tens (30−60 μm) of micrometers which results in droplets sized ≥100 μm. Notably, these droplets, when deposited, will spread and hence form residues well into the micrometer range. These droplet residues could be reduced to the few tens of micrometers if and only if a low viscosity single-phase liquid was deposited on a hydrophobic surface with needles sized finer than 30 μm. However, in the real world, processing single-phase liquids is rather rare and, in most cases, the processing media are in the form of multiphase high viscosity media where a solvent-based suspension carries micro- or nano-sized particles along with polymers to stabilize these suspensions. Hence IJP has successfully processed suspensions having a low particulate loading with residues sized in the micrometer range. Notably the placing of nanosized (<50 nm) droplets on substrates is practically impossible with this jetting route.
Contrary to IJP electrosprays,12, 13 a jetting technique driven entirely by electric fields has recently emerged as a versatile and economical materials processing science.14, 15, 16, 17 Electrosprays unlike IJP employs needles with bores sized in the several hundreds of micrometers that are capable of generating with ease a near-monodistribution of droplets and residues sized in the micro and nanometer range.18, 19, 20 Furthermore, this electrified jetting route has been shown to have the capability for processing highly concentrated particulate suspensions containing either micro- or nanomaterials for both random and controlled deposition at one extreme to the direct processing of living cells at the other.21, 22, 23, 24, 25 Unlike IJP, electrosprays have no dependent relationship between the needle diameters and generated droplet sizes. In the work reported here, we uncover proof of concept from our ongoing developmental studies on a unique and direct three-dimensional biomicrofabrication approach possessing the ability to assemble nanodroplets (<50 nm) to finally directly fabricating structures in the micrometer range having structural complexity (such as overhangs and arches) hitherto both unexplored and unachieved by any other competing jet-based methodology.
MATERIALS AND METHODS
Siloxane sol and electrospraying
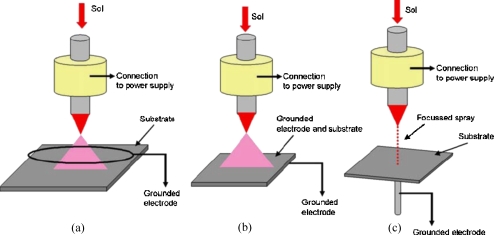
A specially formulated living siloxane sol prepared and characterized as previously described26, 27, 28 was electrosprayed at optimized operational parametric conditions for three different ground electrode configurations, namely, the ring, plate and point types (Fig. 1). These needle to ground electrodes were set up so that the needle exit was ∼15 mm above each grounded electrode. The studies carried out in this present work traversed a wide operational space of applied voltage (∼1–15 kV) to flow rate (∼10−8−10−20 m3 s−1). When the equipment was set up, the ground electrodes gave rise to the formation of stable cone-jet mode of electrospraying at specified conditions.29, 30 In the case of the ring electrode the larger droplets passed through the center while fine droplets recirculated on the extremities of the ring. The plate, much like that of the ring, formed a diverging spray plume of droplets with a large majority collecting at the center of the plate as a function of spray time. In contrast to the ring and plate electrodes, the point type gave rise to the focusing of a large majority of droplets to the tip of the point electrode, which has previously been shown as a route for printing two- and three-dimensional architectures.21, 22, 23 The droplet trajectories for the three ground electrodes directly result due to the formation of the external electric fields for that given needle to ground electrode configuration on the application of an applied voltage.
Figure 1.
A schematic representation of the three ground electrode geometries investigated during jetting in the stable cone-jet mode, namely, (a) the ring-shaped ground electrode which forms a cone-shaped spray diverging droplets with finer droplets based on the extremities of the spray which recirculate, (b) a plate-shaped electrode which has a similar effect much like that of the ring but does not have droplet recirculation, and (c) a point-shaped electrode which converges the spray to the head of the point assisting in the deposition of a large majority of droplet residues with precision.
Generation of operational guides for each ground electrode configuration
Several permutations of flow rates to applied voltages for a given ground electrode distance from the needle exit were studied for all three electrodes. These investigations deduced the optimum operational conditions for generating the finest droplets and residues within the generated spray plumes. The operational spaces were identified by initially setting the flow rate to the lowest possible flow of media to the needle (∼10−20 m3 s−1: present limitation on existing equipment). Later an applied voltage was subjected, and the resulting media behavior was observed in real time by way of high-speed photography. At the lowest possible flow rate (∼10−20 m3 s−1) on the application of an applied voltage, we observed the unstable jetting behavior which is not attractive as it has previous been reported to generate a polydisperse distribution of droplets and residues. We repeated this procedure until we were able to observe stable cone jet mode of jetting while generating the finest droplets and residues. The droplet residue sizes were determined by droplet collection on TEM grids, which were later examined by way of transmission electron microscopy. During these studies, we noticed, for this siloxane sol at this timepoint, stable cone jet mode of electrospraying was achieved for all three ground electrodes at a flow rate in the regime of ∼10−9 m3 s−1 for an applied voltage of ∼11 kV over an electrode distance of ∼15 mm.
RESULTS AND DISCUSSION
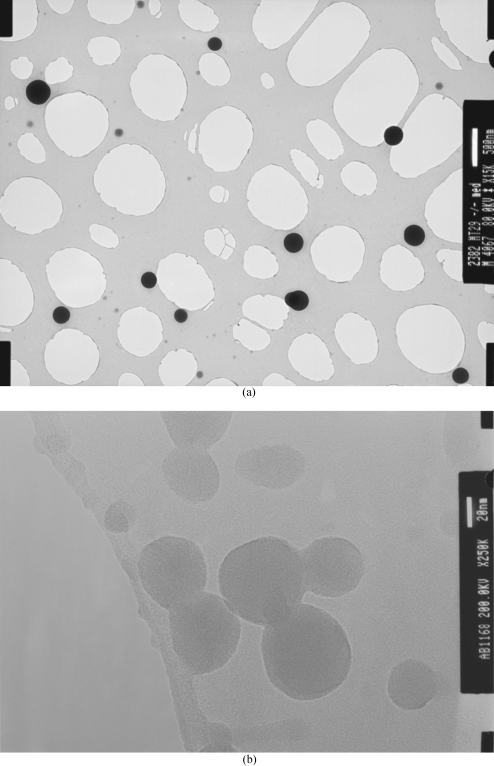
During the generation of the operational guide, it was observed that, for a given applied voltage to flow rate condition, a small window existed where a near mono distribution of nano droplets were generated. At this stage if either parameter was varied, we observed the effect of either an increase in droplet and residue sizes to the generation of droplets having a polydistribution features, which are not attractive. On achieving optimized spraying conditions for a given distance between the ground electrode and the needle exit, several TEM grids, glass microslides to polyester coated substrates (samples), were used for collecting residues for selected substrate to jet exposure times. Our investigations show that at optimized conditions for all three ground electrode geometries, the jetting process is capable of generating droplet residues sized from the micro to the nano range [Figs. 2a, 2b]. It is important to note that the longer the substrate exposure times to the jet the larger the droplet residues. From our previous work, we established that the first generated droplets on deposition form an undulated surface with peaks at heights in the submicrometer range. As the continuous spray of nanodroplets are attracted to the nearest grounded surface, these charged droplets are attracted to the previously formed droplet deposits, which gives rise to the formation of larger residues and deposits. Employing longer exposure times of substrates to these nanodroplets in combination with the point ground electrode would yield the fabrication of a location controlled substrate anchored structure.
Figure 2.
Characteristic transmission electron micrographs of (a) micrometer and (b) nanometer sized droplet residues for an applied voltage to flow rate of ∼10∕11 kV and ∼10−9 m3 s−1 for a ground electrode distance of ∼15 mm from the exit of the needle, respectively.
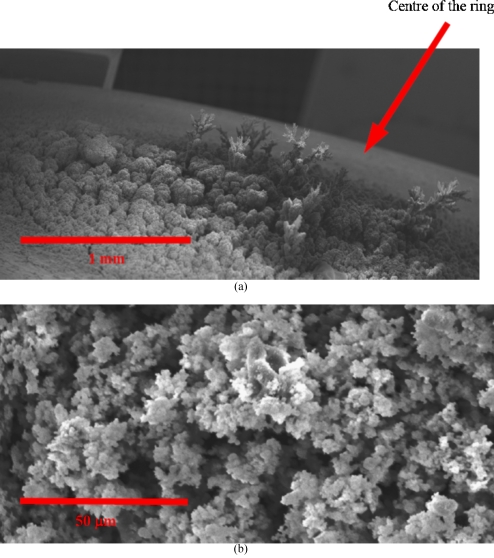
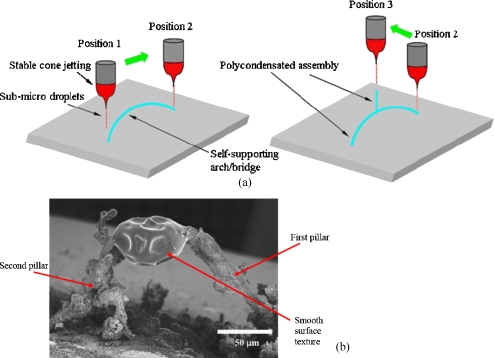
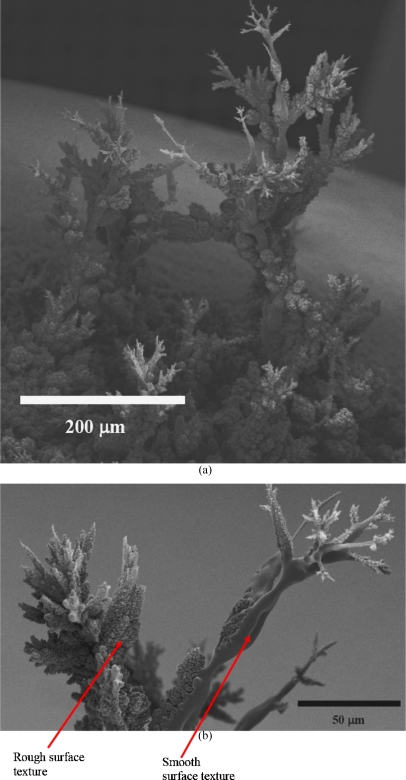
On increasing the substrate to jet exposure times for the ring and plate electrodes, the formation of random structures placed mainly and directly central to the electrodes [Fig. 3a] was observed. The structures resembled a fir-tree-like morphology. On closer examination of the structures formed in either of these ground electrode geometries, we observed that the near central region had fir-tree-like constructs and undulated surfaces at the extremities of the substrate, which had a remarkable resemblance to a coral reef [Fig. 3b]. As the point electrode previously has been investigated for promoting more localized deposition,21 we attempted the formation of a controlled structure. With the point ground electrode in contact below the substrate and in-line with the jetting needle, we exposed the substrate to the generated droplets, which was seen to give rise to the formation of a fine-pillar-like construct after ∼300 s. Subsequently, the substrate was moved in the same plane where a similar substrate anchored pillar structure was formed. Once the second pillar was formed, the jetting needle was moved slowly from the second pillar toward the first in small increments. During this time, the generated droplets were seen to form an overhanging arm to arch from a branch created at the second pillar, which extended by the attraction of additional droplets until the needle reached the first pillar [Fig. 4a]. On reaching the first pillar, the growing and extending arm from the second pillar, fused by the assembly of droplets to form a self-supporting arch [Figs. 4a, 4b]. Although the generated droplets are in the nanorange as shown by the collection of droplets on TEM grids [Fig. 2b], the fabricated structures are in the micrometer range. This is attributed to the fact that these charged droplets are attracted to the nearest grounded surface or structure, and there is also a droplet population that stray with the growth of these structures which gives rise to the disturbance of the electric field. Similarly [as in Fig. 4b], a second structure was created, and, subsequently, the spraying needle was moved to the center of the construct and was seen to promote the growth of a structure, which unlike the previous two, was anchored on the arch, as this was the nearest grounded surface to the charged droplets. On positioning and holding the needle at the center of the structure, a third fir-tree-like construct was grown, which was supported by the arch and was found to be stable [Fig. 5a]. High magnification scanning electron microscopy of the structures fabricated with the aid of the point electrode shows fine features (<50 μm) at the branches of the structure [Fig. 5b]. It should be noted that the jetting needle was always kept at the same distance from the point electrode throughout the fabrication of this three-dimensional structure. High magnification scanning electron microscopy on the fabricated structure showed regions where a mixture of surface textures were observed [Fig. 5b]. The authors are currently trying to establish the reason for this textural mixture and are exploring the various aspects of both the sol and the processing method, which may give rise to this effect. One avenue the authors are in pursuit of is to control the growth of these structures while preventing dendrite-type growth by the incorporation of droplet guiding plates, etc.
Figure 3.
Characteristic scanning electron micrographs of the fabricated structures for a ring electrode geometry, with (a) showing the area which has been fabricated with the region centrally placed below the ring having several assembled but randomly located structures and (b) elucidating a high magnification of the surface topography of the surroundings having a formation much like a coral reef. These structures were very similar when compared with those formed for a plate type electrode configuration.
Figure 4.
(a) Schematic representation of the fabrication path followed by the needle and ground electrode in turn for microfabricating the three-dimensional architecture seen in (b).
Figure 5.
Representative scanning electron micrographs showing (a) the finally fabricated structure following the stages depicted in Fig. 4 and (b) the fine features fabricated by means of this processing technique. This micrograph (5b) depicts in high magnification the top most structure. The micrographs also elucidate the mixed surface texture, which is currently under investigation.
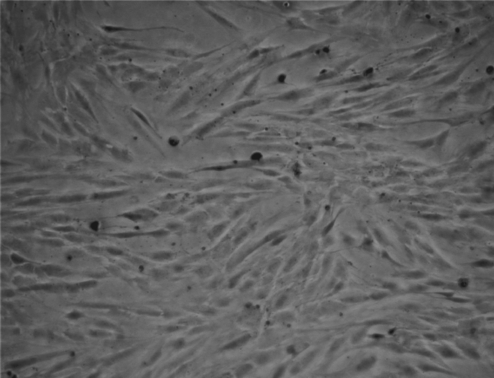
Many structures were later fabricated on several glass microslides, and, subsequently, primary smooth muscle cells were seeded and observed over two days alongside controls (seeded cells on plane glass microslides). Cell seeding was carried out by initially placing the coated glass microslides into several tissue culture plates. Subsequently, the primary smooth muscle cells at known cell density were pipetted onto the center of the coated microslides within the culture plates along with controls, which were seeded directly on plane culture plates. The cells were seen to adhere to the coated glass microslides as in the controls and proliferate over the 48 h (Fig. 6) as expected. The seeded cells showed similar proliferation rates comparable to the controls. Cell phenotype was optically analyzed throughout the period of study. Hence, the undulated surfaces∕structures had no compromising affect on the seeded cells with respect to their growth rates. The fabricated micrometer-sized bio-structures formed in these studies are good cell culturing material as a wide range of adherent cells are sized in the tens of micrometers. The authors, while further developing this fabrication route, in parallel will investigate the formation of preorganized architectures in the hope to promote cell migration to proliferation to those patterns. This would bring about a technique having the potential to assist the proliferation of cells in a controlled direction to finally growing a tissue of desired architectural complexity. Hence, these three-dimensional structures could be explored for a wide range of tissue engineering applications.
Figure 6.
Typical optical micrograph depicting the positively proliferating smooth muscle cells after seeding for 48 h on the microslide which was exposed to the spray of sol for ∼1000 s.
CONCLUSION
Our developmental studies into this direct unique biomicrofabrication approach have not only demonstrated that three-dimensional complex structures could be directly fabricated by means of assembling nano-sized droplets, but the fabricated structures are biocompatible and imply tremendous possibilities for this microfabrication technology. The authors are currently in pursuit of answers to the formation of the mixture of surface textures seen, as well as developing this technique for precision controlled deposition of these nanodroplets with movement in the three axes (Fig. 7). The fabricated structures have shown to be self-supporting and biocompatible, which are promising features for such a direct patterning route. Having demonstrated the constructs are biocompatible, the authors wish to explore this route for the fabrication of controlled matrices, which could give rise to targeted tissue growth. Hence, our results elucidated here show the birth of a powerful biomicrofabrication methodology as a direct jet-based three-dimensional patterning route, which has previously never been demonstrated and will have significant implications spanning the physical to the life sciences ranging from applications as a unique bio-rapid prototyping approach to tissue engineering of complex bio-architectures.
Figure 7.
A schematic representation of the authors intended microfabrication device which will be explored for the fabrication of controlled three-dimensional structures by electric field directed assembly for the creation of complex structures in the micrometer range.
ACKNOWLEDGMENTS
A.C.S. and S.N.J. gratefully acknowledge financial support from both the EPSRC and the Royal Society in the U.K. A.C.S. also wishes to thank PhosphonicS Ltd for the gift of precursors to these sols.
References
- Rosner B., Duenas T., Banerjee D., Shile R., Amro N., and Rendlen J., Smart Mater. Struct. 10.1088/0964-1726/15/1/020 15, S124 (2006). [DOI] [Google Scholar]
- Yonemitsu H., Kawazoe T., Kobayashi K., and Ohtsu M., J. Lumin. 122–123, 230 (2007). [Google Scholar]
- Xia Y. and Whitesides G. M., Angew. Chem. Int. Ed. 37, 550 (1998). [DOI] [PubMed] [Google Scholar]
- Thangawng A. L., Swartz M. A., Glucksberg M. R., and Ruoff R. S., Small 10.1002/smll.200500418 3, 132 (2007). [DOI] [PubMed] [Google Scholar]
- Bao Z., Rogers J. A., and Katz H. E., J. Mater. Chem. 10.1039/a902652e 9, 1895 (1999). [DOI] [Google Scholar]
- Spinelli H. J., Adv. Mater. 10, 1215 (1998). [Google Scholar]
- Gbureck U., Hölzel T., Doillon C. J., Müller F. A., Barralet J. E., Adv. Mater. (Weinheim, Ger.) 19, 795 (2007). [Google Scholar]
- van den Berg A. M. J., Smith P. J., Perelaer J., Schrof W., Koltzenburg S., and Schubert U. S., Soft Matter 10.1039/b610017a 3, 238 (2007). [DOI] [PubMed] [Google Scholar]
- Basaran O. A., AIChE J. 10.1002/aic.690480902 48, 1842 (2002). [DOI] [Google Scholar]
- Zhao Y., Zhou Q., Liu L., Xu J., Yan M., and Jiang Z., Electrochim. Acta 51, 2639 (2006). [Google Scholar]
- Sanjana N. E. and Fuller S. B., J. Neurosci. Methods 10.1016/j.jneumeth.2004.01.011 136, 151 (2004). [DOI] [PubMed] [Google Scholar]
- Hayati I., Bailey A. I., and Tadros T. F., Nature 10.1038/319041a0 319, 41 (1986). [DOI] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. K., and Whitehouse C. M., Science 10.1126/science.2675315 246, 64 (1989). [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N. and Edirisinghe M. J., J. Nanosci. Nanotechnol. 10.1166/jnn.2005.126 5, 923 (2005). [DOI] [PubMed] [Google Scholar]
- Balachandran W., Miao P., and Xiao P., J. Electrost. 10.1016/S0304-3886(00)00039-5 50, 249 (2001). [DOI] [Google Scholar]
- Jayasinghe S. N., Fullerenes, Nanotubes, Carbon Nanostruct. 10.1080/15363830500538524 14, 67 (2006). [DOI] [Google Scholar]
- Jayasinghe S. N., Physica E 10.1016/j.physe.2006.04.011 33, 398 (2006). [DOI] [Google Scholar]
- Hartman R. P. A., Brunner D. J., Camelot D. M. A., Marijnissen J. C. M., and Scarlett B., J. Aerosol Sci. 10.1016/S0021-8502(99)00034-8 31, 65 (2000). [DOI] [Google Scholar]
- Jayasinghe S. N., Int. J. Nanosci. 5, 35 (2006). [Google Scholar]
- Rosell-Llompart J. and Fernandez de la Mora J., J. Aerosol Sci. 10.1016/0021-8502(94)90204-6 25, 1093 (1994). [DOI] [Google Scholar]
- Jayasinghe S. N., Edirisinghe M. J., and de Wilde T., J. Mater. Res. 6, 92 (2005). [Google Scholar]
- Lee D. Y., Hwang E. S., Yu T. U., Kim Y. J., and Hwang J., Appl. Phys. A: Mater. Sci. Process. 10.1007/s00339-005-3452-5 82, 671 (2006). [DOI] [Google Scholar]
- Wang D. Z., Jayasinghe S. N., and Edirisinghe M. J., Rev. Sci. Instrum. 10.1063/1.1942531 76, 075105 (2005). [DOI] [Google Scholar]
- Jayasinghe S. N., Qureshi A. N., and Eagles P. A. M., Small 2, 216 (2006). [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N. and Townsend-Nicholson A., Lab Chip 10.1039/b606508m 6, 1086 (2006). [DOI] [PubMed] [Google Scholar]
- Sullivan A. C. and Wilson J. R. H., Chem. Abstr. 2002, P109980t; PCT Int. Appl., WO02055587.
- Jayasinghe S. N. and Sullivan A. C., J. Phys. Chem. B 110, 2522 (2006). [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N. and Sullivan A. C., J. Sol-Gel Sci. Technol. 10.1007/s10971-006-6727-1 38, 293 (2006). [DOI] [Google Scholar]
- Cloupeau M. and Prunet-Foch B., J. Aerosol Sci. 10.1016/0021-8502(94)90199-6 25, 1021 (1994). [DOI] [Google Scholar]
- Jaworek A. and Krupa A., J. Aerosol Sci. 10.1016/S0021-8502(98)00787-3 30, 873 (1999). [DOI] [Google Scholar]