Abstract
Osteoporosis and vascular calcification frequently coincide. A potential mediator of bone metabolism and vascular homeostasis is the triad cytokine system, which consists of receptor activator of nuclear factor-κB (RANK) ligand (RANKL), its receptor RANK, and the decoy receptor osteoprotegerin. Unopposed RANKL activity in osteoprotegerin-deficient mice resulted in osteoporosis and vascular calcification. We therefore analyzed the effects of RANKL inhibition by denosumab, a human monoclonal antibody against RANKL, on vascular calcium deposition following glucocorticoid exposure. Prednisolone pellets were implanted into human RANKL knock-in (huRANKL-KI) mice, which unlike wild-type mice are responsive to denosumab. No histomorphological abnormalities or differences in aortic wall thickness were detected between wild-type and huRANKL-KI mice, regardless of treatment with prednisolone, denosumab, or both. However, concurrent treatment with denosumab reduced aortic calcium deposition of prednisolone-treated huRANKL-KI mice by up to 50%, based on calcium measurement. Of note, aortic calcium deposition in huRANKL-KI mice was correlated negatively with bone mineral density at the lumbar spine (P = 0.04) and positively with urinary excretion of deoxypyridinoline, a marker of bone resorption (P = 0.01). In summary, RANKL inhibition by denosumab reduced vascular calcium deposition in glucocorticoid-induced osteoporosis in mice, which is further evidence for the link between the bone and vascular systems. Therefore, the prevention of bone loss by denosumab might also be associated with reduced vascular calcification in certain conditions.
Osteoporosis is frequently associated with vascular calcification, and there is a positive association between the severity of aortic calcification and bone loss.1 Bona fide bone tissue has been detected in areas of vascular calcification, including bone trabeculae, bone marrow, osteoblast- and osteoclast-like cells, and various bone-related extracellular matrix proteins,2,3 suggesting that vascular calcification has similarities with osteogenesis.4 However, the molecular mechanisms, the involved signaling pathways of vascular calcification, and their cross talk with bone metabolism are still poorly defined.
Receptor activator of nuclear factor (NF)-κB ligand (RANKL) is an essential cytokine for osteoclast differentiation and activation that binds to its cellular receptor, receptor activator of NF-κB (RANK). Osteoprotegerin (OPG) is a soluble decoy receptor for RANKL, which inhibits osteoclastogenesis.5 Patients on systemic glucocorticoid therapy commonly develop osteoporosis, at least in part due to an increased RANKL-to-OPG ratio, which translates into an increased number and activity of osteoclasts.5,6 Interestingly, a number of in vitro studies have also implicated glucocorticoids in the induction of vascular calcification, mainly by promoting osteogenic transdifferentiation of vascular wall-derived cells.7,8
To inhibit RANKL effects in metabolic bone diseases of humans, denosumab, a fully human monoclonal antibody with a high affinity and specificity for RANKL was developed. Denosumab inhibits the differentiation, activity, and survival of osteoclasts. Consistently, denosumab treatment of postmenopausal women with low bone mass decreased biochemical markers of bone resorption and increased bone mass at various skeletal sites.9,10 To test the effects of RANKL inhibition by denosumab in a murine model, human RANKL knock-in (huRANKL-KI) mice were developed.11 Homozygous huRANKL-KI mice exclusively express a chimeric murine/human RANKL protein that is endogenously regulated and is fully inhibited by denosumab.11 In a previous study using huRANKL-KI mice, we demonstrated that prednisolone treatment caused bone loss by enhancing bone resorption, resulting in reduced bone strength.12 Here, we used this model to test the hypothesis that denosumab would reduce prednisolone-induced vascular calcification in the aorta, and that this reduction might be related to the extent to which denosumab inhibited bone resorption.
Materials and Methods
Animal Procedures
Denosumab does not bind to murine RANKL that is produced by wild-type mice, as described by Kostenuik et al.11 Therefore, huRANKL-KI mice were generated by gene targeting.11 In brief, huRANKL-KI mice carry the human instead of the murine exon 5 in their RANKL gene. This exon encodes most of the extracellular receptor binding domain of the RANKL molecule. The chimeric murine/human RANKL protein expressed by huRANKL-KI mice has normal affinity for each of its potential binding partners (murine RANK, murine OPG, and denosumab).11
Eight-month-old mice (wild-type and huRANKL-KI, n = 6 per group) received subcutaneously implanted slow-release pellets (Innovative Research of America, Sarasota, FL) that released a calculated prednisolone dose of 2.1 mg/kg/d or placebo. HuRANKL-KI mice were subcutaneously injected with placebo (PBS, n = 6) or denosumab (n = 6) at a dose of 10 mg/kg, twice weekly for 4 weeks.13 All animal procedures were approved by the Ethical Committee of the University of Veterinary Medicine Vienna.
Histology
To analyze aortic histology, cryosections of 4 μm were generated by a Leica CM 3050S microtome (Leica Micosystems, Wetzlar, Germany) and stained with H&E (Merck, Darmstadt, Germany). One abdominal and one thoracic ring were analyzed from each animal. The thickness of each specimen was measured at 10 different sites of the vascular specimen using ImageJ program (National Institutes of Health, Bethesda, MD).
Assessment of the Aortic Calcium and Phosphate Content
Calcium content of the aorta was measured using the calcium liquicolor kit from Greiner Diagnostics (Bahlingen, Germany). In brief, 5 to 6 mm segments of the aorta were cut into cross-sections of 10 μm each. To elute calcium, the aortic rings were incubated in a 0.5 N HCl solution over night. Calcium was measured from the supernatant. After 10 minutes of incubation at room temperature, absorbance at 570 nm was measured using a microplate reader (BioRad, Munich, Germany) and compared with a standard curve generated by known calcium concentrations.
Phosphate content of the aorta was measured using a NH4 molybdate-based assay from Greiner Diagnostics. Aortic rings were incubated with a 0.5 N HCl solution over night. Supernatant was used for phosphate measurement. After incubation at room temperature for 10 minutes, the phosphate content was measured by measuring absorbance at 340 nm with a microplate reader (BioRad). The results were compared with a standard curve generated by known phosphate concentrations.
Mineral content in nmol was calculated from linear regression analysis (y = ax + b, R2 > 0.99) and was normalized to the length of the aortic specimen in mm. All samples were analyzed in a blinded manner.
Alizarin Red S Staining for Mineralized Matrix
Mineralized aortic matrix was determined using alizarin red staining as previously described.14 In brief, aortic rings were stained with 2% alizarin red S (pH 4.2; Sigma-Aldrich, Munich, Germany) for 20 minutes at room temperature. Excess dye was removed by washing with distilled water. Alizarin red S was eluted from the cell matrix with 100 mmol/L cetylpyridinium chloride (pH 5.2, Sigma-Aldrich) for 20 minutes at room temperature. Aliquots were taken and measured with a spectrophotometer at 530 nm in triplicates for each mouse.
Evaluation of Bone Mineral Density and Biochemical Bone Markers
Bone mineral density of the forth lumbar vertebrae (L4) was measured using peripheral quantitative computed tomography with a XCT Research M+ pQCT (Stratec Medizintechnik, Pforzheim, Germany).13 Urinary creatinine concentration was analyzed in a Hitachi 766 Autoanalyzer (Boehringer-Ingelheim, Ingelheim, Germany), and total deoxypyridinoline concentration was measured after acid hydrolysis by enzyme-linked immunosorbent assay (Metra Biosystems, Mountain View, CA).12
Statistical Analysis
Data are given as the mean ± SEM. Statistical analysis between wild-type and prednisolone-treated wild-type mice were performed using an unpaired t-test. P values < 0.05 were considered statistically significant. A two-way factorial analysis of variance procedure was used for data analysis of the huRANKL-KI mice for evaluating the effects of prednisolone and denosumab. The correlation analyses were performed according to Pearson with SPSS 15.0 (SPSS, Chicago, IL).
Results
Morphological Characterization of Aortic Rings
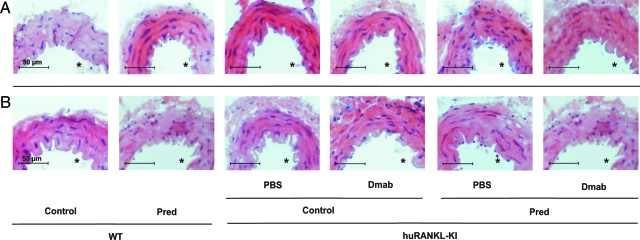
Both untreated wild-type and huRANKL-KI mice exhibited a normal aortic morphology with comparable appearances of the different vascular wall layers of the thoracic (Figure 1A) and abdominal aorta (Figure 1B). Neither treatment of wild-type and huRANKL-KI mice with prednisolone nor treatment with denosumab over a 4-week period altered the morphology of the aorta. Furthermore, computer-assisted measurement of the aortic thickness showed no differences between the groups at both sites (data not shown).
Figure 1.
Histomorphology of the aorta from wild-type (WT) and human RANKL knock-in (huRANKL-KI) mice treated with prednisolone (Pred), denosumab (Dmab), or both. Representative sections from the thoracic (A) and abdominal (B) aorta are shown. Scale bar = 50 μm. Asterisk denotes aortic lumen. Original magnification, ×40.
Calcium and Phosphate Content of the Aorta
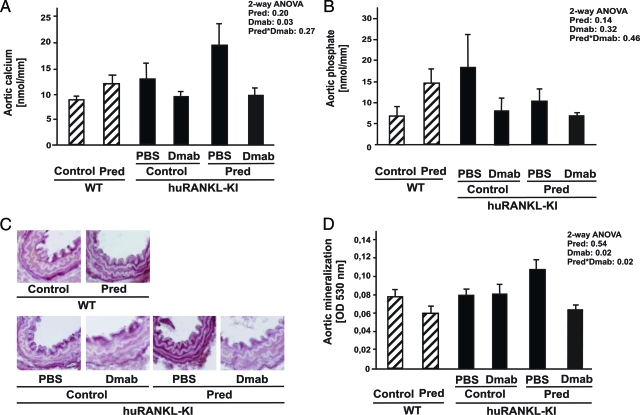
To examine the effects of denosumab, we measured calcium and phosphate content in the aorta. Prednisolone treatment of wild-type mice resulted in a trend toward higher aortic calcium (Figure 2A), as well as phosphate content (Figure 2B). Direct colorimetric calcium measurement demonstrated an increased calcium content in the aorta of prednisolone-treated huRANKL-KI mice compared with untreated huRANKL KI mice (19.3 ± 4.35 nmol/mm vs. 12.5 ± 2.94 nmol/mm, n = 6), which was lowered by concurrent treatment with denosumab (9.6 ± 1.41 nmol/mm, n = 6, P = 0.03). Of note, in huRANKL-KI mice that did not receive prednisolone, denosumab treatment also reduced calcium content in the aortic wall compared with PBS-treated animals (n = 6, P = 0.03, Figure 2A). The phosphate content within the aortic wall revealed no significant differences across the different treatment groups (Figure 2B). To confirm the effects of denosumab on prednisolone-induced vascular deposition of calcium, we assessed mineralized matrix in the thoracic vascular wall histologically (Figure 2C) and semiquantitatively (Figure 2D) by alizarin red S staining. Denosumab reduced aortic mineral deposition in prednisolone-treated huRANKL-KI compared with untreated mice by 42% (n = 6, P = 0.02, Figure 2D). Histologically, alizarin red staining was mainly located at the medial layer, and was reduced in prednisolone-treated huRANKL-KI mice by denosumab treatment (Figure 2C), whereas von Kossa staining showed no aortic mineral deposition (data not shown).
Figure 2.
Analysis of aortic calcium and phosphate content. Wild-type (WT) and human RANKL knock-in (huRANKL-KI) mice were treated with prednisolone (Pred), denosumab (Dmab), or both. A: Aortic calcium content was measured photometrically at 570 nm (n = 6). B: Aortic phosphate content was measured using a photometric assay at 340 nm (n = 6). Mineral content of aortas was evaluated histologically (C) and semiquantitatively (D) using alizarin red S (n = 6). Bars represent mean ± SEM. Insets show results of two-way analysis of variance.
Of note, denosumab treatment reduced calcium content in cardiac tissue, as compared with PBS-treated huRANKL-KI mice (n = 6, P = 0.01, data not shown). In the heart, phosphate content was not affected by the denosumab treatment (data not shown).
Correlation of Calcium Deposition with Bone Markers
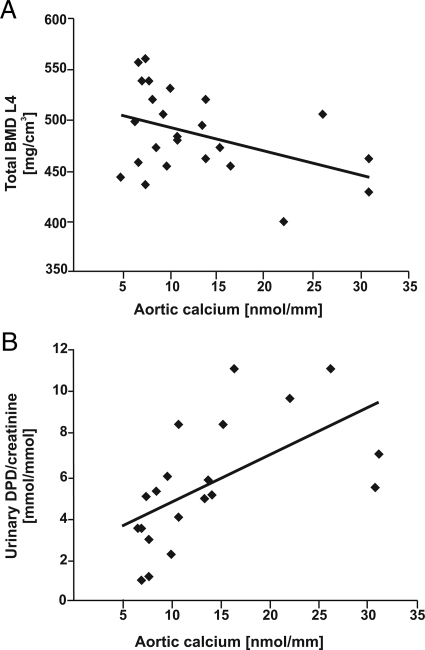
Since bone loss and arterial calcification are closely linked, we analyzed the correlation of determinants of bone metabolism with vascular calcium content in the whole cohort of huRANKL-KI mice analyzed in the current study. Total bone mineral density of the L4 vertebrae was negatively correlated with aortic calcium content (r = −0.42, P = 0.04, n = 24, Figure 3A). In addition, urinary excretion of deoxypyridinoline/creatinine, a biochemical marker of bone resorption, was positively correlated with aortic calcium deposition (r = 0.59, P = 0.01, n = 20, Figure 3B).
Figure 3.
Correlation of calcium deposition in the aortic wall with bone mineral density and biochemical markers of bone resorption in human RANKL knock-in mice (huRANKL-KI) treated with prednisolone, denosumab, or both. A: Negative correlation between aortic calcium deposition and total bone mineral density (BMD) at the fourth lumbar spine in huRANKL-KI mice (r = −0.42, P = 0.04, n = 24). B: Positive correlation between aortic calcium deposition and urinary deoxypyridinoline/creatinine excretion in huRANKL-KI mice (r = 0.59, P = 0.01, n = 20). Correlation analyses were performed according to Pearson.
Discussion
The huRANKL-KI mouse model enabled us to test the specific effects of RANKL inhibition by denosumab, a fully human monoclonal antibody against RANKL in mice.11 Here, we analyzed the effects of denosumab on the vascular system of glucocorticoid-treated huRANKL-KI mice and found that denosumab reduced calcium deposition in the aortic wall without affecting vascular architecture or histomorphology. Previously, we found that denosumab treatment prevented glucocorticoid-induced bone loss, suppressed bone resorption, and maintained bone strength.12 While systemic glucocorticoid treatment is not associated with the rapid and severe arterial calcification or ossification observed in rats treated with warfarin or supraphysiological doses of vitamin D,15 it caused substantial bone loss due to enhanced bone resorption along with calcium accumulation in the aorta. These vascular changes were subtle, accompanied by normal morphology, and may be among the earliest pathological changes in the multistep process of vascular calcification. As reported recently, these subtle changes may precede apoptosis and osteogenic transformation of vascular smooth muscle cells, although we did not assess these endpoints in our study.16 Despite the increase in osteoclastic bone resorption induced by prednisolone, we did not observe alterations in serum mineral concentrations with our experimental design,13 suggesting that the changes in aortic calcium content occurred in the absence of a disturbed systemic mineral homeostasis. Thus, the vascular changes we describe might be more typical of early clinical changes, in contrast to the rapid and gross abnormalities seen with warfarin treatment or vitamin D excess in vivo.15 Of note, normal vessel morphology does not exclude osteogenic transdifferentiation or apoptosis of vascular cells, which we did not specifically address. It is clear that a limitation of our study is its short duration. However, the major reason to limit the study to just 4 weeks was to minimize the potential appearance of antibodies against the fully human denosumab protein in these immunocompetent huRANKL KI mice.11
The huRANKL-KI mouse model allowed us to study the concurrent processes of osteoporosis and vascular calcification, and its potential reversibility via the specific inhibition of RANKL, a cytokine that has been implicated in both disorders. Selective inhibition of bone resorption with bisphosphonates was also able to inhibit vascular calcification in rats, which is further support for the hypothesis that vascular calcification is linked to bone loss.17 Our study demonstrates a positive correlation between aortic calcium deposition and urinary excretion of deoxypyridinoline, a biochemical marker of bone resorption, and a negative correlation between aortic calcium deposition and bone mineral density, suggesting that an increased efflux of mineral from bone caused by enhanced bone resorption is associated with increased vascular calcification.
Other animal models with targeted deletion of a single gene leading to a combined phenotype of osteoporosis and vascular calcification include mice deficient in fetuin-A,18 an important circulating calcification inhibitor, or mice deficient in Klotho, a regulator of phosphate homeostasis.19 In recent years, OPG deficiency has been implicated in the process of vascular calcification, and animal models suggest a protective role for OPG in the vascular system.14,20,21 OPG can also bind and inhibit the cytotoxic ligand tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).22 The differential effects of OPG as an inhibitor of RANKL versus TRAIL are unclear. Recombinant OPG was recently shown to bind to recombinant TRAIL, whereas denosumab showed no TRAIL binding.9 The current study therefore provides the first vascular calcification data obtained with a RANKL-specific inhibitor.
Many human studies have assessed systemic OPG serum levels in patients with vascular diseases and cardiovascular risk factors such as arterial hypertension or diabetes mellitus.23 In these studies, high OPG serum levels were found in patients with advanced arterial disease and were thought to represent an insufficient counterregulatory mechanism to prevent further vascular damage. The current data are consistent with other interventional studies, which suggested that RANKL, rather than RANKL inhibition, was responsible for the exacerbation of vascular disease.3,20,24
In conclusion, we demonstrate a reduction of vascular calcium deposition in glucocorticoid-induced osteoporotic mice by administering denosumab, a specific inhibitor of RANKL that does not recognize TRAIL. Whether denosumab treatment of patients with osteoporosis also confers vascular protective effects remains to be determined.
Acknowledgments
We thank Dr. Monika Skalicky (University of Veterinary Medicine, Vienna, Austria) for help with the statistical analyses, and Ms. Andrea Lohse-Fischer (Technical University, Dresden, Germany) and Claudia Bergow (University of Veterinary Medicine, Vienna, Austria) for technical assistance.
Footnotes
Address reprint requests to Lorenz C. Hofbauer, M.D., Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine III, Technical University of Dresden, Fetscherstr. 74, D-01307 Dresden, Germany. E-mail: lorenz.hofbauer@uniklinikum-dresden.de.
Supported by a seed grant from the DFG Research Center and Cluster of Excellence for Regenerative Therapy Dresden to S.H., H.M., and L.C.H., a grant from Deutsche Forschungsgemeinschaft (Ho 1875/5-2) to M.S. and L.C.H., and a grant from DFG/SFB TRR 67 to L.C.H. and U.H.
References
- Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis–from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- Kirton JP, Wilkinson FL, Canfield AE, Alexander MY. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: implications for vascular calcification. Circ Res. 2006;98:1264–1272. doi: 10.1161/01.RES.0000223056.68892.8b. [DOI] [PubMed] [Google Scholar]
- Mori K, Shioi A, Jono S, Nishizawa Y, Morii H. Dexamethasone enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2112–2118. doi: 10.1161/01.atv.19.9.2112. [DOI] [PubMed] [Google Scholar]
- McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Nguyen HQ, McCabe J, Warimgton K, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G, Scully S, Elliott R, Grisanti M, Morony S, Tan HL, Asuncion F, Li X, Ominsky MS, Stolina M, Dwyer D, Dougall WC, Hawkins N, Boyle WJ, Simonet WS, Sullivan JK. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases bone density in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2008;24:153–161. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what’s circulating? Kidney Int. 2008;73:384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Zeitz U, Schoppet M, Skalicky M, Schueler C, Stolina M, Kostenuik PJ, Erben RG. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60:1427–1437. doi: 10.1002/art.24445. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Schrader J, Niebergall U, Viereck V, Burchert A, Horsch D, Preissner KT, Schoppet M. Interleukin-4 differentially regulates osteoprotegerin expression and induces calcification in vascular smooth muscle cells. Thromb Haemost. 2006;95:708–714. [PubMed] [Google Scholar]
- Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–824. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- Nybo M, Rasmussen L. The capability of plasma Osteoprotegerin as a predictor of cardiovascular disease: A systematic literature review. Eur J Endocrinol. 2008;159:603–608. doi: 10.1530/EJE-08-0554. [DOI] [PubMed] [Google Scholar]
- Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/ − mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]