Abstract
Prostate cancers that progress during androgen-deprivation therapy often overexpress the androgen receptor (AR) and depend on AR signaling for growth. In most cases, increased AR expression occurs without gene amplification and may be due to altered transcriptional regulation. The transcription factor nuclear factor (NF)-κB, which is implicated in tumorigenesis, functions as an important downstream substrate of mitogen-activated protein kinase, phosphatidylinositol 3-kinase, AKT, and protein kinase C and plays a role in other cancer-associated signaling pathways. NF-κB is an important determinant of prostate cancer clinical biology, and therefore we investigated its role in the regulation of AR expression. We found that NF-κB expression in prostate cancer cells significantly increased AR mRNA and protein levels, AR transactivation activity, serum prostate-specific antigen levels, and cell proliferation. NF-κB inhibitors decrease AR expression levels, prostate-specific antigen secretion, and proliferation of prostate cancer cells in vitro. Furthermore, inhibitors of NF-κB demonstrated anti-tumor activity in androgen deprivation-resistant prostate cancer xenografts. In addition, levels of both NF-κB and AR were strongly correlated in human prostate cancer. Our data suggest that NF-κB can regulate AR expression in prostate cancer and that NF-κB inhibitors may have therapeutic potential.
Androgens play a critical role in normal prostate function and in the development and progression of prostate cancer.1,2,3 Prostate cancer cells are typically androgen dependent, and androgen deprivation is the standard systemic therapy for this disease. Virtually all prostate cancer patients treated with anti-androgens eventually progress, an ominous clinical state for which no consistently effective therapy exists. The mechanisms involved in the development of resistance are poorly understood. AR mutations, AR gene amplification, and activation of the AR through other signal transduction pathways have been implicated.2
The majority of tumors progressing during androgen deprivation therapy (referred to here as androgen deprivation-resistant prostate cancer or ADRPC) express higher levels of AR transcript and protein suggesting that a marked increase in AR expression is a critical event in therapy resistance.4,5,6,7 Recent studies also demonstrate that increased AR expression is both necessary and sufficient to convert prostate cancer growth from a hormone therapy-sensitive to a resistant state in xenograft models.8 Since AR mRNA levels are often increased in ADRPC without gene amplification,6,7 it is likely mediated by transcription factors and transcription regulating signal transduction pathways that are altered during progression.
Nuclear Factor (NF)-κB is a family of transcription factors composed of homo- and hetero-dimers initially identified as an enhancer binding protein for the κ immunoglobulin light chain in B lymphocytes.9 Five known mammalian subunits have been identified and include p65 (RelA), RelB, c-Rel, p50/p105, and p52/p100. NF-κB dimers are retained in the cytoplasm complexed with inhibitory (I)κB proteins. Upon cellular stimulation, IκB is phosphorylated by IκB kinases and dissociates from NF-κB subunits. As a consequence, NF-κB can translocate to the nucleus and regulate gene expression via interaction with κB enhancer elements.10
The NF-κB family of transcription factors are implicated in oncogenesis by stimulating cell proliferation, inhibiting apoptosis, and promoting metastasis and angiogenesis.11 There is growing evidence that NF-κB proteins play important roles in the development and progression of a number of human malignancies.11,12,13 Constitutive NF-κB activity is common in solid tumors and has been demonstrated in cell lines of head and neck squamous cell carcinoma,14 pancreatic adenocarcinoma,15 melanoma,16 and breast cancer.17,18 Nuclear localization of p65, which is indicative of NF-κB activation, has also been observed in tumor samples from pancreatic adenocarcinoma,15 melanoma,19 hepatocellular carcinoma,20 thyroid C-cell carcinoma,21 and breast cancer.18 Furthermore constitutive activation of NF-κB has been proposed to be involved in the progression of breast cancers to hormone independent growth.17,18,22
Several studies have examined the expression of NF-κB in human prostate cancer and its relationship to clinical features of the disease. NF-κB/p65 is overexpressed in prostatic intraepithelial neoplasia and cancer compared with benign epithelium.23 Nuclear levels of NF-κB/p65 correlate with NF-κB-dependent expression of BclII, cyclin D1, matrix metalloproteinase-9, and vascular endothelial growth factor.24 Recent work indicates that NF-κB/p65 expression is predictive of biochemical recurrence in patients with positive surgical margins after radical prostatectomy and nuclear localization of NF-κB is increased in prostate cancer lymph node metastasis25 and can be used to predict patient outcome.26 These results demonstrate that NF-κB/p65 is frequently activated in human prostate adenocarcinoma and expression may be related to progression.
NF-κB is constitutively activated in ADRPC xenografts where AR expression is also up-regulated.8,27 In addition, apigenin treatment, which inhibits the growth of androgen-responsive LNCaP human prostate carcinoma cells, results in a significant decrease in AR protein expression and down-modulation of the constitutive expression of NF-κB/p65.28 These studies suggest there is a positive correlation between the expression of NF-κB and AR in prostate cancer cell lines and xenograft models although the underlying molecular relationship is not entirely clear. Furthermore, NF-κB has been shown to regulate transcription of the AR gene in rat Sertoli cells.29 No prior study has addressed the direct transcriptional regulatory interaction between NF-κB and AR in prostate cancer. We investigated the molecular mechanisms of regulation of AR by NF-κB using cellular and animal models and demonstrated that NF-κB can directly regulate AR expression and activity and proliferation of human prostate cancer cells. Our data suggest that inhibition of NF-κB might be a novel therapeutic strategy appropriate for prostate cancers with activated NF-κB and AR signaling pathways.
Materials and Methods
Cell Culture
The human prostate cancer cell line LNCaP was obtained from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). The LNCaP/C5 and LNCaP/IκBαM cell lines were kindly provided by Edward Gelmann (Georgetown University)30 and maintained in RPMI 1640 medium supplemented with 10% FBS and 200 μg/ml of G418. The LNCaP/V3 and LNCaP/p65 cell lines were described previously31 and maintained in RPMI 1640 medium supplemented with 10% FBS and 200 μg/ml of G418.
Plasmids
The MMTV-Luc reporter and constructs encoding NF-κB p50 and p65 subunits were kindly provided by Terry Brown (The Johns Hopkins University). The pNFκB-TA-Luc plasmid, as well as the plasmids encoding IκBα and IκBαM (it contains amino acid substitutions of serines 32 and 36 to alanine) were purchased from BD Biosciences (Palo Alto, CA).
Transfection
LNCaP cells was plated at 200 × 103 cells/well in 12-well plates and transfected 2 days later in accordance with the manufacturer’s instructions using Lipofectamine Plus reagent (Invitrogen Life Technologies, Carlsbad, CA). To minimize interference from androgen, transfected cells were maintained in RPMI 1640 medium supplemented with 10% charcoal-stripped serum. Two days after transfection, cells were harvested and the luciferase activity of the reporter constructs was measured with a dual luciferase assay kit (Promega, Madison, WI). Luciferase activity was normalized to Renilla luciferase from pRL-TK plasmid used as the normalization control.
Transfection with Small Interfering RNA
ON-TARGETplus SMARTpool p65 (RelA) and p50 (NFκB1) small interfering (si)RNAs each consists of four double-stranded siRNAs commercially designed and tested by Dharmacon, Inc. (Dallas, TX). The pool of siRNAs contained the p65-specific sequences 5′-GGAUUGAGGAGAAACGUAA-3′, 5′-CCCACGAGCUUGUAGGAAA-3′, 5′-GGCUAUAACUCGCCUAGUG-3′, and 5′-CCACACAACUGAGCCCAUG-3′. The pool of siRNAs contained the p50 specific sequences 5′-GGAGACAUCCUUCCGCAAA-3′, 5′-GAUGGGAUCUGCACUGUAA-3′, 5′-GAAAUUAGGUCUGGGGAUA-3′, and 5′-GCAGGAAGGACCUCUAGAA-3′. Control (nonsilencing) siRNA (Qiagen, Germantown, MD) was used as the negative control (Cat# 1027281). LNCaP cells at 50% to 70% confluent were transfected with p65 (RelA) and p50 SMARTpool siRNAs (100 nmol/L each) or control siRNA (100 nmol/L) by LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) for 4 to 6 hours at 37°C in six-well culture plates in Opti-MEM (Invitrogen). Following transfection, cells were grown in complete RPMI 1640 medium and collected 48 hours later.
Immunohistochemistry
Multitissue blocks of formalin-fixed, paraffin-embedded tissue from 52 cases of androgen-independent metastatic prostate cancer were prepared using a tissue arrayer (Beecher Instruments, Silver Spring, MD). The blocks contained three representative 0.6-mm cores from diagnostic areas of each case. Immunohistochemical detection of p65 (SC-8008, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and AR (Clone AR441, DAKO Corporation, Carpinteria, CA) was performed with standard streptavidin-biotin-peroxidase methodology as described.32 Immunohistochemical studies were manually scored on a semiquantitative scale: negative (0), weak, (1) moderate (2), and strong (3).
Western Blot
Whole cell protein was extracted using the T-PER Tissue Protein Extraction Reagent (Cat# 78510, Pierce Biotechnology Inc., Rockford, IL). Protein concentration was determined (Bio-Rad, Hercules, CA) and Western blot analysis was performed using standard procedures33 with antibodies against human AR (Clone AR441, DAKO Corporation, Carpinteria, CA), NF-κB p65 (SC-109, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), NF-κB p50 (SC-1190, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and β-actin (C-15, Sigma, Saint Louis, MO).
Proliferation Assay
Cells were maintained in RPMI 1640 supplemented with 10% FBS. Equal numbers of LNCaP cells and LNCaP/p65 cells (100,000 cells/well) were seeded into 12-well tissue culture plates. Cells were stained by trypan blue and cell numbers were determined by direct counting on hemacytometers. For the treatment of Parthenolide, LNCaP cells (10,000 cells/well) were seeded in 96-well microtiter plates and maintained in RPMI supplemented with 10% FBS for 48 hours. Cells were treated with Parthenolide (0, 2, 5, and 10 μg/ml) for 6 hours. Cell viability and proliferation were measured using the 3-(4,5 dimethylthiazol-2-yl)−2,5- diphenyl tetrazolium bromide colorimetric assay (American Type Culture Collection, Manassas, VA) and quantified by measuring absorbance at 570 nm (Victor V7 microplate reader, Perkin Elmer, Wellesley, MA).
Real-Time Reverse Transcription-PCR
Total RNA from cultured cells or mouse tissues was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and 0.25 μg of total RNA was reverse transcribed to cDNA using the SuperScript III One-Step RT-PCR System with Platium TaqDNA Polymerase (Invitrogen Life Technologies, Carlsbad, CA). To construct the AR recombinant plasmid as standards, the primer/probe mix (Applied Biosystems, Foster City, CA) was used for reverse transcription (RT)-PCR to amplify the target gene. The PCR fragment was gel purified and inserted into the pGEM-T Easy Vector according to manufacturer’s instructions (Promega Corporation, Madison, WI). To make TATA-binding protein (TBP) recombinant plasmid, similar strategy was used and the PCR product was cloned into TOPO cloning vector pCR2.1 according to manufacturer’s instructions (Invitrogen Inc., Carlsbad, CA). Plasmid constructs were verified by DNA sequencing. Real-time quantitative RT-PCR was performed using iCycler (Bio-Rad Laboratories, Inc., Hercules, CA). Briefly, serially diluted plasmid DNA (10 ∼ 106 copies) was used to generate standard curves for absolute quantitation of the target genes, AR and TBP, in each sample. The AR PCR primer/probe mix (20×) was purchased from ABI (Hs00171172_ml, Applied Biosystems, Foster City, CA). The sequence of primers for TBP is: (Forward) 5′-CACGAACCACGGCACTGATT-3′; (Reverse) 5′-TTTTCTTGCTGCCAGTCTGGAC-3′. The sequence of the probe for TBP is 5′mGTGCACAGGAGCCAAGAGTGAAGA xp-3′(m: 6-FAM; xp: TAMRA) (Sigma, Saint Louis, MO). Each PCR reaction contained 25 μl TaqMan Universal PCR Master Mix (Part number: 4304437, Applied Biosystems, Foster City, CA), 1× primer and probe mix for AR and 0.2 μmol/L of each primer and probe for TBP, 10 μl cDNA (1:5 dilution) or 10 μl of serially diluted plasmid DNA as standards, and water to make the final volume of 50 μl. Real time PCR reactions were performed in 96-well PCR plates (Bio-Rad Laboratories, Inc., Hercules, CA). Cycling conditions were 95°C for 8 minutes, 95°C for 30 seconds (40 ×), and 60°C for 1 minute (40×).
Measurements of PSA
To measure total PSA (tPSA), we used the commercialized version of a previously reported dual-label assay (DELFIA Prostatus PSA F/T Dual Assay, Perkin Elmer Life Sciences, Turku, Finland) that measures tPSA on an equimolar basis using H117/H50 monoclonal antibodies.34 Detection limit was 0.05 ng/ml.
Chromatin Immunoprecipitation
Chromatin Immunoprecipitation (ChIP) was performed using the ChIP kit (Cat# 17-295, Upstate Biotechnology, Inc., Charlottesville, VA) according to manufacturer’s instruction. 4 μg of rabbit polyclonal antibody specific to p50 (Cat# ab7971-1, Abcam Inc., Cambridge, MA) or the same amount of normal rabbit IgG (SC-2027, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used in each reaction of ChIP. For PCR, 1 to 5 μl of DNA was used in 25 to 30 cycles of amplification. The primer sequences for the putative NF-κB binding motifs were as follows: 1F: 5′-ACAGTCTTCATCAAAGAAAG-3′; 1R: 5′-GGTATACACCTACTTTTGTAAG-3′; 2F: 5′-CTACTTACATATGGTGAGGTAT-3′; 2R: 5′-AAACCTAAACTAGTCTTCCA-3′; 34F: 5′-GCACAAGATAAAGCTACAAC-3′; 34R: 5′-ATGGAAAGAGAGAGAAAATG-3′; 56F: 5′-GGAGGCCCAGGGTCTCTACTGACAT-3′; 56R: 5′-TGCAGCCGCTTGCTTTTCCA-3′; 7F: 5′-CTTGGAAAAGCAAGCGGCTGCATA-3′; 7R: 5′-TAGATCGGGCCTCGTGGCATTG-3′; 8F: 5′-ACAATGCCACGAGGCCCGAT-3′; 8R: 5′-AAACCAACAGGGTGGAGGCGAG-3′. To demonstrate the binding specificity, two control PCRs were performed using primers that bind to the region located ∼5 kb region upstream of the AR transcription start site. The primer sequences were: 1C F:5′-CAGCCAGGGGAGAAG TTAG G-3′; 1C R: 5′-CCTCTGCTGATTTGTGGACATC-3′; 2C F: 5′-GATGAGAAAGTAGTCCATTATAAGC-3′; 2C R:5′-TGAACTGGATTGGAGATGAA-3′.
Tumor Animal Model Studies
Animal studies have been approved by the institutional review board of Memorial Sloan-Kettering Cancer Center. Athymic nude mice (NCI) aged 6 to 8 weeks old were procured and allowed to acclimatize for one week. The androgen independent prostate cancer line, CWR22Rv1 (kindly provided by Dr. Yuhong Shi, Antitumor Assessment Core, Memorial Sloan-Kettering Cancer Center) was implanted into the subcutaneous tissue of the flank. Tumors were allowed to establish for 6 days before drug treatment. The mice were treated with solvent control or parthenolide alone. Parthenolide was administered by oral gavage daily (QDx5/week) at 40 mg/kg daily. The mice were maintained in a pathogen-free environment with free access to food. Body weight and tumor volume were measured twice weekly in three dimensions using calipers. At the end of experiment, mouse blood and tissues were collected for measurement of PSA and AR levels.
Results
NF-κB Activates the AR Promoter in LNCaP Cells
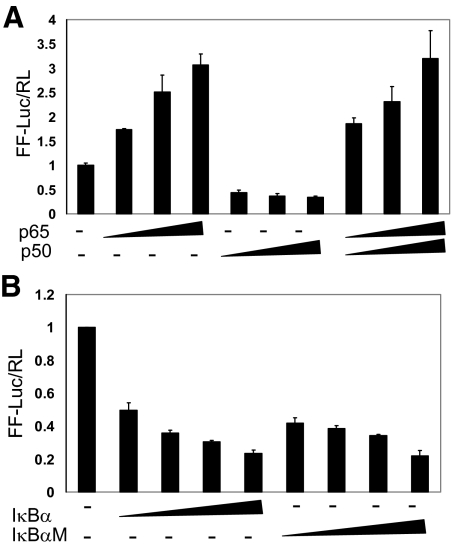
Recent studies demonstrating that the accumulation of NF-κB is associated with increased AR expression and higher binding activity in androgen-independent xenografts,8,27 and the regulation of AR transcription by NF-κB in rat Sertoli cells29 suggest NF-κB modulates AR function possibly through transcriptional regulation. In addition a query of the 5′ regulatory region of the AR gene (GenBank accession no.X78592) using the Transcription Element Search System (http://www.cbil.upenn.edu/cgi-bin/tess/tess) identified eight putative NF-κB cis-acting, consensus binding elements within 3.6 kb upstream of the transcription start site. To determine whether NF-κB plays a regulatory role in AR function, we asked whether NF-κB is sufficient to activate transcription from the AR promoter in prostate cancer cells. LNCaP cells were transiently transfected with expression vectors for the p65 and p50 subunits along with the AR promoter-reporter construct hARp-Luc. Over expression of p65, or the combination of p65 and p50, activated the AR promoter in a dose-dependent manner. Expression of p50 alone, the NF-κB subunit without a transcriptional activation domain, slightly inhibited the human AR promoter activity (Figure 1A). Furthermore, over expression of the NF-κB inhibitor, IκBα, or IκBαM (a dominant-active form of IκBα containing alanine substitutions at serines 32 and 36), inhibited the AR promoter activation in a dose-dependent manner (Figure 1B). These results indicated that NF-κB is sufficient to activate the AR promoter in prostate cancer cells and suggest that p65 could be involved in the physiological and pathophysiologic regulation of AR expression.
Figure 1.
Overexpression of NF-κB activates the AR promoter and IκBα or IκBαM inhibits the AR promoter. A: LNCaP cells were transfected with the reporter construct hARp-Luc (250 ng) with increasing amounts of human p65 (50, 100, and 250 ng), human p50 (50, 100, and 250 ng), or a combination of p50 and p65 (50, 100, and 250 ng). pRL-TK (2 ng) was included as an internal control. The promoter activity was expressed as the ratio of firefly luciferase reporter to Renilla luciferase internal control. The data shown represent at least two independent experiments. B: LNCaP cells were cotransfected with the reporter construct hARp-Luc (500 ng) and increasing amount of IκBα or IκBαM (100, 250, 500, 1000 ng) as indicated. Luciferase activity was measured 2 days after transfection and normalized to Renilla luciferase. The data shown represent at least two independent experiments.
p65 Induces Endogenous AR Expression in LNCaP Cells and Promotes Cell Proliferation
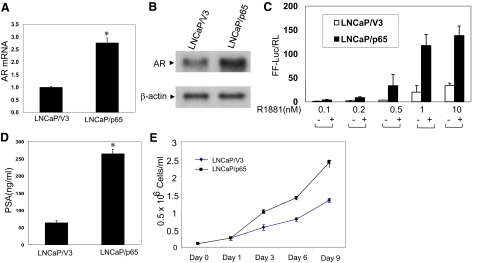
Since overexpression of p65 is sufficient to activate an AR promoter construct in LNCaP cells, we next asked if p65 could regulate endogenous AR expression in prostate cancer cells. To test this, we used LNCaP cells stably transfected with the expression vector pcDNA3 containing p65, the major activation subunit of NF-κB (LNCaP/p65), or the empty vector (LNCaP/V3). Several independent transfected cell lines were established, and protein extracts from each were subjected to Western blot analyses with anti-p65 antibody for confirmation of expression.31 The results are representative of two p65 and control clones. As expected, ectopic expression of p65 significantly enhanced NF-κB transcriptional activity compared with control LNCaP/V3 cells when transiently transfected with the pNF-κB-TA-Luc reporter (P < 0.05) (data not shown). To determine whether overexpression of p65 had an effect on AR expression, we measured AR mRNA level using real-time RT-PCR. LNCaP/p65 lines expressed a significantly higher level of AR (∼2.5-fold), as compared with the control LNCaP/V3 (P < 0.05) (Figure 2A). Consistent with the increased AR transcript level, the AR protein is also significantly increased in LNCaP/p65 cells (Figure 2B). The increased level of AR in cells overexpressing p65 was associated with enhanced transactivation activity particularly in the presence of the non-metabolizable AR ligand R1881 (Figure 2C). Importantly, the increased sensitivity to low concentrations of androgen observed in LNCaP/p65 cells indicates that activation of p65 could contribute to enhanced growth in the setting of low androgen levels during androgen-deprivation therapy. About 0.5 nmol/L of androgen is required to give a half maximal response. To determine whether the increased transactivation activity was physiologically relevant, we monitored the secretion of PSA, a well-known AR target gene. About fourfold more PSA was secreted into the cell culture media from LNCaP/p65 cells than from LNCaP/V3 cells (P < 0.05) (Figure 2D).
Figure 2.
Overexpression of p65 (RelA) enhances AR expression in LNCaP cells and promotes cell proliferation. A: AR mRNA level is significantly higher in LNCaP/p65 compared with LNCaP/V3 cells. Real-time RT-PCR was performed to examine the AR mRNA level using iCycler. The AR mRNA level was expressed relative to TBP internal housekeeping control. The level of AR mRNA in LNCaP/p65 cells was normalized to that in LNCaP/V3 cells. B: Western blot analysis for human AR performed on protein extracts derived from LNCaP/V3 and LNCap/p65. C: AR transcriptional activity. LNCaP/V3 and LNCap/p65 cells were transfected with MMTV-Luc and pRL-TK constructs and cultured in 10% charcoal-stripped FBS in the presence or absence of R1881 for 2 days. Data were normalized to Renilla luciferase. The data shown is representative of at least two independent experiments. D: Overexpression of p65 (RelA) induces endogenous PSA expression. LNCaP/V3 and LNCaP/p65 cell lines were cultured in RPMI1640 media supplemented with 10% FBS to about 70% to 80% confluence. The media were subjected to enzyme-linked immunosorbent assay to determine PSA expression. E: p65 promotes prostate cancer cell proliferation. Equal numbers of LNCaP/V3 and LNCap/p65 cells (100,000 cells/well) were seeded into 12-well tissue culture plates. Cells were stained by trypan blue (1, 3, 6, and 9 days) and cell numbers were determined. The data represent means ± SD from triplicate sets of three independent experiments.
The androgen response pathway is a critical growth regulatory mechanism in prostate cancer and knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression.35 It follows that regulation of AR activity by NF-κB may have a similar effect. To further explore the biological significance of p65 induced AR expression in prostate cancer cells, we determined the effects on cell growth. Overexpression of p65 resulted in an increased viable cell number, detected as early as day 3 that reflected a near doubling of growth (Figure 2E). Of interest, a similar, but more modest, increase in cell growth was detected in androgen-depleted media (data not shown). Cumulatively, these data confirm that p65 can induce expression of the endogenous AR in human prostate cancer cells, and that induction is associated with increased expression of downstream AR targets and enhanced growth and/or survival of prostate cancer cells.
NF-κB Inhibition Decreases Endogenous AR Expression, AR Transactivation Activity, and NF-κB Binding in the AR Promoter in Prostate Cancer Cells
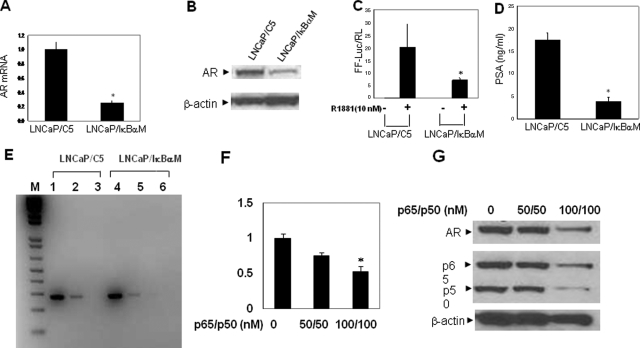
NF-κB activity is specifically regulated by its inhibitor IκBα. To further validate the direct role of the NF-κB signaling pathway on AR expression in prostate cancer, we compared LNCaP cells stably transfected with an expression construct for IκBαM, a constitutively active mutant NF-κB inhibitor (LNCaP/IκBαM) and LNCaP/C5, which harbors the empty expression vector. The results are representative of two IκBα clones (LNCaP/IκBα and LNCaP/IκBαM) and two control clones. The results of LNCaP/IκBα (data not shown) is very similar to those of LNCaP/IκBαM. The inhibitory effect of IκBαM on transcriptional activity of endogenous NF-κB was shown by reporter gene assay. As expected, ectopic expression of IκBαM blocked NF-κB activity in LNCaP cells as shown by transient transfection with the NF-κB responsive reporter construct, pNF-κBTA-Luc (Data not shown). Overexpression of IκBαM also significantly inhibited expression of the endogenous AR gene at the mRNA level as determined by real-time RT-PCR (Figure 3A) and decreased AR protein (Figure 3B). To examine the effects of IκBαM on AR transactivation function, we transiently transfected LNCaP/IκBαM and LNCaP/C5 cells with the AR dependent MMTV-Luc reporter construct. Consistent with the reduced AR level in LNCaP/IκBαM, the transactivation activity of AR in these cells is significantly decreased even in the presence of R1881 (Figure 3C). Reduced AR activity by inhibition of NF-κB is also demonstrated by decreased PSA secretion (Figure 3D), a well validated endogenous transcriptional target of AR. To further demonstrate the inhibition of AR expression by IκBαM at the transcriptional level, we performed ChIP assay and observed decreased binding of p50 (the DNA binding subunit of NF-κB p65/p50 heterodimer) to one of the NF-κB binding sites, site 5/6 (compare lane 5 v.s. lane 2, Figure 3E). Based on the inhibitory effects of IκBαM on AR expression, we next examined if specific inhibition of p65 and p50 expression could affect AR expression in LNCaP cells. Co-transfection of p65 and p50 siRNA (100 nmol/L each) significantly inhibited AR mRNA (P < 0.05) and protein expression (Figure 3F and 3G). Decreased expression AR by specific inhibition of NF-κB (overexpression of IκBαM or co-transfection of p65 and p50 siRNAs) provided strong evidence that this transcription factor plays an important regulatory role in this process.
Figure 3.
Overexpression of IκBαM inhibits AR expression, AR transactivation activity, and NF-κB binding in the AR promoter in LNCaP cells. NF-κB inhibition by p65 and p50 siRNAs also inhibits endogenous AR expression. AR mRNA (A), AR protein level (B), AR transcriptional activity (C), and PSA secretion (D) were determined in LNCaP/p65 (LNCaP cells stably transfected with p65) and LNCaP/V3 (which harbors empty expression vector) as outlined in Materials and Methods. The data represent means ± SD from triplicate sets of three independent experiments. *P < 0.05. E: Overexpression of IκBαM decreased the binding of p50 to the AR promoter region. M: 1kb plus DNA marker; Lanes 1 and 4, input control; Lanes 2 and 5, immunoprecipitation with p50 specific antibody; Lanes 3 and 6, immunoprecipitation with control antibody (normal rabbit IgG). F: Co-transfection of p65 and p50 siRNAs (100 nmol/L each) significantly inhibited AR mRNA expression in LNCaP cells. G: Co-transfection of p65 and p50 siRNAs (100 nmol/L each) inhibited AR protein expression in LNCaP cells.
NF-κB p65 Is Coordinately Expressed with the AR in Human ADRPC
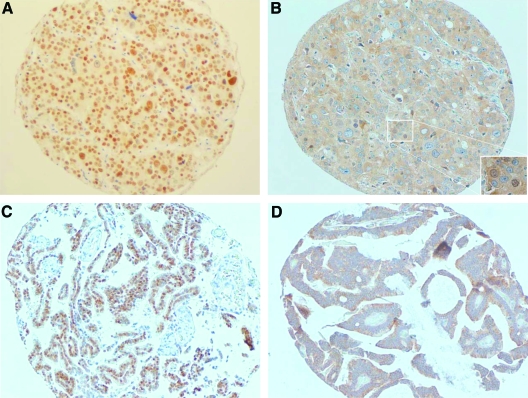
Recent studies indicate that NF-κB is overexpressed in prostate cancer and its expression correlates with disease progression during androgen deprivation therapy.23,24,36,37 Our experiments have further shown that NF-κB can regulate expression of the AR in prostate cancer cell lines even in the setting of androgen depletion. To determine the relationship between NF-κB and AR expression in human prostate cancers progressing during androgen deprivation therapy, we examined protein expression of AR and p65 by immunohistochemistry in 52 metastatic ADRPC (Figure 4a–d). In 22 out of 52 (42%) cases examined, p65 and AR were coordinately expressed at moderate to high levels (spearman rho P = 0.03). Twelve out of 51 (23%) are weakly stained or negative for both p65 and AR, and in 18 cases (35%), the immunostaining was moderate for one protein and weak/negative for the other protein (Table 1). To a certain extent, heterogeneity was observed for both AR and p65 staining in human ADRPC. Besides the overall correlation between AR and p65 expression, prostate cancer cells with moderate or strong staining of p65 also have corresponding moderate or strong nuclear AR staining (Figure 4). In addition, when AR is strongly stained in the nucleus, we observed p65 nuclear staining (Figure 4, A and B). Although circumstantial, the common coincident expression of p65 and AR in human ADRPC is consistent with a causal relationship as suggested by the in vitro experimental data presented here.
Figure 4.
Correlation between AR and p65 immunostaining in human ADRPC. Moderate AR labeling (2+) is shown in (A), this corresponds to moderate p65 labeling (B). Nuclear p65 is shown in the inset. Strong AR labeling (3+) in (C) corresponds to strong p65 immunostaining (D).
Table 1.
Expression of NF-κB/p65 and Nuclear AR in Metastatic Prostate Cancer Specimens
| AR nuclear immunoreactivity | NF-κB/p65 immunoreactivity
|
||||
|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | Total | |
| Negative | 6 | 2 | 1 | 0 | 9 |
| Weak | 2 | 2 | 2 | 0 | 6 |
| Moderate | 3 | 8 | 6 | 5 | 22 |
| Strong | 1 | 3 | 6 | 5 | 15 |
| Total | 12 | 15 | 15 | 10 | 52 |
The expression of NF-κB/p65 and nuclear AR was evaluated on staining intensity of the tissues as: negative (0), weak (1), moderate (2) and strong (3). In 22 out of 52 (42%) cases examined, p65 and AR were coordinately expressed at moderate to high levels (spearman rho P = 0.03). Twelve out of 51 (23%) are weakly stained or negative for both p65 and AR, and in 18 cases (35%), the immunostaining was moderate for one protein and weak/negative for the other protein.
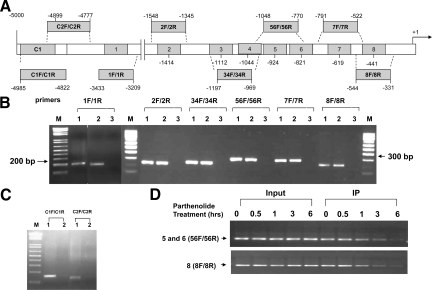
NF-κB Binds the 5′ Regulatory Region of the AR Gene, which Can Be Inhibited by Parthenolide
We identified eight putative NF-κB binding sites within the −3.6 kb 5′ regulatory region of the AR gene (Figure 5A). To determine whether transcriptional regulation of AR by NF-κB is mediated by these binding motifs, we conducted ChIP experiments to define the occupancy of NF-κB on the AR promoter. Soluble chromatin was prepared from LNCaP/p65 cells after formaldehyde treatment and specific antibody against p50 (the subunit that contains the DNA-binding domain) was used to immunoprecipitate p50-bound genomic DNA fragments. Four micrograms of rabbit polyclonal antibody specific to p50 or the same amount of normal rabbit IgG were used in each reaction of ChIP. To demonstrate the binding specificity, two control PCRs were performed to amplify fragments within the −5 kb region that do not contain a putative NF-κB sites (Figure 5C). NF-κB recruitment to the AR promoter was detected in all of the putative NF-κB binding sites assayed by PCR (Figure 5B) and not detected in the negative controls, indicating that the binding of p50 to these sites was specific. As the average length of the genomic DNA fragments produced in these experiments is ∼1000 bp and some NF-κB binding sites are very close to each other, we cannot distinguish with certainty whether some or all of the NF-κB binding motifs are bound. Direct binding of the NF-κB transcription factor to the AR promoter is consistent with a role in transcriptional regulation of this gene.
Figure 5.
NF-κB interacts with the 5′ regulatory region of the AR gene, which can be inhibited by Parthenolide treatment. A: Schematic diagram of the AR gene regulatory region. Solid boxes depict putative NF-κB binding sites, and 1F/1R, 2F/2R, 34F/34R, 56F/56R, 7F/7R, 8F/8R, C1F/C1R, C2F/C2R are primer pairs used for amplifying corresponding DNA fragments. Numbers are the positions upstream of the AR gene transcription start site. B: ChIP assays of NF-κB occupancy on the AR gene regulatory region. LNCaP/p65 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. Soluble chromatin was prepared from formaldehyde-cross-linked and sonicated cell cultures. Specific antibody against p50, as well as control serum (normal rabbit IgG), was used to immunoprecipitate protein-bound DNA fragments. These fragments were amplified by PCR using primers described in (A). M, 1-kb plus marker (for 1F/1R) or 100-bp markers (for the other sites); 1, input; 2, p50 IP; 3, rabbit serum IP. C: Two control PCRs were performed using primers that bind to the region located ∼5 kb region upstream of the AR transcription start site. M: 1kb plus marker; 1, input; 2, p50 IP. D: Inhibition of AR expression by parthenolide is associated with alterations in the recruitment of NF-κB to the 5′ regulatory region of the AR gene.
Parthenolide, the active ingredient of the herbal remedy feverfew (Tanacetum parthenium), is able to inhibit NF-κB DNA binding and prostate cancer cell growth in vitro.23 This effect is at least in part due to inhibition of NF-κB signaling38,39 and we therefore expected that this drug is able to interfere with NF-κB transcriptional regulation of the AR gene. ChIP assays were performed after treatment with parthenolide and NF-κB occupancy of the NF-κB binding sites was markedly decreased (Figure 5D). The inhibition of NF-κB occupancy correlated with the inhibition of AR transcription by parthenolide, which was maximal at 6 hours (data not shown). These data add support to the hypothesis that NF-κB regulates AR. Furthermore, parthenolide could be used to test this hypothesis in vitro and in vivo.
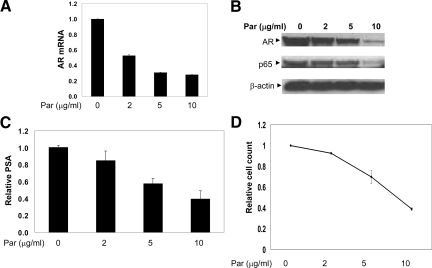
Pathenolide, an NF-κB Inhibitor, Decreases AR Expression, PSA Secretion and Cell Proliferation in Prostate Cancer Cells
To test whether parthenolide is able to regulate AR expression, we analyzed LNCaP cells treated with parthenolide (Figure 6, A and B). There was a dose-dependent decrease in AR mRNA and protein after 6 hours of parthenolide treatment, a previously determined time of maximal inhibition. Interestingly, parthenolide also decreased levels of p65 while having no observable effects on β-actin (Figure 6B). The inhibition of AR expression correlated with reduction of PSA secretion (Figure 6C) and resulted in a dose-dependent decrease in viable prostate cancer cells (Figure 6D). These experiments were also performed in LAPC4, a prostate cancer cell line with a wild-type AR, with similar results (data not shown).
Figure 6.
Parthenolide, a NF-κB inhibitor, inhibits AR expression, PSA secretion and cell proliferation in prostate cancer cells. A: Inhibition on AR transcription in LNCaP cells treated with parthenolide. AR mRNA levels measured using real-time RT-PCR. LNCaP cells (50% to 60% confluent) were treated with parthenolide (0, 2, 5, and 10 μg/ml) for 6 hours in RPMI 1640 supplemented with 10% FBS. The data represent at least two independent experiments. B: Parthenolide significantly inhibited AR and p65 expression in LNCaP cells in a dose-dependent manner. LNCaP cells (50% to 60% confluent) were treated with parthenolide (0, 2, 5, and 10 μg/ml) for 6 hours in RPMI 1640 supplemented with 10% FBS before cell harvest and Western blot analysis. C: PSA secretion was significantly inhibited in LNCaP cells treated with parthenolide using enzyme-linked immunosorbent assay. D: Proliferation assay demonstrating dose-dependent inhibition of prostate cancer cell proliferation with the NF-κB inhibitor, parthenolide. The readings are normalized to controls. Data represent at least two independent experiments.
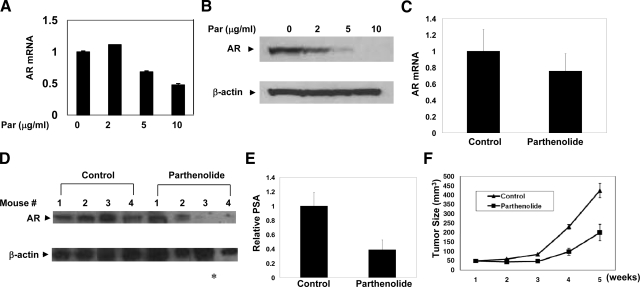
Parthenolide Treatment Inhibits AR Expression, PSA Secretion, and Tumor Growth in a Xenograft Model of ADRPC
Our data have demonstrated that parthenolide inhibits AR expression and prostate cancer cell proliferation in vitro using the androgen-dependent LNCaP cell line (Figure 6). To test whether parthenolide treatment is effective in the setting of ADRPC, we used the CWR22Rv1 prostate cancer xenograft model.40,41,42 We first tested whether treatment of parthenolide inhibited the AR expression in vitro. There was a dose-dependent decrease in AR mRNA and protein after 6 hours of parthenolide treatment in CWR22Rv1 cells (Figure 7A and 7B). We then examined the effect of parthenolide treatment in vivo. CWR22Rv1 tumors were allowed to establish for 6 days before drug treatment. The mice were treated with solvent control or parthenolide (QDx5/week at 40 mg/kg daily). Body weight and tumor volume were measured twice weekly and mouse blood and tumor tissues were collected for measurement of PSA and AR levels. Parthenolide inhibited AR expression significantly (both mRNA and protein) in most of the CWR22Rv1 tumors (Figure 7, C and D). In concordance with the inhibition in AR expression, mice treated with parthenolide produced significantly lower levels of circulating PSA in the serum (Figure 7E). Furthermore, parthenolide administration resulted in a consistent inhibition of tumor growth (Figure 7F). The in vivo effects recapitulate those seen in vitro and suggest that NF-κB regulation of AR activity is physiologically relevant in the setting of ADRPC and potentially provides a therapeutic strategy.
Figure 7.
Parthenolide treatment inhibits AR expression, PSA secretion and tumor growth in ADRPC. A: Inhibition on AR transcription in CWR22Rv1 cells treated with parthenolide. AR mRNA levels measured using real-time RT-PCR. CWR22Rv1 cells (50% to 60% confluent) were treated with parthenolide (0, 2, 5, and 10 μg/ml) for 6 hours in RPMI 1640 supplemented with 10% FBS. B: Parthenolide significantly inhibited AR expression in CWR22Rv1 cells in a dose-dependent manner. C: AR mRNA in CWR22Rv1 cells in mice treated with parthenolide. CWR22Rv1 cells were implanted in female mice (four mice per group) 6 days before drug treatment. The mice were treated with solvent control or parthenolide alone (QDx5/week at 40 mg/kg daily). At the end of day 35, mice were sacrificed and tissues were collected for RNA extraction and RT-PCR. TBP was used as internal control. Expression of AR was normalized to TBP in each sample. D: AR protein level in mice tumors treated with parthenolide. Mice tissues were collected for protein extraction and Western blot analysis. E: Parthenolide inhibits serum PSA level in mouse xenograft model. Mouse blood was collected for measurement of PSA levels using enzyme-linked immunosorbent assay method. F: Parthenolide inhibits tumor growth in tumor model. Body weight and tumor volume were measured twice weekly in three dimensions using calipers. The data represent means ± SD from all of the mice included in this experiment. *P < 0.05.
Discussion
There is growing evidence that the expression of NF-κB/p65 correlates with prostate cancer progression and the development of androgen deprivation resistance.24,26,27 It is tempting to speculate that constitutive activation of NF-κB, which is known to induce potent anti-apoptotic effects, may contribute to prostate cancer cell survival following androgen withdrawal. Increased AR expression is also a critical event in the development of prostate cancer resistance to androgen deprivation therapy. However, the signaling pathways and transcription factors involved in the regulation of AR expression are not clear. Our findings provide mechanistic support for a role of NF-κB in regulation of the AR in human prostate cancer that could contribute to prostate cancer growth and ADRPC. NF-κB directly bound to the AR promoter and enhancer, and regulated AR expression and function. Inhibitors of NF-κB decreased AR expression and prostate cancer cell growth in vitro and in vivo. Furthermore, NF-κB/p65 levels correlated with that of AR in human ADRPC. These findings suggest that NF-κB is part of a signaling network regulating AR expression in prostate cancer and targeting NF-κB may be an effective therapeutic strategy.
Several signal transduction pathways that regulate NF-κΒ activity have emerged as important in prostate cancer.43 In particular the phosphatidylinositol 3-kinase/AKT pathway has been implicated in protection from apoptosis in response to growth factors, cytokines, c-myc overexpression, and matrix detachment. AKT can also lead to activation of NF-κΒ by phosphorylation of the IκB kinase, a positive regulator of NF-κB.44 Given that loss of heterozygosity and decreased expression of phosphatase and tensin homolog, an inhibitor of the phosphatidylinositol 3-kinase/AKT pathway, is frequent in prostate cancer,45,46 this pathway may be a primary mechanism for activation of NF-κB. We have previously detected decreased phosphatase and tensin homolog expression specifically in ADRPC, that correlates with increased expression of AR and implicates this pathway in development of resistance.6 Furthermore, DIM, 3,3′-diindolylmethane, a compound that significantly inhibits AKT activation and NF-κΒ DNA binding activity, decreases AR phosphorylation and the expression of the AR and PSA.47 This suggests that an integrated signaling network including phosphatidylinositol 3-kinase, AKT, NF-κΒ, and AR may be of primary importance in prostate cancer and especially in the development of ADRPC.
NF-κB is a member of a family of transcription factors that are involved in stimulating cell proliferation, inhibiting apoptosis, and promoting metastasis and angiogenesis.11 More than 180 NF-κB target genes have been proposed and many of them, such as BclII, cyclin D1, matrix metalloproteinase-9, and vascular endothelial growth factor, regulate critical steps of cancer progression. Therefore, it is important to point out that AR may not be the only downstream target of NF-κΒ that contributes to prostate cancer growth or that is inhibited by NF-κΒ antagonists. Further study is needed to elucidate the genes and signaling pathways that are critical mechanisms of NF-κΒ activity that participate in prostate cancer development and progression.
Recently, proteosome inhibitors and IκBα kinase inhibitors have been used to target the NF-κB pathway in both preclinical models and clinical trials involving patients with prostate cancer and other solid tumors.48,49,50 Our studies provide improved understanding of the relationship of NF-κΒ to AR regulation and the potential for these interactions as therapeutic targets. The data suggest that NF-κB inhibition is a promising strategy for the treatment of prostate cancer, especially for ADRPC with activation of AKT, NF-κΒ, and AR. However, further preclinical and clinical studies are needed to fully appreciate the therapeutic index and ultimate value of NF-κΒ inhibitors in this disease.
Acknowledgments
We thank Edward Gelmann for LNCaP/IkBa, LNCaP/IkBaM and control cell lines, Charles L.Sawyers for LAPC4 cell line, Terry Brown for MMTV-Luc reporter and NF-κB p50 and p65 expression vectors. We thank Hui-Kuan Lin and Xiaodi Ren for advice and technical assistance.
Footnotes
Address reprint requests to Liying Zhang, M.D., Ph.D., Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 36, New York, NY 10065. E-mail: zhangl2@mskcc.org.
Supported by National Institutes of Health/NCI (CA84999) to W.L.G. and DOD postdoctoral fellowship award (W81XWH-05-1-0107) to L.Z.
This paper is dedicated to the memory of Dr. William L. Gerald who provided strong leadership and support in this project.
References
- Culig Z, Hobisch A, Hittmair A, Peterziel H, Cato AC, Bartsch G, Klocker H. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Montgomery JS, Price DK, Figg WD. The androgen receptor gene and its influence on the development and progression of prostate cancer. J Pathol. 2001;195:138–146. doi: 10.1002/1096-9896(200109)195:2<138::AID-PATH961>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Edwards J, Krishna NS, Mukherjee R, Watters AD, Underwood MA, Bartlett JM. Amplification of the androgen receptor may not explain the development of androgen-independent prostate cancer. BJU Int. 2001;88:633–637. doi: 10.1046/j.1464-410x.2001.02350.x. [DOI] [PubMed] [Google Scholar]
- Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci. 2000;25:434–440. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N, Van Waes C. Constitutive activation of transcription factors NF-(kappa)B. AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26:119–129. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- Shattuck-Brandt RL, Richmond A. Enhanced degradation of I-kappaB alpha contributes to endogenous activation of NF-kappaB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, McCauley LK, Keller ET, Pienta KJ. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97:739–747. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- Ludwig L, Kessler H, Wagner M, Hoang-Vu C, Dralle H, Adler G, Bohm BO, Schmid RM. Nuclear factor-kappaB is constitutively active in C-cell carcinoma and required for RET-induced transformation. Cancer Res. 2001;61:4526–4535. [PubMed] [Google Scholar]
- Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3, Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail HA, Lessard L, Mes-Masson AM, Saad F. Expression of NF-kappaB in prostate cancer lymph node metastases. Prostate. 2004;58:308–313. doi: 10.1002/pros.10335. [DOI] [PubMed] [Google Scholar]
- Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91:417–420. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22:2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B. Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- Zhang L, Charron M, Wright WW, Chatterjee B, Song CS, Roy AK, Brown TR. Nuclear factor-kappaB activates transcription of the androgen receptor gene in Sertoli cells isolated from testes of adult rats. Endocrinology. 2004;145:781–789. doi: 10.1210/en.2003-0987. [DOI] [PubMed] [Google Scholar]
- Kimura K, Gelmann EP. Propapoptotic effects of NF-kappaB in LNCaP prostate cancer cells lead to serine protease activation. Cell Death Differ. 2002;9:972–980. doi: 10.1038/sj.cdd.4401049. [DOI] [PubMed] [Google Scholar]
- Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res. 2003;63:7106–7112. [PubMed] [Google Scholar]
- Lee SB, Kolquist KA, Nichols K, Englert C, Maheswaran S, Ladanyi M, Gerald WL, Haber DA. The EWS-WT1 translocation product induces PDGFA in desmoplastic small round-cell tumour. Nat Genet. 1997;17:309–313. doi: 10.1038/ng1197-309. [DOI] [PubMed] [Google Scholar]
- Sambrook JE, Russell DW. Molecular cloning: A laboratory manual, 3rd. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, N.Y.; 2001:A9.28. [Google Scholar]
- Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–1120. [PubMed] [Google Scholar]
- Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- Ross JS, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Stringer B. Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res. 2004;10:2466–2472. doi: 10.1158/1078-0432.ccr-0543-3. [DOI] [PubMed] [Google Scholar]
- Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–3046. [PubMed] [Google Scholar]
- Sirotnak FM, She Y, Lee F, Chen J, Scher HI. Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res. 2002;8:3870–3876. [PubMed] [Google Scholar]
- Wainstein MA, He F, Robinson D, Kung HJ, Schwartz S, Giaconia JM, Edgehouse NL, Pretlow TP, Bodner DR, Kursh ED, Resnick MI, Seftel A, Pretlow TG. CWR22: androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 1994;54:6049–6052. [PubMed] [Google Scholar]
- Graff JR. Emerging targets in the AKT pathway for treatment of androgen-independent prostatic adenocarcinoma. Expert Opin Ther Targets. 2002;6:103–113. doi: 10.1517/14728222.6.1.103. [DOI] [PubMed] [Google Scholar]
- Gustin JA, Maehama T, Dixon JE, Donner DB. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor kappa B activity. J Biol Chem. 2001;276:27740–27744. doi: 10.1074/jbc.M102559200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res. 1998;4:811–815. [PubMed] [Google Scholar]
- Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- Adams J. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol Med. 2002;8:S49–S54. doi: 10.1016/s1471-4914(02)02315-8. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Ross JS. The proteasome: a new target for novel drug therapies. Am J Clin Pathol. 2001;116:637–646. doi: 10.1309/44HW-5YCJ-FLLP-3R56. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer, Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]