Abstract
Purpose
Since activity of sorafenib was observed in sarcoma patients in a phase I study, we performed a multicenter phase II study of daily oral sorafenib in patients with recurrent or metastatic sarcoma.
Patients and Methods
We employed a multiarm study design, each representing a sarcoma subtype with its own Simon optimal two-stage design. In each arm, 12 patients who received 0 to 1 prior lines of therapy were treated (0 to 3 for angiosarcoma and malignant peripheral-nerve sheath tumor). If at least one Response Evaluation Criteria in Solid Tumors (RECIST) was observed, 25 further patients with that sarcoma subtype were accrued.
Results
Between October 2005 and November 2007, 145 patients were treated; 144 were eligible for toxicity and 122 for response. Median age was 55 years; female-male ratio was 1.8:1. The median number of cycles was 3. Five of 37 patients with angiosarcoma had a partial response (response rate, 14%). This was the only arm to meet the RECIST response rate primary end point. Median progression-free survival was 3.2 months; median overall survival was 14.3 months. Adverse events (typically dermatological) necessitated dose reduction for 61% of patients. Statistical modeling in this limited patient cohort indicated sorafenib toxicity was correlated inversely to patient height. There was no correlation between phosphorylated extracellular signal regulated kinase expression and response in six patients with angiosarcoma with paired pre- and post-therapy biopsies.
Conclusion
As a single agent, sorafenib has activity against angiosarcoma and minimal activity against other sarcomas. Further evaluation of sorafenib in these and possibly other sarcoma subtypes appears warranted, presumably in combination with cytotoxic or kinase-specific agents.
INTRODUCTION
Sarcomas are a heterogeneous family of malignancies of soft tissue, with biologic behavior and clinical outcomes distinct for each subtype. For soft tissue sarcomas other than gastrointestinal stromal tumors (GIST), doxorubicin and ifosfamide remain the most active agents against these diseases.1 Gemcitabine and docetaxel are an active chemotherapy combination against selected sarcoma histologies as well.2–7
Patients with metastatic GIST show notable sensitivity to kinase inhibitors imatinib and sunitinib.8,9 However, the activity of tyrosine kinase inhibitors is less well examined in patients with other soft tissue sarcomas. Imatinib has only anecdotal activity in non-GIST sarcomas except for dermatofibrosarcoma protuberans,10–12 and studies of inhibitors of mammalian target of rapamycin (mTOR) show only low Response Evaluation Criteria in Solid Tumors (RECIST) response rates.13–16
Sorafenib, a small molecule B-raf and vascular endothelial growth factor (VEGF) receptor inhibitor, is potentially useful in several specific sarcoma subtypes, such as malignant peripheral-nerve sheath tumors (MPNST) with loss of NF1 and activation of the ras-raf signaling pathway.17–19 Angiosarcomas are inherently a target for antiangiogenic agents. Further, a phase I study of sorafenib in patients with solid tumors indicated a promising 30% 12-week nonprogression rate in patients with metastatic sarcomas.20
Accordingly, we sought to examine the activity of sorafenib in sarcoma subtypes for which there appeared to be a biologic rationale. We used a multiarm phase II study design to evaluate specific sarcoma subtypes independently. We performed biopsies in a limited number of patients with angiosarcoma and MPNST before and after starting therapy to determine changes in downstream targets of sorafenib, and examined trough sorafenib levels and soluble mediators of angiogenesis before and after starting therapy, in particular in angiosarcoma patients.
PATIENTS AND METHODS
This was a six-arm, multicenter phase II study of oral sorafenib in patients with advanced sarcomas. Patients were accrued from 11 institutions. Institutional review board or ethics committee approval of the protocol and informed consent form was required. Each participant provided written informed consent. RECIST response determinations were made by study radiologists at the treating institutions; images were not reviewed centrally. Pathologists at treating institutions defined sarcoma subtype; central review of pathology was not performed. Responding patients at Memorial Hospital were subject to confirmatory review by an independent committee.
Study Design
Patients received a starting dose of sorafenib 400 mg oral twice per day continuously. A cycle of therapy was defined as 28 days of treatment. Dose reductions to 400 mg oral daily and then 200 mg oral daily were permitted for patients experiencing National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) version 3.0 is as follows1: grade 2 dermatological reaction,2 asymptomatic grade 2 hypertension on at least two consecutive measurements, or any symptomatic grade 2 or any grade 3 to 4 hypertension,3 grade 3 to 4 drug-related nonhematologic toxicity, and4 grade 4 drug-related neutropenia or thrombocytopenia. The clinicaltrials.gov study identifier was NCT00245102.
Clinical examinations and laboratory testing were performed at a screening visit, at the start of treatment, and at the start of each cycle of therapy for six months, every 6 weeks for the next 6 months, then every 12 weeks. Blood pressure was examined weekly for 4 weeks and at each clinic visit. Physical examination, Eastern Cooperative Oncology Group (ECOG) performance status, complete blood count, and biochemical profile were performed every 4 weeks, and at each visit after 6 months. Tumors were re-evaluated after every 8 weeks of therapy for 24 weeks, then after every 12 weeks of therapy.
The first 10 patients with angiosarcoma and MPNST treated on the study had pre- and two-week post-treatment biopsies and limited sequencing of BRAF. All MPNST and angiosarcoma patients were eligible to participate in correlative studies before treatment and after 2 weeks of treatment, including determination of sorafenib and metabolite trough levels, and circulating endothelial cell levels. Patients gave written informed consent for these studies.
Sorafenib was provided to participating sites by the National Cancer Institute Cancer Therapy Evaluation Program through a Cooperative Research and Development Agreement between Bayer AG, Onyx Pharmaceuticals Inc, and the National Cancer Institute Division of Cancer Treatment and Diagnosis (protocol 7060).
Patient Eligibility
Inclusion criteria included: histologically confirmed sarcoma other than GIST; presence of measurable metastatic and/or locally advanced or locally recurrent disease; 0 to 1 prior lines of chemotherapy for metastatic disease (0 to 3 lines for angiosarcoma or MPNST); no prior treatment with small molecule kinase inhibitors; age older than 18 years; ECOG performance status 0 to 2; adequate hematologic, hepatic, and renal function; use of adequate contraception; resolution of prior adverse events related to tumor-specific therapy resolved to grade 1 or less before study entry (except alopecia); and ability to understand and the willingness to give written informed consent.
Exclusion criteria included prior chemotherapy or radiotherapy within 3 weeks before entering the study, simultaneously use of other systemic agents, known brain metastases, use of therapeutic anticoagulation, cytochrome P450 enzyme-inducing antiepileptic drugs, rifampin, or St John's wort, uncontrolled intercurrent illness, significant swallowing dysfunction, evidence of a bleeding diathesis, pregnancy, and known positivity for HIV.
Correlative Studies
Sorafenib day 15 trough plasma concentration was examined in 67 patients using a validated liquid chromatography-mass spectrometry assay, with a lower limit of quantification of 10 ng/mL.20
Circulating endothelial cell measurement was performed in five angiosarcoma patients using a Cell Track Analyzer II system (Varidex, Raritan, NJ).21 Details of the technique are provided in the Appendix (online only).
Immunohistochemical staining was performed on 5 to 6 μ sections cut from the paraffin-embedded tumor samples, and analyzed using a rabbit polyclonal antibody for phosphorylated extracellular signal regulated kinase (pERK) (phospho-p44/42 mitogen-activated protein kinase [Thr202/Tyr204]; Cell Signaling Technology Inc, Danvers, MA).22 Slides from each biopsy were stained with a species, isotype (immunoglobulin G), and concentration-matched negative control antibody. A hematoxylin-eosin slide was also stained for each sample. Localization of pERK staining to cell nuclei or cytoplasm was evaluated qualitatively. Nuclear pERK staining intensity was graded using a five-point scale: 0, no staining; 1+, weak; 2+, moderate; 3+, strong and 4+, intense.
For exon 15 BRAF DNA sequencing, tumor DNA was extracted from fresh frozen tissue or paraffin-embedded tissue, and genomic exon 15 BRAF sequence amplified by polymerase chain reaction and sequenced using standard techniques.23 Exon 15 was of interest given the known V600E mutation in this exon, using forward primer 5′-TCATAATGCTTGCTCTGATAGGA-3′ and reverse primer 5′-GGCCAAAAATTTAATCAGTGGA-3′.
Statistical Methods
Given more than 70 different types of sarcoma, we assumed that activity of sorafenib against one sarcoma subtype did not imply activity against another. Based on preclinical data,24 we examined as separate Simon two-stage designs arms for patients with1 leiomyosarcoma,2 malignant peripheral-nerve sheath tumor (MPNST),3 synovial sarcoma,4 vascular sarcomas (includes angiosarcoma, hemangiopericytoma-solitary fibrous tumors patient [n = 2] and giant hemangioma [n = 1]),5 high-grade undifferentiated pleomorphic sarcoma [HGUPS], formerly termed malignant fibrous histiocytoma [MFH], and6 an “other” sarcoma arm (included patients with epithelioid hemangioendothelioma [n = 4]), to insure adequate inclusion of a variety of sarcoma subtypes.
A Simon optimal two-stage design was used for each arm of the study.25 The primary end point was response rate using RECIST. A 5% response rate was considered not promising; a 20% response rate was considered promising. The probability of type I and type II errors were set at 0.10 each. Twelve eligible patients were initially accrued. If at least one RECIST response were observed, the arm would be opened to enroll 25 more eligible patients. If there were at least four responses in 37 eligible patients within 6 months of starting therapy, the stratum was declared to have a positive result for that study arm. This design gave a 90% probability of a positive result if the true response rate were at least 20%, and a 90% probability of a negative result if the true response rate were 5% or lower.
Secondary end points included: progression-free survival (PFS; defined as rate of RECIST complete response [CR] + partial response [PR] + stable disease [SD]); overall survival (OS); day 15 trough pharmacokinetics of sorafenib and its relationship to toxicity; assessment of pre- and post-treatment inhibition of ERK phosphorylation (n = 10 patients with MPNST or angiosarcoma); circulating endothelial cells (n = 5 angiosarcoma patients) and soluble cytokine levels (n = 13, angiosarcoma and MPNST) as metrics of angiogenesis before and after starting therapy.
Kaplan-Meier curves for OS and PFS were constructed for each stratum and for all patients.26 Analysis of dose reductions with respect to prognostic variables was first examined using univariate analysis, specifically Kruskal-Wallis rank-sum test for continuous variables and Fisher's exact test for categoric variables, and then by multivariate analysis. Logistic regression was used to examine any dose reduction as a function of the variables from the univariate analysis, while the number of dose reductions (0, 1, ≥ 2 [including those who needed a third dose reduction after at least one clinical evaluation but were taken off study for toxicity]) was analyzed using proportional-odds logistic regression.
RESULTS
Demographics
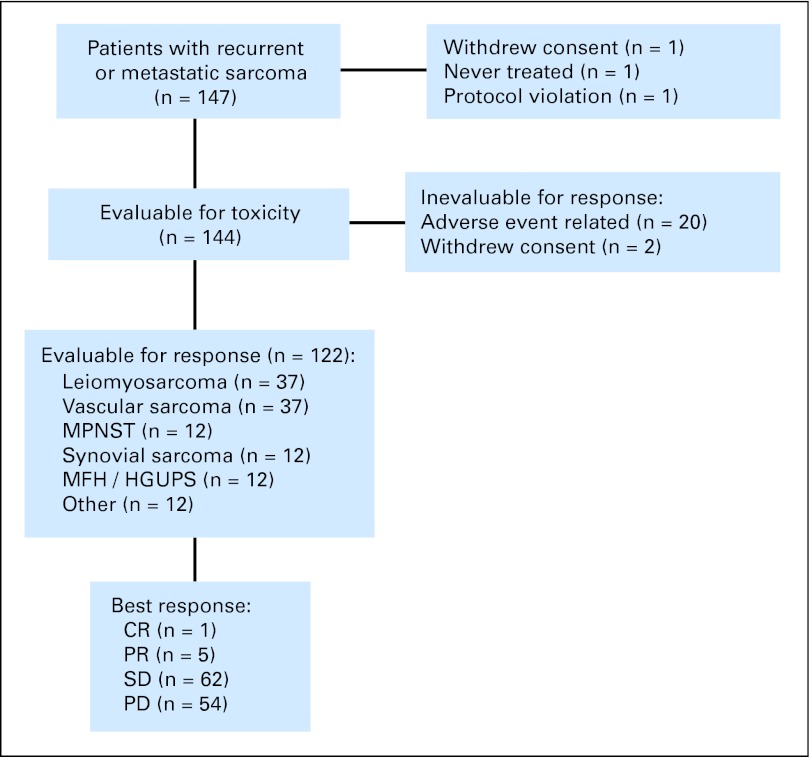
Patient characteristics are presented in Table 1 and Figure 1. The study enrolled 147 patients at 11 sites in the United States between October 6, 2005 and November 14, 2007. A total of 145 patients received at least one dose of sorafenib; 144 were assessable for toxicity. Twenty-two patients discontinued therapy for adverse events did not resolve within 2 weeks and could not be construed as disease progression. These 22 patients were assessable for toxicity, but not response. Among 144 assessable patients for toxicity, median age was 55 (range, 18 to 90), and 92 were women (64%). Median ECOG performance status was 0. Chemotherapy had been given to 91 patients (63%) before enrollment on study.
Table 1.
Patient Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Patients registered | 147 | |
| Ineligible for toxicity | 3 | |
| Never treated | 1 | |
| Withdrew consent before treatment | 1 | |
| Protocol violation (arm misassignment) | 1 | |
| Patients assessable for toxicity | 144 | |
| Patients unassessable for response | 22 | |
| Adverse event | ||
| Dermatologic | 9 | |
| Rash or palmar-plantar erythrodysesthesia and/or blistering | 6 | |
| Dermatologic and other toxicity | 3 | |
| Hemorrhage | 4 | |
| Unsuspected CNS metastasis | 1 | |
| Lung metastases | 1 | |
| Axillary primary | 1 | |
| Gastric primary | 1 | |
| Deep venous thrombosis | 1 | |
| Perforation | 2 | |
| Ileum | 1 | |
| Tension pneumothorax | 1 | |
| Other | 4 | |
| Extremity pain | 1 | |
| Congestive heart failure | 1 | |
| Transient ischemic attack | 1 | |
| Reversible posterior leukoencephalopathy syndrome | 1 | |
| Withdrew consent | 2 | |
| Assessable for response | 122 | |
| Median age | 55 | |
| Range | 18-90 | |
| Sex, female | 92 | 64 |
| ECOG performance status | ||
| 0 | 83 | |
| 1 | 54 | |
| 2 | 7 | |
| Prior chemotherapy | 91 | 63 |
| Histology | ||
| Leiomyosarcoma | 42 | |
| Angiosarcoma | 40 | |
| MPNST | 15 | |
| HGUPS | 14 | |
| Synovial sarcoma | 14 | |
| Other | 19 | |
| Epithelioid hemangio-endothelioma | 4 | |
| Fibrosarcoma | 4 | |
| Liposarcoma | 3 | |
| Chondrosarcoma | 2 | |
| Other | 6 | |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MPNST, malignant peripheral nerve sheath tumor; HGUPS, high grade undifferentiated pleomorphic sarcoma, formerly termed malignant fibrous histiocytoma.
Fig 1.
CONSORT diagram for patients registered for this study. MPNST, malignant peripheral nerve sheath tumor; MFH, malignant fibrous histiocytoma; HGUPS, high grade undifferentiated pleomorphic sarcoma, formerly termed malignant fibrous histiocytoma; CR, complete response; PR, partial response; SD, stable disease; PD, progresive disease.
Treatment Efficacy
Figures 2A to 1D and Table 2 indicate PFS and OS for all patients and by histology. Median follow-up time was 6 months (range, 0 to 31). By investigator assessment of 122 assessable patients, PFS was 3.2 months (95% CI, 2.5 to 3.7 months). Median number of treatment cycles was 3 (range, 1 to 33), and 20% of patients received 6 or more treatment cycles (25 of 122 patients). PFS for the study population was 53% at 3 months and 22% at 6 months (Kaplan-Meier estimates), and is indicated by stratum in Table 2, and for the leiomyosarcoma and angiosarcoma patients in Appendix Tables A1 and A2 (online only), respectively. PFS was superior in those patients with angiosarcoma who had received no prior chemotherapy versus those who had (P = .04, Mann-Whitney U test), while this was not the case for leiomyosarcoma (P = .16). Of the 122 assessable patients who received sorafenib, 56 (46%) were alive as of May 2008; median OS was 14.3 months (95% CI, 12.2 to 19.2 months). The histology of patients still on study after 6 months or more is indicated in Appendix Table A3 (online only).
Fig 2.
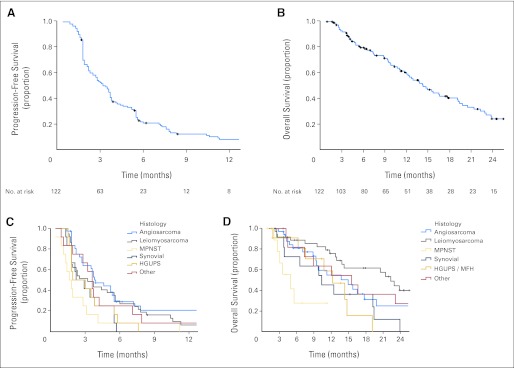
(A, C) Progression-free survival and (B, D) overall survival for (A, B) all patients and by (C, D) stratum. (A) Progression-free survival for all assessable patients (n = 122). (B) Overall survival for all assessable patients (n = 122). (C) Progression-free survival by arm. (D) Overall survival by arm.
Table 2.
Clinical Outcomes in Assessable Patients (n = 122)
| Histology | Progression-Free Survival |
Overall Survival |
Kaplan-Meier Estimate for Progression-Free Survival (%) |
|||
|---|---|---|---|---|---|---|
| Duration (months) | 95% CI | Duration (months) | 95% CI | 3 Months | 6 Months | |
| All patients | 3.2 | 2.5 to 3.7 | 14.3 | 12.2 to 19.2 | 53 | 22 |
| Angiosarcoma | 3.8 | 2.8 to 5.5 | 14.9 | 9.4 to ∞ | 64 | 31 |
| Leiomyosarcoma | 3.2 | 1.9 to 5.6 | 22.4 | 14.2 to ∞ | 54 | 30 |
| MPNST | 1.7 | 1.3 to ∞ | 4.9 | 3.0 to ∞ | 25 | 8 |
| Synovial sarcoma | 2.5 | 1.8 to ∞ | 10.3 | 6.5 to ∞ | 42 | 0 |
| HGUPS | 2.4 | 1.8 to ∞ | 12.2 | 7.5 to ∞ | 42 | 8 |
| Other histologies | 3.6 | 2.8 to ∞ | 15.5 | 8.9 to ∞ | 67 | 25 |
Abbreviations: MPNST, malignant peripheral nerve sheath tumor; HGUPS, high grade undifferentiated pleomorphic sarcoma, formerly termed malignant fibrous histiocytoma.
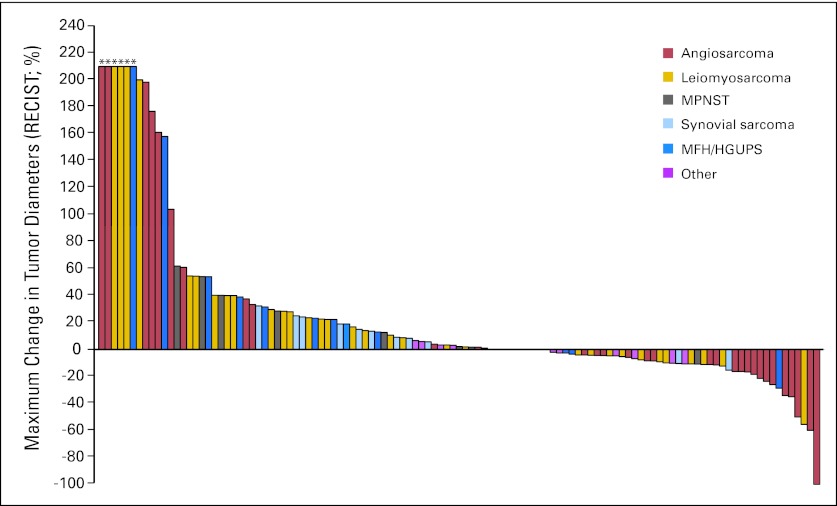
Best RECIST responses on treatment are indicated in Table 3. Four of 37 eligible patients with vascular sarcomas experienced a PR, and one experienced a CR, for a response rate of 14%. The RECIST response rate is 10% (four of 40 patients), using an intention-to-treat analysis patients who were assessable for toxicity. Three patients with epithelioid hemangioendothelioma of four treated had stable disease as a best result. Patients with angiosarcoma had the greatest degree to tumor shrinking overall, as is apparent in the plot of best RECIST response by tumor subtype (Fig 3).
Table 3.
Best Response Evaluation Criteria in Solid Tumors Response to Therapy (n = 122 assessable)
| Histology | No. of Patients |
||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | Total | |
| Angiosarcoma | 1 | 4 | 21 | 11 | 37 |
| Leiomyosarcoma | 1 | 18 | 18 | 37 | |
| MPNST | 3 | 9 | 12 | ||
| Synovial sarcoma | 6 | 6 | 12 | ||
| HGUPS | 5 | 7 | 12 | ||
| Other | 9 | 3 | 12 | ||
| Total | 1 | 5 | 62 | 54 | 122 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; MPNST, malignant peripheral nerve sheath tumor; HGUPS, high grade undifferentiated pleomorphic sarcoma, formerly termed malignant fibrous histiocytoma.
Fig 3.
Plot of best radiological outcome by Response Evaluation Criteria in Solid Tumors (RECIST) by subject. (*) The discovery of new disease or growth of index lesions over 200% from baseline as the basis of progression. MPNST, malignant peripheral nerve sheath tumor; MFH, malignant fibrous histiocytoma; HGUPS, high grade undifferentiated pleomorphic sarcoma, formerly termed malignant fibrous histiocytoma.
One confirmed PR was observed in 37 eligible patients with leiomyosarcoma. The RECIST response rate is 3%, and is 2% by an intention-to-treat analysis of the 42 patients who were assessable for toxicity. No RECIST PR or CR was seen in 12 eligible patients each with malignant fibrous histiocytoma, synovial sarcoma, MPNST, or “other” sarcomas, although patients with MPNST and synovial sarcoma experience minor RECIST responses.
Toxicity and Dose Reductions
For 144 patients eligible for toxicity assessment, the most common selected grade 2, and all grade 3, 4, and 5 adverse events possibly, probably, or definitely related to sorafenib are presented in Table 4, including fatigue, dermatologic reactions, hypertension, and gastrointestinal symptoms. Eighty-nine patients (61%) required dose reductions for toxicity, of which 75% were due to dermatologic toxicity. Eighteen patients (12%) required two dose reductions, and three patients (2%) were taken off study for the need for three protocol-specified dose reductions for dermatologic toxicity.
Table 4.
National Cancer Institute Common Toxicity Criteria for Adverse Events Version 3.0 Grade 2-4 Adverse Events With at Least 5% Incidence Possibly, Probably, or Definitely Related to Sorafenib (as determined by investigator; n = 144 assessable for toxicity)
| Toxicity | Grade |
Total |
||||||
|---|---|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Hand-foot skin reaction | 29 | 20 | 18 | 13 | 47 | 33 | ||
| Hypophosphatemia | 23 | 16 | 7 | 5 | 1 | 1 | 31 | 22 |
| Rash/desquamation | 11 | 8 | 17 | 12 | 28 | 19 | ||
| Lymphopenia | 7 | 5 | 14 | 10 | 3 | 2 | 24 | 17 |
| Low hemoglobin | 16 | 11 | 7 | 5 | 23 | 16 | ||
| Fatigue | 14 | 10 | 9 | 6 | 23 | 16 | ||
| Low albumin | 18 | 13 | 1 | 1 | 19 | 13 | ||
| Hyperglycemia | 13 | 9 | 3 | 2 | 16 | 11 | ||
| Hypertension | 9 | 6 | 6 | 4 | 15 | 10 | ||
| Leukopenia | 11 | 8 | 2 | 1 | 2 | 1 | 15 | 10 |
| Rash: erythema multiforme | 12 | 8 | 1 | 1 | 13 | 9 | ||
| Elevated ALT | 9 | 6 | 3 | 2 | 1 | 1 | 13 | 9 |
| Elevated AST | 7 | 5 | 2 | 1 | 1 | 1 | 10 | 7 |
| Diarrhea | 6 | 4 | 4 | 3 | 10 | 7 | ||
| Hypocalcemia | 7 | 5 | 3 | 2 | 10 | 7 | ||
| Hyperbilirubinemia | 6 | 4 | 2 | 1 | 8 | 6 | ||
Fatal GI hemorrhage was observed in one patient with abdominal disease, as was one episode of grade 4 bowel perforation in another patient; one episode of fatal tension pneumothorax was seen in a patient with metastatic disease to the lungs. One patient experienced a grade 4 decrease in cardiac ejection fraction, but recovered after discontinuation of sorafenib. Much of the severe toxicity was observed within 2 weeks of the start of treatment, accounting for the significant number of patients assessable for toxicity only.
Pharmacokinetics
Trough pharmacokinetics after 2 weeks of therapy was examined in 67 patients receiving sorafenib. Patients with toxicity requiring a dose reduction had trough levels examined after approximately 2 weeks of therapy on the reduced dose.
Of the patients who had trough levels examined between 6 and 24 hours from the prior dose of sorafenib (n = 41), the median trough level of sorafenib (±median absolute deviation) was 4,300 ± 3,400 ng/mL. There was no difference in trough levels in those patients who received dose reductions versus those who did not (P = .55), suggesting adequate serum levels were present once a tolerable dose had been identified. There was no difference in PFS for those patients with sorafenib level above versus below the median trough level (P = .16).
Modeling Risk Factors
We examined demographic features and their association with toxicity. By univariate analysis, lean body mass, height, body-surface area, sex, serum creatinine, and weight were statistically significant factors associated with sorafenib dose reductions. We fitted a number of multivariate models including gender, serum creatinine, and one of the following: lean body mass, body surface area, or height. Height and lean body mass were significant when adjusting for serum creatinine by logistic regression for any dose reduction or by proportional odds logistic regression for number of dose reduction (Appendix Tables A4 and A5, online only).
Other Translational Studies
Tumor biopsies were taken from 10 patients with MPNST and angiosarcoma before treatment and after 12 to 15 days of sorafenib therapy. There was no consistent change in pERK staining in six patients with interpretable paired samples. There were no exon 15 mutations in BRAF in the seven samples with interpretable data. There was no consistent change in circulating endothelial cell number between pre- and post-treatment specimens in four of five patients with angiosarcoma with paired data.
DISCUSSION
Sorafenib is active against metastatic angiosarcoma, and had minor activity against leiomyosarcoma. Although the response rates were lower with sorafenib in this study than standard cytotoxic agents, PFS for leiomyosarcoma and angiosarcoma patients was comparable,27–31 supporting the activity of sorafenib in these diagnoses. In comparison, imatinib was inactive in 16 angiosarcoma patients as part of a phase II study,32 supporting sorafenib's VEGF receptor blockade as its key mechanism of action. There were no RECIST responses among the epithelioid hemangioendothelioma or hemangiopericytoma-solitary fibrous tumor patients on study, implying that the biology of these tumors is distinct from angiosarcoma.
We were struck with two findings beyond the RECIST responses. Two patients with MPNST had regression or cystification of metastatic disease without a RECIST response; one patient with synovial sarcoma with significant hemoptysis followed by a minor response to sorafenib given at a lower dose off study. Whether these effects were associated with VEGF or ras-raf signaling effects of sorafenib is speculative, given inconsistent changes in phospho-ERK observed in tumor biopsies performed before and after staring sorafenib. However, sorafenib inhibits MAPK signaling, cell growth, and induces G1 cell cycle arrest in MPNST cell lines in vitro in a B-raf-dependent fashion,24 supporting the importance of the NF1 deletion common in MPNST. More potent inhibitors of raf may be appropriate agents to examine in MPNST, and combination therapy including sorafenib may be a more fruitful way to use these agents in other sarcomas.
Secondly, we also noted tumor flares in patients with angiosarcoma who stopped therapy for progression or toxicity, as has been observed with other targeted agents, such as patients with GIST who stop imatinib or patients with other cancers who stop antiangiogenic therapies. This observation is consistent with increases in VEGF observed after use of antiangiogenic agents seen in other studies.33
Except in rare sarcoma histologies,10–12,34 other kinase inhibitors have also shown only minor activity in patients with sarcomas.13,14,32,35–37 Nonetheless, as with renal cell and hepatocellular carcinoma patients treated with sorafenib, PFS observed on trials of kinase-specific agents such as sorafenib supports its use as a meaningful end point in clinic trials of such agents.
The toxicity of sorafenib in our patients at the US Food and Drug Administration–approved dose and schedule suggests that many patients would have tolerated therapy better if started at a lower dose, with dose escalation later. Plasma trough levels of sorafenib were not different between people tolerating 400 mg daily versus those tolerating 800 mg daily, suggesting that trough level may be used to help adjust dose. Patient stature correlated to toxicity in our multivariate model, and may be a means to select patients for higher versus lower daily doses of sorafenib. However, our correlative studies were too small to draw any firm conclusions, and are thus only hypothesis-generating.
In summary, we observed activity of sorafenib in sarcoma patients, in particular those with angiosarcoma. These results, taken together with striking responses seen with imatinib in patients with dermatofibrosarcoma protuberans10–12 and pigmented villonodular synovitis,34 with insulin-like growth factor inhibitors in Ewing sarcoma,38,39 and with anti-RANK ligand antibodies in patients having recurrent giant cell tumors of bone40 give investigators hope that more effective single agent and combinations of kinase-directed therapeutics can be identified for different sarcoma subtypes. Enthusiasm is tempered by the low response rate observed in this study, and with other tyrosine kinase inhibitors.
Acknowledgment
We are grateful to all the patients and their families for participating in this study. Dr John J. Wright coordinated the study and answered innumerable questions at Cancer Therapy Evaluation Program. We also acknowledge the contribution of Sant Chawla, MD, and Shirish Gadgeel, MD, MBBS, for entering patients on the study, research nurses Gloria Wasilewski, Kelly Scheu, Lisa Caltieri, and Linda Ahn. We thank Martin Fleisher, Rita Gonzalez-Espinoza, Juana Gonzalez, and Vipin Agarwal for assistance with correlative studies, Alex Lash, and Manda Wilson and colleagues for the development of the Study Tracker platform for data entry, and the assistance in protocol and data management by Jennifer Kachel, Gauri Bhuchar, and Susan Puleio.
Appendix
Circulating endothelial cell quantitation.
Circulating endothelial cell (CEC) measurement was performed in five patients as described previously for circulating tumor cells (Shaffer DR, Leversha MA, Danila DC, et al: Clin Cancer Res 13:2023-2029, 2007). The methodology for automated immunomagnetic selection of CECs, based on capture with an anti-CD146 and immunofluorescent staining and analysis, has been described (Allard WJ, Matera J, Miller MC, et al: Clin Cancer Res 10:6897-6904, 2004; Kagan M, Howard D, Bendele T, et al: J Clin Ligand Assay 25:104-110, 2002; Cristofanilli M, Budd GT, Ellis MJ, et al: N Engl J Med 351:781-791, 2004). In short, samples were drawn in tubes containing cell preservatives, maintained at room temperature, incubated with CD146 antibody-covered ferroparticles at room temperature, and processed on the CellTracks Autoprep (Veridex, Raritan, NJ). CECs expressing CD146 were isolated by a magnetic field without centrifugation. After the supernatant containing unbound cells was removed, the enriched samples were processed for fluorescent staining. Nucleic acids were stained with 4′,6-diamidino-2-phenylindole, and endothelial cells were stained with anti–CD105-phycoerythrin. Leukocytes were excluded with an allophycocyanin-conjugated anti-CD45 antibody as previously described. Stained cells were analyzed on a fluorescence microscope using the Cell Track Analyzer II (Immunicon). Automatically selected images were reviewed by the operator for identification and counting of CECs, which were defined as CD105-positive and 4′,6-diamidino-2-phenylindole–positive nucleated cells lacking CD45. Quality control was maintained via standard procedures. In control assays using blood from healthy controls spiked with human umbilical cord vein endothelial cells, assay sensitivity was 1 CEC/7.5 mL peripheral blood. The CellSearch System is available from Veridex LLC (Warren, NJ).
Table A1.
Features of Angiosarcoma Patients on Study
| Diagnosis | Grade | Site | Special Features | Best Response | Prior Lines of Systemic Therapy | Agents Employed |
|---|---|---|---|---|---|---|
| RT angiosarcoma | High | Breast | CR | 0 | None | |
| RT angiosarcoma | High | Breast | PR | 0 | None | |
| RT angiosarcoma | High | Breast | PR | 0 | None | |
| Angiosarcoma | High | Head-neck | PR | 1 | 1 Paclitaxel | |
| Angiosarcoma | High | Head-neck | PR | 3 | 1 Gemcitabine-docetaxel-carboplatin; 2 gemcitabine-docetaxel-bevacizumab; 3 liposomal doxorubicin | |
| Giant hemangioma | Low | Thorax | SD | 0 | None | |
| Angiosarcoma | High | Head-neck | SD | 0 | None | |
| Angiosarcoma | High | spleen | SD | 0 | None | |
| Angiosarcoma | High | Soft tissue, NOS | SD | 0 | None | |
| Angiosarcoma | High | Retroperitoneal | SD | 0 | None | |
| Angiosarcoma | High | Soft tissue, NOS | SD | 0 | None | |
| Angiosarcoma | High | Bone, NOS | SD | 0 | None | |
| Angiosarcoma | High | Head-neck | SD | 0 | None | |
| Angiosarcoma | High | Head-neck | SD | 1 | 1 Doxorubicin | |
| Angiosarcoma | High | Retroperitoneal | SD | 1 | 1 Doxorubicin-ifosfamide | |
| RT angiosarcoma | High | Breast | SD | 1 | 1 Liposomal doxorubicin | |
| Angiosarcoma | High | Head-neck | SD | 1 | 1 Paclitaxel | |
| RT angiosarcoma | High | Breast | SD | 1 | 1 Doxorubicin-ifosfamide; 2 paclitaxel | |
| Angiosarcoma | High | Breast | SD | 1 | 1 Gemcitabine-docetaxel-cisplatin | |
| Angiosarcoma | High | Lower extremity | SD | 1 | 1 Gemcitabine-docetaxel | |
| RT angiosarcoma | High | Breast | +Epithelioid | SD | 2 | 1 Liposomal doxorubicin; 2 paclitaxel |
| RT angiosarcoma | High | Head-neck | SD | 2 | 1 Liposomal doxorubicin; 2 paclitaxel | |
| RT angiosarcoma | High | Breast | SD | 2 | 1 Liposomal doxorubicin-paclitaxel; 2 doxorubicin | |
| Angiosarcoma | High | Thorax | SD | 2 | 1 Ifosfamide; 2 paclitaxel-estramustine | |
| Angiosarcoma | High | Head-neck | SD | 2 | 1 Doxorubicin-ifosfamide; 2 paclitaxel | |
| Angiosarcoma | High | Thorax | SD | 3 | 1 Doxorubicin-ifosfamide-dacarbazine; 2 bevacizumab; 3 gemcitabine-docetaxel | |
| Angiosarcoma | High | Mediastinum | PD | 0 | None | |
| Angiosarcoma | High | Soft tissue, NOS | PD | 1 | 1 Paclitaxel | |
| Angiosarcoma | High | Head-neck | PD | 1 | 1 Paclitaxel | |
| Angiosarcoma | High | Soft tissue, NOS | PD | 1 | 1 Doxorubicin-paclitaxel | |
| Angiosarcoma | High | spleen | PD | 1 | 1 Doxorubicin-paclitaxel | |
| Angiosarcoma | High | Head-neck | PD | 2 | 1 Paclitaxel; 2 doxorubicin | |
| RT angiosarcoma | High | Breast | PD | 2 | 1 Liposomal doxorubicin; 2 paclitaxel | |
| Angiosarcoma | High | Head-neck | +Epithelioid | PD | 2 | 1 Paclitaxel; 2 doxorubicin |
| HPC-SFT | Low | Retroperitoneal | PD | 2 | 1 Doxorubicin-ifosfamide; 2 gemcitabine-docetaxel-cisplatin | |
| HPC-SFT | Low | Lower extremity | PD | 2 | 1 Doxorubicin-ifosfamide; 2 gemcitabine-docetaxel | |
| Angiosarcoma | High | Lower extremity | +Epithelioid | PD | 3 | 1 Liposomal doxorubicin; 2 ifosfamide; 3 gemcitabine-docetaxel |
NOTE. Progression-free survival was superior in those patients who had received no prior chemotherapy versus those who did, P = .04, Mann-Whitney U test.
Abbreviations: RT, radiation therapy–associated (angiosarcoma); HPC-SFT, hemangiopericytoma-solitary fibrous tumor; CR, complete response; PR, partial response; SD, stable disease; PD, progression of disease; NOS, not otherwise specified.
Table A2.
Features of Leiomyosarcoma Patients on Study
| Diagnosis | Grade | Site | Special Features | Best Response | No. of Prior Lines of Systemic Therapy | Agents Employed |
|---|---|---|---|---|---|---|
| Leiomyosarcoma | Low | Retroperitoneum | PR | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | Low | Retroperitoneum | SD | 0 | None | |
| Leiomyosarcoma | High | Retroperitoneum | SD | 0 | None | |
| Leiomyosarcoma | High | Inferior vena cava | SD | 0 | None | |
| Leiomyosarcoma | High | Retroperitoneum | SD | 0 | None | |
| Leiomyosarcoma | High | Lower extremity | SD | 0 | None | |
| Leiomyosarcoma | High | Uterus | SD | 0 | None | |
| Leiomyosarcoma | High | Bone, lower extremity | SD | 0 | None | |
| Leiomyosarcoma | — | Soft tissue, NOS | SD | 0 | None | |
| Leiomyosarcoma | — | Uterus | SD | 1 | 1 Doxorubicin-ifosfamide | |
| Leiomyosarcoma | High | Retroperitoneum | SD | 1 | 1 Doxorubicin-dacarbazine | |
| Leiomyosarcoma | High | Inferior vena cava | SD | 1 | 1 Epirubicin-ifosfamide | |
| Leiomyosarcoma | High | Uterus | SD | 1 | 1 Gemcitabine | |
| Leiomyosarcoma | High | Retroperitoneum | SD | 1 | 1 Gemcitabine-docetaxel-cisplatin | |
| Leiomyosarcoma | High | Intra-abdominal | SD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Intra-abdominal | SD | 1 | 1 Gemcitabine-docetaxel-cisplatin | |
| Leiomyosarcoma | High | Intra-abdominal | SD | 1 | 1 Temsirolimus | |
| Leiomyosarcoma | High | Uterus | SD | 2 | 1 Doxorubicin; 2 investigational agent | |
| Leiomyosarcoma | High | Intra-abdominal | SD | 2 | 1 Doxorubicin-ifosfamide; 2 gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 0 | None | |
| Leiomyosarcoma | High | Uterus | PD | 0 | None | |
| Leiomyosarcoma | High | Head-neck | PD | 0 | None | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 0 | None | |
| Leiomyosarcoma | High | Intra-abdominal | PD | 0 | None | |
| Leiomyosarcoma | High | Uterus | PD | 1 | 1 Doxorubicin | |
| Leiomyosarcoma | Low | Uterus | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 1 | 1 Doxorubicin-ifosfamide | |
| Leiomyosarcoma | High | Retroperitoneum | +Epithelioid | PD | 1 | 1 Doxorubicin |
| Leiomyosarcoma | High | Uterus | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 1 | 1 Doxorubicin | |
| Leiomyosarcoma | High | Uterus | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Uterus | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 1 | 1 Gemcitabine-docetaxel-cisplatin | |
| Leiomyosarcoma | High | Retroperitoneum | PD | 1 | 1 Doxorubicin-ifosfamide | |
| Leiomyosarcoma | High | Uterus | PD | 1 | 1 Gemcitabine-docetaxel | |
| Leiomyosarcoma | High | Uterus | PD | 2 | 1 Doxorubicin; 2 gemcitabine-docetaxel |
NOTE. Progression-free survival was not superior in those patients who had received no prior chemotherapy (v those who did), P = .16, Mann-Whitney U test.
Abbreviations: PR, partial response; SD, stable disease; PD, progression of disease; NOS, not otherwise specified.
Table A3.
Summary of Patients With Nonprogressive Disease At 6 Months and Beyond
| Treatment Arm | Best Result | No. of Patients |
|---|---|---|
| Vascular sarcoma | ||
| Angiosarcoma | CR | 1 |
| Angiosarcoma | PR | 3 |
| Angiosarcoma | SD | 3 |
| Giant hemangioma | SD | 1 |
| Leiomyosarcoma | PR | 1 |
| Leiomyosarcoma | SD | 11 |
| MPNST | SD | 1 |
| Synovial sarcoma | SD | 1 |
| Other | ||
| Extraskeletal myxoid chondrosarcoma | SD | 1 |
| Conventional chondrosarcoma | SD | 1 |
| Fibrosarcoma | SD | 1 |
| Total | 25/122 assessable patients (20%) |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progression of disease; MPNST, malignant peripheral nerve sheath tumor.
Table A4.
Univariate Analysis of Demographic Factors Associated With Any Dose Reduction or Number of Dose Reductions and Statistical Significance
| Variable | Median |
|||
|---|---|---|---|---|
| Range | P | Any Dose Reduction | No. of Dose Reductions | |
| Lean body mass, kg | 50 | 34.7-79.8 | .006 | .007 |
| Height, cm | 166 | 148-200 | .009 | .008 |
| Serum creatinine, mg/dL | 0.9 | 0.5-1.5 | .016 | .023 |
| Body surface area | 1.86 | 1.37-2.83 | .033 | .013 |
| Sex | .035 | .014 | ||
| Female | 92 | |||
| Male | 52 | |||
| Weight, kg | 75.6 | 42.8-196.4 | .08 | .04 |
| Total bilirubin, mg/dL | 0.6 | 0.1-1.4 | .20 | .30 |
| Total white blood cell count, ×103/μL | 6.2 | 2.6-16.7 | .27 | .32 |
| Histology* | .43 | .64 | ||
| Age | 55 | 18-90 | .82 | .37 |
| Calculated creatinine clearance, mL/min† | 89.9 | 30.9-354.4 | .83 | .95 |
| Serum albumin, mg/dL | 3.9 | 2.2-4.8 | .94 | .84 |
NOTE. Bold font indicates variables used in multivariate analysis.
Six arms of study indicated above.
Cockcroft DW, Gault MH: Nephron 16:31-41, 1976.
Table A5.
Logistic Regressions to Model Factors Important in Toxicity Observed in This Study
| Logistic Regression | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Stepwise* | |||
| Height | 0.960 | 0.923 to 0.998 | .034 |
| Creatinine | 0.344 | 0.068 to 1.729 | .186 |
| Proportional odds† | |||
| Height | 0.956 | 0.921 to 0.992 | .014 |
| Creatinine | 0.301 | 0.065 to 1.403 | .119 |
For model selection (any dose reduction). Variables noted in Table A4. Similar findings were noted when examining lean body mass instead of height.
For model selection (number of dose reduction). Variables noted in Table A4. Similar findings were noted when examining lean body mass instead of height.
Footnotes
Supported by the National Cancer Institute (NCI) Program Project Grant No. CA47179, NCI phase II contract CM62202, Cycle for Survival, the Shuman Family Fund for GIST research, and the Sarcoma Research Fund.
Presented in part in oral format at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available JCO.org.
Clinical trial information can be found for the following: NCT00245102.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Chris H. Takimoto, Johnson & Johnson/Ortho Biotech Oncology R + D (Centocor) (C) Consultant or Advisory Role: Chris H. Takimoto, Amgen (C) Stock Ownership: None Honoraria: David R. D'Adamo, Bayer AG; Chris H. Takimoto, Amgen Research Funding: David R. D'Adamo, Bayer AG; Chris H. Takimoto, Bayer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Robert G. Maki
Financial support: Gary K. Schwartz
Administrative support: Robert G. Maki, Michael Saulle, Gary K. Schwartz
Provision of study materials or patients: Robert G. Maki, David R. D'Adamo, Mary L. Keohan, Michael Saulle, Scott M. Schuetze, Samir D. Undevia, Michael B. Livingston, Matthew M. Cooney, Martee L. Hensley, Monica M. Mita, Chris H. Takimoto, Andrew S. Kraft, Anthony D. Elias, Bruce Brockstein, Gary K. Schwartz
Collection and assembly of data: Robert G. Maki, David R. D'Adamo, Michael Saulle, Scott M. Schuetze, Samir D. Undevia, Michael B. Livingston, Matthew M. Cooney, Martee L. Hensley, Monica M. Mita, Anthony D. Elias, Anthony D. Elias, Bruce Brockstein, Nathalie E. Blachère, Mark A. Edgar, Lawrence H. Schwartz, Li-Xuan Qin, Cristina R. Antonescu
Data analysis and interpretation: Robert G. Maki, David R. D'Adamo, Nathalie E. Blachère, Mark A. Edgar, Lawrence H. Schwartz, Li-Xuan Qin, Cristina R. Antonescu
Manuscript writing: Robert G. Maki, Gary K. Schwartz
Final approval of manuscript: Robert G. Maki, David R. D'Adamo, Mary L. Keohan, Michael Saulle, Scott M. Schuetze, Samir D. Undevia, Michael B. Livingston, Matthew M. Cooney, Martee L. Hensley, Monica M. Mita, Chris H. Takimoto, Andrew S. Kraft, Anthony D. Elias, Bruce Brockstein, Nathalie E. Blachère, Mark A. Edgar, Lawrence H. Schwartz, Li-Xuan Qin, Cristina R. Antonescu, Gary K. Schwartz
REFERENCES
- 1.Brennan MF, Singer S, O'Sullivan B, et al. Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. ed 8. Philadelphia, PA: Wolters Kluwer; 2008. pp. 1741–1794. [Google Scholar]
- 2.Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: A retrospective analysis. Int J Cancer. 2006;119:706–711. doi: 10.1002/ijc.21867. [DOI] [PubMed] [Google Scholar]
- 3.Hensley ML, Blessing JA, Degeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley ML, Blessing JA, Mannel R, et al. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 6.Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706–1712. doi: 10.1200/JCO.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of Sarcoma Alliance for Research through Collaboration study 002. J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 9.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 10.Maki RG, Awan RA, Dixon RH, et al. Differential sensitivity to imatinib of 2 patients with metastatic sarcoma arising from dermatofibrosarcoma protuberans. Int J Cancer. 2002;100:623–626. doi: 10.1002/ijc.10535. [DOI] [PubMed] [Google Scholar]
- 11.McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866–873. doi: 10.1200/JCO.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 12.Rubin BP, Schuetze SM, Eary JF, et al. Molecular targeting of platelet-derived growth factor B by imatinib mesylate in a patient with metastatic dermatofibrosarcoma protuberans. J Clin Oncol. 2002;20:3586–3591. doi: 10.1200/JCO.2002.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Chawla SP, Tolcher AW, Staddon AP, et al. Survival results with AP23573, a novel mTOR inhibitor, in patients with advanced soft tissue or bone sarcomas: Update of phase II trial. J Clin Oncol. 2007;25(suppl):563s. abstr 10076. [Google Scholar]
- 14.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 15.Okuno SH, Mahoney MR, Bailey HH, et al. A multicenter phase 2 consortium study of the mTOR inhibitor CCI-779 in advanced soft tissue sarcomas. J Clin Oncol. 2006;24:9504. [Google Scholar]
- 16.Schuetze SM, Baker LH, Maki RG. Sirolimus reduced tumor-related morbidity and resulted in biochemical and radiographic response in patients with progressive sarcoma. J Clin Oncol. 2006;24(suppl):520s. abstr 9503. [Google Scholar]
- 17.Jhanwar SC, Chen Q, Li FP, et al. Cytogenetic analysis of soft tissue sarcomas. Recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (MPNST) Cancer Genet Cytogenet. 1994;78:138–144. doi: 10.1016/0165-4608(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MR, DeClue JE, Felzmann S, et al. Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol Cell Biol. 1994;14:641–645. doi: 10.1128/mcb.14.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori S, Satoh T, Koide H, et al. Inhibition of Ras/Raf interaction by anti-oncogenic mutants of neurofibromin, the neurofibromatosis type 1 (NF1) gene product, in cell-free systems. J Biol Chem. 1995;270:28834–28838. doi: 10.1074/jbc.270.48.28834. [DOI] [PubMed] [Google Scholar]
- 20.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Teruya-Feldstein J, Bonner P, et al. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;67:7106–7112. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- 23.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosini G, Cheema HS, Seelman S, et al. Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther. 2008;7:890–896. doi: 10.1158/1535-7163.MCT-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 28.Fata F, O'Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034–2037. [PubMed] [Google Scholar]
- 29.Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–247. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Penel N, Lansiaux A, Adenis A. Angiosarcomas and taxanes. Curr Treat Options Oncol. 2007;8:428–434. doi: 10.1007/s11864-007-0042-0. [DOI] [PubMed] [Google Scholar]
- 31.Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361–366. doi: 10.1002/cncr.21140. [DOI] [PubMed] [Google Scholar]
- 32.Chugh R, Thomas D, Wathen K, et al. Imatinib mesylate in soft tissue and bone sarcomas: Interim results of a Sarcoma Alliance for Research Through Collaboration phase II trial. J Clin Oncol. 2004;22:9001. [Google Scholar]
- 33.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 34.Blay JY, El Sayadi H, Thiesse P, et al. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT) Ann Oncol. 2008;19:821–822. doi: 10.1093/annonc/mdn033. [DOI] [PubMed] [Google Scholar]
- 35.Heinrich MC, Joensuu H, Demetri GD, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. 2008;14:2717–2725. doi: 10.1158/1078-0432.CCR-07-4575. [DOI] [PubMed] [Google Scholar]
- 36.Keohan ML, Morgan JA, D'Adamo DR, et al. Continuous daily dosing of sunitinib in patients with metastatic soft tissue sarcomas other than GIST: Results of a phase II trial. J Clin Oncol. 2008;26:10533. [Google Scholar]
- 37.Sleijfer S, Papai Z, Le Cesne A, et al. Phase II study of pazopanib (GW786034) in patients with relapsed or refractory soft tissue sarcoma: EORTC 62043. J Clin Oncol. 2007;25(suppl):552s. doi: 10.1200/JCO.2008.21.3223. abstr 10031. [DOI] [PubMed] [Google Scholar]
- 38.Leong S, Gore L, Benjamin RS, et al. A phase I study of R1507, a human monoclonal antibody IGF1R antagonist given weekly in patients with advanced solid tumors. AACR-NCI-EORTC Molecular Targets Meeting; October 22-26, 2007; San Francisco, CA. abstr A78. [Google Scholar]
- 39.Olmos D, Okuno S, Schuetze SM, et al. Safety, pharmacokinetics and preliminary activity of the anti-IGF-IR antibody CP-751,871 in patients with sarcoma. J Clin Oncol. 2008;26(suppl):553s. doi: 10.1016/S1470-2045(09)70354-7. abstr 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas D, Chawla SP, Skubitz K, et al. Denosumab treatment of giant cell tumor of bone: Interim analysis of an open-label phase II study. J Clin Oncol. 2008;26(suppl):553s. abstr 10500. [Google Scholar]