Abstract
Purpose
The genomic grade index (GGI) is a 97-gene measure of histological tumor grade. High GGI is associated with decreased relapse-free survival in patients receiving either endocrine or no systemic adjuvant therapy. Herein we examined whether GGI predicts pathologic response to neoadjuvant chemotherapy in patients with HER-2–normal breast cancer.
Methods
Gene expression data (gene chips) was generated from fine-needle aspiration biopsies (n = 229) prospectively collected before neoadjuvant paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy. Pathologic response was quantified using the residual cancer burden (RCB) method. The association between the GGI and pathologic response was assessed in univariate and multivariate analyses. The performance of a response predictor combining clinical variables and GGI was evaluated under cross-validation.
Results
Eighty-five percent of grade 1 tumors had low GGI, 89% of grade 3 tumors had high GGI, and 63% of grade 2 tumors had low GGI. Among both estrogen receptor (ER)-positive and -negative cancers, high GGI score was associated with pathologic complete response (RCB-0) or minimal residual disease (RCB-1). A multivariate model combining GGI and clinical parameters had an overall accuracy of 71%, compared with 58% for the GGI alone, for prediction of pathologic response. However, high GGI score was also associated with significantly worse distant relapse-free survival in patients with ER-positive cancer (P = .005), and was not associated with survival in patients with ER-negative cancer.
Conclusion
High GGI is associated with increased sensitivity to neoadjuvant paclitaxel plus fluorouracil, adriamycin, and cyclophosphamide chemotherapy in both ER-negative and ER-positive patients, but it remains a predictor of worse survival in ER-positive patients.
INTRODUCTION
Histologic grading of breast carcinomas is an important prognostic factor.1 High tumor grade is associated with decreased overall survival,2 but it also predicts increased response to neoadjuvant chemotherapy.3 Lack of sufficient reproducibility and objectivity limit the clinical utility of histologic grade as a prognostic or predictive factor.4 Particularly, tumors of intermediate grade display a low degree of reproducibility and are of poor prognostic and predictive value. These imperfections have recently been addressed by the development of a multigene index representing a genomic correlate of histologic tumor grade.5 Comparison of gene expression profiles between breast cancers of low versus high histologic grade identified a panel of differentially expressed genes, 97 of which were combined into the genomic grade index (GGI). It has been demonstrated that a high GGI is capable of assigning breast cancers of intermediate grade into two groups, whose prognoses resemble those of either high or low histologic grade and predictive of higher risk of recurrence than a low index in both untreated and tamoxifen-treated patients.6
Neoadjuvant chemotherapy refers to administration of chemotherapy before breast cancer surgery, with the goal to reduce the extent of surgery or to enable surgery at all. Response to neoadjuvant chemotherapy is usually dichotomized as pathological complete response (pCR; ie, absence of invasive breast cancer in both the primary tumor bed and regional lymph nodes at the time of definitive surgery) and residual disease (RD). Achievement of pCR is strongly correlated with increased overall and disease-free survival.7 It has been recognized, however, that dichotomization of chemotherapy response into pCR versus RD does not capture the complete spectrum of response as seen at the time of surgery; patients with a marked reduction in tumor volume, but with a few remaining invasive cells may show significantly improved outcome compared to those with no response at all. This issue has recently been addressed through the development of a continuous measurement of the residual cancer burden (RCB), which can also be categorized into a semiquatitative, four-tiered response score. It has been demonstrated that patients with minimal residual disease (ie, RCB-I) carry an equally favorable long-term relapse-free prognosis compared to patients with pCR (RCB-0).8 High histologic tumor grade is a known predictor of increased response to neoadjuvant chemotherapy.9–12 Consequently, we hypothesized that breast cancers with high genomic grade (ie, high GGI index), would also show increased response rates to chemotherapy.
The aim of this study was to investigate whether high GGI predicts increased response to neoadjuvant chemotherapy measured by using the RCB score. As neoadjuvant or adjuvant therapy with trastuzumab has shown to be effective in patients with HER-2–overexpressing breast cancer in addition to chemotherapy,13–14 this combined regimen is currently recommended for HER-2–positive breast cancer. Given that we wanted to analyze the predictive value of the GGI with regards to chemotherapy alone, only patients with HER-2–normal breast cancer were included in this study.
METHODS
Patients
Gene expression profiles from patients with breast cancer treated with a neoadjuvant chemotherapy regimen as part of a pharmacogenomic research program15,16 were included in this study. Inclusion criteria comprised of HER-2–normal status, uniform neoadjuvant therapy with 12 cycles of weekly paclitaxel followed by four cycles of three-weekly fluorouracil, adriamycin, and cyclophosphamide and availability of pathologic response information. Fine-needle aspiration samples were obtained before initiation of chemotherapy and gene expression profiles were obtained using Affymetrix U133A Genechips (Affymetrix, Santa Clara, CA). RNA extraction, transcription, and hybridization were described previously.15,17
Pathology
Pathologic response was measured using the RCB method (www.mdanderson.org/breastcancer_RCB) and RCB scores were categorized into four groups (RCB 0, I, II, and III). RCB-0 indicates pCR defined by the absence of any invasive cancer in both the breast and lymph nodes.18 Patients with RD were categorized as having minimal residual disease (RCB-I), intermediate residual disease (RCB-II), and extensive residual disease (RCB-III). Given that the categories RCB-0 and RCB-I have previously been shown to carry an equally favorable prognosis,8 these two groups were combined and compared to patients with either RCB-II or RCB-III. Estrogen receptor (ER) status was assessed using immunohistochemistry (IHC; 6F11; Novocastra Laboratories Ltd, Newcastle, United Kingdom); HER-2 status was determined by either fluorescence in situ hybridization (FISH) or IHC (Dako North America Inc, Carpinteria, CA). The cutoff for ER positivity was ≥ 10% positive tumor cells with nuclear staining. HER-2 negativity was defined as either lack of HER-2 gene amplification by FISH or an IHC score of 0 or 1+. Tumor grade was assessed using the modified Black's nuclear grading system.19
Statistical Analysis
For each case, a GGI score was assigned using the previously published algorithm.5 GGI was used as a dichotomized value and cases were assigned to either GGI “high risk” or GGI “low risk” categories using a cutoff representing the midpoint between the mean values associated with high nuclear grade (grade 3) and low grade (grade 1) cases from this cohort as in the original publication.5 The GGI scores were also assessed as continuous variable in multivariate models and as part of the combined prediction models of GGI and clinical parameters. All clinical variables were dichotomized including age (> 50 v ≤ 50 years), ER status (ER negative v ER positive), nodal status (node negative v node positive), tumor grading (grade 3 v grade 1/2), American Joint Commission on Cancer stage (1 v 2/3). The association of GGI with clinical parameters and chemotherapy response (ie, RCB scores) was evaluated in univariate and multivariate logistic regression analyses. Multivariate logistic models that included all the clinical covariates with or without the continuous GGI were compared based on the likelihood-ratio test for nested models. The logistic regression models were then used to predict response class based on a probability threshold of .5. The predictive performance of the multivariate clinical model and of the combined model was evaluated in random three-fold cross-validation with 1,000 random splits and receiver operating characteristic (ROC) curves were also plotted. Survival analysis was performed to evaluate the effect of GGI on distant recurrence-free survival (DRFS) among patients with ER-positive and ER-negative breast cancer separately. DRFS was defined as the interval between the date of the biopsy and the first documented distant metastatic relapse. All statistical computations were performed in R version 2.5.1 (R Development Core Team; http://www.R-project.org).
RESULTS
Patient Characteristics
Gene expression profiles were available for 274 patients. Overall, 45 patients had to be excluded from the study because their gene expression results did not pass quality control or path review/RCB scorings were not available (n = 12) or they had overexpression/amplification of HER2 (n = 33). The remaining 229 patients with HER-2–normal breast cancer were included in this study. Of these patients, 132 patients had ER-positive tumors and 97 tumors were ER negative.
Table 1 summarizes the patient characteristics. Table 2 shows the association between GGI risk categories and clinical parameters. High GGI risk categories were significantly correlated with high tumor grade (P < .001) and ER-negative status (P < .001). The majority of grade 1 tumors (84.6%) were assigned to the GGI low risk category whereas the majority of grade 3 tumors (88.3%) belonged to the GGI high risk category; 62.7% of grade 2 tumors had low GGI. Grade 2 tumors were reclassified mostly as GGI low risk category (63.2%). Sixty patients (44.8%) with ER-positive breast cancer and 86 patients (89.6%) with ER-negative tumors were assigned to the GGI high-risk category.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Median age at diagnosis, years | 50 | |
| Range | 26-75 | |
| Lymph node status | ||
| Negative | 62 | 27 |
| Positive | 167 | 73 |
| Tumor size | ||
| T1 | 21 | 9 |
| T2 | 133 | 58 |
| T3 | 35 | 15 |
| T4 | 40 | 18 |
| Tumor grade | ||
| 1 | 13 | 6 |
| 2 | 94 | 41 |
| 3 | 122 | 53 |
| ER status | ||
| Positive | 133 | 58 |
| Negative | 96 | 42 |
| PR status | ||
| Positive | 103 | 45 |
| Negative | 126 | 55 |
| Response to neoadjuvant chemotherapy | ||
| pCR/RCB-0 | 43 | 19 |
| RCB-I | 26 | 11 |
| RCB-II | 101 | 44 |
| RCB-III | 59 | 26 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; pCR, pathologic complete response; RCB, residual cancer burden.
Table 2.
Association Between GGI Risk Categories and Clinical Variables
| Characteristic | GGI Risk Category |
P* | |||
|---|---|---|---|---|---|
| Low |
High |
||||
| No. | % | No. | % | ||
| Age at diagnosis, years | |||||
| ≤ 50 | 45 | 54.2 | 73 | 50.0 | .54 |
| > 50 | 38 | 45.8 | 73 | 50.0 | |
| Lymph node status | |||||
| 0 | 26 | 31.3 | 36 | 24.7 | |
| 1 | 39 | 47.0 | 67 | 45.9 | |
| 2 | 7 | 8.4 | 26 | 17.8 | |
| 3 | 11 | 13.3 | 17 | 11.6 | |
| Tumor size | .32 | ||||
| Tis | 0 | 0.0 | 2 | 1.4 | |
| T1 | 8 | 9.6 | 11 | 7.5 | |
| T2 | 54 | 65.1 | 79 | 54.1 | |
| T3 | 10 | 12.0 | 25 | 17.1 | |
| T4 | 11 | 13.3 | 29 | 19.9 | |
| Tumor grade | < .001 | ||||
| 1 | 11 | 13.3 | 2 | 1.4 | |
| 2 | 59 | 71.1 | 35 | 24.0 | |
| 3 | 13 | 15.6 | 109 | 74.7 | |
| ER status | < .001 | ||||
| Negative | 10 | 12.0 | 86 | 58.9 | |
| Positive | 73 | 88.0 | 60 | 41.1 | |
| Response to neoadjuvant chemotherapy | < .001 | ||||
| pCR‡/RCB-I§ | 10 | 11.9 | 59 | 40.4 | |
| RCB-II | 47 | 56.0 | 54 | 37.0 | |
| RCB-III | 26 | 31.0 | 33 | 22.6 | |
Abbreviations: GGI, genomic grade index; pCR, pathologic complete response; RCB, residual cancer burden.
As per χ2 test.
Association Between GGI and Response to Neoadjuvant Chemotherapy
GGI hig- risk category was associated with significantly higher response rates to neoadjuvant paclitaxel plus fluorouracil, adriamycin, and cyclophosphamide chemotherapy compared to GGI low risk category (40% v 12%; P < .001). Among patients with excellent response to chemotherapy (ie, pCR/RCB-I), as many as 85.5% were assigned to the GGI high-risk category, whereas among cases with RCB-II and RCB-III, only 53.6% and 55.9%, respectively, were designated as GGI high-risk category (Table 2). The association between the continuous GGI and RCB following stratification by ER status is illustrated in Figure 1. Increased GGI is significantly associated with increased response to neoadjuvant chemotherapy (ie, low RCB score) in both ER-positive and ER-negative breast cancers (P = .047 and P = .012, respectively). Continuous GGI is plotted against continuous RCB scores in Appendix Figure A1 for ER-positive and ER-negative breast cancers separately.
Fig 1.
Distribution of the continuous genomic grade index (GGI) within response groups defined by the residual cancer burden (RCB) for (A) estrogen receptor (ER)-positive (n = 133) and (B) ER-negative patients (n = 96). 25th and 75th percentiles are indicated by the box plot hinges and the horizontal line corresponds to the median. P values are derived from an unequal variance t-test of GGI in pathologic complete response (pCR) or RCB-I as compared to RCB-II or RCB-III groups.
GGI and Clinical Covariates As Predictors of the Likelihood of Response to Neoadjuvant Chemotherapy
In univariate analysis, ER-negative status (P < .001), low prechemotherapy nodal status (P = .042), high prechemotherapy nuclear grade (P < .001), and continuous GGI (P < .001) were significantly associated with increased response to chemotherapy (ie, pCR or RCB-I). No significant association was observed for patient age at diagnosis and prechemotherapy tumor size. In multivariate logistic regression analysis (Table 3) without inclusion of continuous GGI, ER positivity (odds ratio [OR], 0.34; 95% CI, 0.16 to 0.72; P = .004) and positive nodal status (OR, 0.35; 95% CI, 0.17 to 0.72; P = .005) remained predictive of decreased chance of response, whereas high tumor grade remained associated with increased response to neoadjuvant chemotherapy (OR, 3.33; 95% CI, 1.51 to 7.33; P = .003). When the continuous GGI was included in the multivariate model, GGI was independently associated with chemotherapy response (OR, 1.86; 95% CI, 1.15 to 3.00; P = .011). ER positivity (OR, 0.43; 95% CI, 0.20 to 0.91; P = .028) and positive nodal status (OR, 0.32; 95% CI, 0.16 to 0.68; P = .003) still remained significant predictors of decreased chemotherapy response whereas high nuclear grade became borderline insignificant (OR, 2.26; 95% CI, 0.97 to 5.24; P = .059). To evaluate whether the GGI added predictive information over and above the clinical covariates, we performed a likelihood ratio test comparing the performance of multivariate models with or without the continuous GGI. This test yielded highly significant results (P = .008; χ2 = 6.93), demonstrating that the continuous GGI is a significant independent predictor of the likelihood of response to neoadjuvant T/FAC chemotherapy.
Table 3.
Multivariate Logistic Regression Analyses for Predicting the Probability of Excellent Pathologic Response After Neoadjuvant Chemotherapy With or Without the Continuous GGI
| Variable | Analysis Without Continuous GGI |
Analysis With Continuous GGI |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age, > 50 v ≤ 50 years | 0.88 | 0.47 to 1.64 | .692 | 0.94 | 0.50 to 1.77 | .839 |
| ER status, positive v negative | 0.34 | 0.16 to 0.72 | .004 | 0.43 | 0.20 to 0.91 | .028 |
| Nodal status, positive v negative | 0.35 | 0.17 to 0.72 | .005 | 0.32 | 0.16 to 0.68 | .003 |
| Grade, 3 v 1 or 2 | 3.33 | 1.51 to 7.33 | .003 | 2.26 | 0.97 to 5.24 | .059 |
| T stage, 2 or 3 v 1 | 0.85 | 0.43 to 1.67 | .635 | 0.81 | 0.40 to 1.62 | .550 |
| Continuous GGI* | — | — | — | 1.86 | 1.15 to 3.00 | .011 |
NOTE. Likelihood ratio test for the comparison between the analysis with and without the GGI: P = .008 and χ2 = 6.93.
Abbreviations: GGI, genomic grade index; OR, odds ratio; ER, estrogen receptor.
GGI used as a continuous covariate.
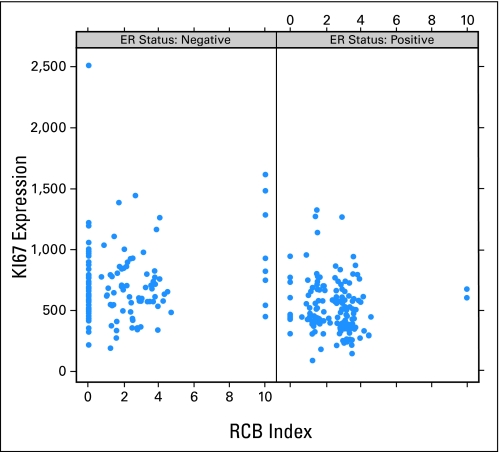
We compared the performance of GGI as predictor of neoadjuvant chemotherapy response to that of Ki67 mRNA expression (measured by probe set 212021_s_at). Appendix Figure A2 (online only) shows Ki67 plotted as a continuous variable against RCB values, Appendix Figure 3 (online only) shows Ki67 plotted as a continuous variable against GGI. In a multivariate model including age, ER status, nodal status, nuclear grade, and T stage, Ki67 was not associated with chemotherapy response (OR, 0.94; 95% CI, 0.53 to 1.67; P = .845). When adding GGI to this model, GGI remained independently significantly associated with neoadjuvant chemotherapy response (OR, 2.37; 95% CI, 1.36 to 4.16; P = .002; Appendix Table A1 online only).
Fig 3.
Kaplan-Meier curves for distant relapse-free survival after neoadjuvant paclitaxel plus fluorouracil, adriamycin, and cyclophosphamide by genomic grade (GG) risk group: (A) estrogen receptor (ER)-positive patients; (B) ER-negative patients (P values are derived from the log-rank test).
Prediction Models for Response to Neoadjuvant Chemotherapy
In order to evaluate how well the clinical covariates alone or in combination with the GGI predict the probability of pathologic response to chemotherapy (ie, pCR/RCB-I v RCB-II/RCB-III), we dichotomized the probability outputs of the logistic regression models described in the previous section using a threshold of 0.5 (probability of > 50% for pCR/RCB-I and ≤ 50% for RCB-II/RCB-III). We then evaluated the performance of these models in random three-fold cross validation with 1,000 random data splits. As a baseline comparison we used the predictions of the dichotomized GG (high or low). Comparison of the performance of these three predictors is given in Appendix Table A1 (online only). Combination of GGI with clinical variables (including histologic tumor grade) compared to clinical variables alone increased prediction accuracy (71% v 69%), sensitivity (38% v 28%), positive predictive value (53% v 48%), and negative predictive value (76% v 74%), respectively. However, none of these differences was significant at the 5% level since the 95% CIs overlapped. In contrast, the combination of GGI and clinical variables compared to GGI alone lead to significantly increased accuracy (71% v 58%) and specificity (86% v 46%), but significantly reduced sensitivity (38% v 75%) and NPV (76% v 88%). Figure 2 shows the cross-validated ROC curves for the multivariate logistic regression model using clinical characteristics only and the multivariate model in combination with GGI. The area under the ROC curve (AUC) for the model that included only the clinical covariates was lower at 0.714 (95% CI, 0.685 to 0.743), whereas that of the full model combining clinical covariates and GGI was 0.735 (95% CI, 0.702 to 0.768) but the difference was not significant at the 5% level. For comparison, the AUC for the continuous GG alone was 0.715 (95% CI, 0.638 to 0.791). Comparison of AUC results is generally less sensitive to detect modest but potentially important differences in predictor performance than some other metrics of comparison. This explains why the likelihood ratio test of the multivariate logistic regression models was significant for the combined model whereas the AUC values only indicated a numerical improvement but no significant difference.
Fig 2.
Cross-validated receiver operating characteristic (ROC) curves for the multivariate logistic regression models for chemotherapy response (pathologic complete response or residual cancer burden [RCB]-I) shown in Appendix Table A1. The area under the ROC curve (AUC) for the model that included only the clinical covariates (age at diagnosis, estrogen receptor status, nodal status, histologic grade, and tumor stage) was 0.714 (95% CI, 0.685 to 0.743), whereas that of the full model combining clinical covariates and continuous genomic grade index (GGI) was 0.735 (95% CI, 0.702 to 0.768). Box plots show for a series of false positive rates the distribution of true-positive rates estimated under cross validation.
GGI and Clinical Covariates As Predictors of DRFS
At a median follow-up time of 28 months (maximum, 68 months) GGI high-risk category was associated with significantly worse distant metastasis relapse-free survival compared to GGI low risk for patients with ER-positive breast cancer (5-year DRFS, 67.5 v 93.2 months; P = .005). Among patients with ER-negative breast cancer, no difference in survival was observed by GGI status (70.3 v 67.5 months, P = .918, Fig 3).
DISCUSSION
In this study we examined the association between neoadjuvant chemotherapy response and the GGI as a multigene surrogate of histological tumor grade. We applied the GGI to gene expression profiles from 229 patients with breast cancer treated with a taxane- and anthracycline-containing neoadjuvant chemotherapy. High GGI score predicted significantly and independently for increased probability of response to chemotherapy (OR, 1.86; 95% CI, 1.15 to 3.00; P = .011).
It has been recognized that ER-negative and ER-positive breast cancers represent two molecularly20 and clinically21 distinct entities and molecular features associated with either response or resistance to chemotherapy may differ between patients with ER-positive and ER-negative breast cancer.22 Thus, we examined if the association between GGI and response to chemotherapy remained significant if analyzed separately by ER status. The majority of patients with ER-negative cancers were assigned to the high GGI category (90%) whereas among patients with ER-positive cancers a substantial proportion (45%) had low GGI scores. Both ER-positive and ER-negative breast cancers with high GGI scores experienced significantly higher rates of excellent response to chemotherapy than cases with low GGI scores (P = .047 and P = .012, respectively). Many of the genes included in the GGI index are related to cell cycle progression, therefore it was important to examine if a single proliferation marker such as Ki67 could provide the same predictive information as the complex GGI signature. Our results indicate that mRNA levels of Ki67 alone are not as predictive of response as the GGI. This suggests that measuring multiple components of a proliferation pathway may be more robust clinical predictor than measuring a single gene alone. However, many patients with ER-negative tumors and extensive residual disease had a high mean GGI score despite their obviously poor response to neoadjuvant chemotherapy. This clearly indicates in these tumors some other forms of resistance override chemotherapy sensitivity that is generally present in high proliferative tumors.
To investigate whether the GGI contains predictive information in addition to clinical parameters we built a multivariate model of clinical factors with and without GGI. Inclusion of GGI into this multivariate model increased its accuracy (χ2 = 6.93; likelihood ratio test P = .008), therefore providing evidence that GGI is an independent predictor of response to chemotherapy.
We also examined the prognostic value of GGI in our study. Patients with ER-positive and high GGI cancer had worse DRFS, despite their higher chemotherapy sensitivity. This is the same inverse relationship between greater predicted chemotherapy sensitivity and poorer overall survival than seen with histologic grade itself. A similar relationship was observed for the OncotypeDX (Genomic Health Inc, Redwood City, CA) recurrence score in ER-positive patients; higher recurrence score indicate greater benefit from adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy but higher overall risk of relapse. These observations highlight the complexity of interpreting survival curves when a molecular marker interacts with several biologic features of a cancer that each effect survival. Survival after adjuvant systemic therapy is influenced not only by chemotherapy sensitivity but also by baseline prognosis and sensitivity to endocrine therapy (among the ER-positive tumors). The same molecular marker can interact to different degrees and with an opposing effect with several of these factors. For example, high GGI is associated with poor baseline prognosis, lower endocrine sensitivity, and greater sensitivity to chemotherapy. Because of its modest positive predictive value of around 50% to predict near complete pathologic response, the increased chemotherapy sensitivity does not fully compensate for the worse baseline prognosis and lower endocrine sensitivity among those who achieve less than excellent response. This explains why patients with low GGI (ie, better baseline prognosis and greater endocrine sensitivity among ER-positive patients) had better survival despite including a lower proportion of patients with highly chemotherapy sensitive tumors. Interestingly, among the ER-negative patients where endocrine therapy was not used, the GGI category was no longer prognostic when chemotherapy was administered. These paradoxical interactions between existing pCR predictors and survival underscore the need to develop more accurate predictors separately for ER-negative and -positive patients that do not interact with prognosis and endocrine responsiveness.
In summary, high GGI correlates with increased sensitivity to neoadjuvant paclitaxel plus fluorouracil, adriamycin, and cyclophosphamide chemotherapy in both ER-negative and ER-positive patients. GGI provides modest but significant additional information with regards to response to chemotherapy compared to clinical variables. A multivariate prediction model of pathologic response to chemotherapy including combination of the GGI and clinical parameters had an overall accuracy of 71% (95% CI, 67.7 to 74.2). The GGI continues to predict for poor prognosis among ER-positive patients even after systemic chemotherapy.
Appendix
Fig A1.
Continuous genomic grade index (GGI) plotted against residual cancer burden (RCB) scores for estrogen receptor (ER)-positive and ER-negative patients separately.
Fig A2.
Continuous Ki67 plotted against residual cancer burden (RCB) scores for estrogen receptor (ER)-positive and ER-negative patients separately.
Fig A3.
Continuous genomic grade index plotted against Ki67 for estrogen (ER)-positive and ER-negative patients separately. RCB, residual cancer burden.
Table A1.
Multivariate Logistic Regression Analyses for Predicting the Probability of Excellent Pathologic Response After Neoadjuvant Chemotherapy With or Without the Continuous GGI Including Ki67
| Variable | Analysis Without GGI |
Analysis With GGI* |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P | |
| Age, > 50 v ≤ 50 years | 0.88 | 0.47 to 1.64 | .679 | 0.88 | 0.46 to 1.68 | .709 |
| ER status, positive v negative | 0.34 | 0.16 to 0.72 | .005 | 0.39 | 0.18 to 0.84 | .016 |
| Nodal status, positive v negative | 0.35 | 0.17 to 0.72 | .005 | 0.32 | 0.15 to 0.68 | .003 |
| Grade, 3 v 1 or 2 | 3.37 | 1.52 to 7.48 | .003 | 2.22 | 0.95 to 5.16 | .065 |
| T stage, 2 or 3 v 1 | 0.85 | 0.43 to 1.67 | .633 | 0.79 | 0.39 to 1.60 | .517 |
| Ki67, log2 transformed | 0.94 | 0.53 to 1.67 | .845 | 0.54 | 0.27 to 1.08 | .082 |
| Continuous GGI | — | — | — | 2.37 | 1.36 to 4.16 | .002 |
NOTE. Likelihood ratio test for the comparison between the analysis with and without the GGI: P = .002 and χ2 = 9.97.
Abbreviations: GGI, genomic grade index; ER, estrogen receptor.
Table A2.
Predictive Performance of Clinical and Genomic Predictors of Excellent Pathologic Response After Neoadjuvant Chemotherapy
| Predictive Model | Accuracy |
Sensitivity |
Specificity |
PPV |
NPV |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Clinical factors alone* | 69.1 | 65.5 to 72.0 | 27.6 | 18.8 to 37.7 | 87.0 | 80.0 to 92.5 | 48.2 | 38.9 to 57.1 | 73.6 | 71.7 to 75.7 |
| Clinical factors + continuous GGI | 71.2 | 67.7 to 74.2 | 37.9 | 30.4 to 46.4 | 85.6 | 81.2 to 88.8 | 53.1 | 45.1 to 60.4 | 76.2 | 73.9 to 78.4 |
| GGI risk† categories alone | 57.6 | 51.0 to 64.1 | 85.5 | 75.0 to 92.8 | 45.6 | 37.7 to 53.7 | 40.4 | 32.4 to 48.8 | 88.0 | 79.0 to 94.1 |
NOTE. For the logistic regression models the 95% CIs of the performance statistic represent the cross-validated CIs (ie, they contain 95% of the cross-validated values of the statistic). For the single-marker model (GGI), the 95% CI is based on the exact binomial interval.
Abbreviations: GGI, genomic grade index; PPV, positive predictive value; NPV, negative predictive value.
Age at diagnosis, estrogen receptor status, nodal status, histologic grade, and tumor stage.
Dichotomized as GGI high risk compared to GGI low risk.
Footnotes
Supported by grants from the Deutsche Forschungsgemeinschaft (C.L.); Grant No. RO1-CA106290 from the National Cancer Institute (L.P.); the Breast Cancer Research Foundation; and the Goodwin Foundation.
Presented in poster format at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30 to June 3, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Cornelia Liedtke, Christos Hatzis, Christos Sotiriou, Lajos Pusztai
Administrative support: Gabriel N. Hortobagyi, Martine Piccart-Gebhart, Christos Sotiriou, Lajos Pusztai
Provision of study materials or patients: William Fraser Symmans, Christine Desmedt, Benjamin Haibe-Kains, Henry Kuerer, Martine Piccart-Gebhart, Lajos Pusztai
Collection and assembly of data: Cornelia Liedtke, William Fraser Symmans, Christine Desmedt, Benjamin Haibe-Kains, Vicente Valero, Henry Kuerer, Christos Sotiriou, Lajos Pusztai
Data analysis and interpretation: Cornelia Liedtke, Christos Hatzis, Christine Desmedt, Vicente Valero, Gabriel N. Hortobagyi, Christos Sotiriou, Lajos Pusztai
Manuscript writing: Cornelia Liedtke, Christos Hatzis, William Fraser Symmans, Christos Sotiriou, Lajos Pusztai
Final approval of manuscript: Cornelia Liedtke, Christos Hatzis, William Fraser Symmans, Christine Desmedt, Benjamin Haibe-Kains, Vicente Valero, Henry Kuerer, Gabriel N. Hortobagyi, Martine Piccart-Gebhart, Christos Sotiriou, Lajos Pusztai
REFERENCES
- 1.Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau ME, Pritchard KI, Chapman JA, et al. Prognostic factors affecting the natural history of node-negative breast cancer. Breast Cancer Res Trt. 2005;89:35–45. doi: 10.1007/s10549-004-1368-y. [DOI] [PubMed] [Google Scholar]
- 3.Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 4.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 6.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 7.Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol. 2001;28:389–399. doi: 10.1016/s0093-7754(01)90132-0. [DOI] [PubMed] [Google Scholar]
- 8.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Powles TJ, Allred DC, et al. Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol. 1999;17:3058–3063. doi: 10.1200/JCO.1999.17.10.3058. [DOI] [PubMed] [Google Scholar]
- 10.Ellis P, Smith I, Ashley S, et al. Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol. 1998;16:107–114. doi: 10.1200/JCO.1998.16.1.107. [DOI] [PubMed] [Google Scholar]
- 11.Vincent-Salomon A, Rousseau A, Jouve M, et al. Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline-based preoperative chemotherapy. Eur J Cancer. 2004;40:1502–1508. doi: 10.1016/j.ejca.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- 13.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: An update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 14.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess KR, Anderson K, Symmans WF, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236–4244. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 16.Pusztai L, Symmans FW, Hortobagyi GN. Development of pharmacogenomic markers to select preoperative chemotherapy for breast cancer. Breast Cancer. 2005;12:73–85. doi: 10.1007/BF02966817. [DOI] [PubMed] [Google Scholar]
- 17.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 18.Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- 19.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 20.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Hess KR, Pusztai L, Buzdar AU, et al. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 22.Tordai A, Wang J, Andre F, et al. Evaluation of biological pathways involved in chemotherapy response in breast cancer. Breast Cancer Res. 2008;10:1–9. doi: 10.1186/bcr2088. [DOI] [PMC free article] [PubMed] [Google Scholar]