Abstract
Oxidative stresses are believed to play an important role in the induction of both cell adhesion molecules and pro-inflammatory cytokines, a key event in a variety of inflammatory processes. The enzyme heme oxygenase-1 (HO-1) functions as an antioxidant and serves to protect against tissue injury. In this study, we report that HO-1 was induced in cultured human tracheal smooth muscle cells after either treatment with a potent inducer of HO-1 activity, cobalt protoporphyrin IX, or infection with a recombinant adenovirus that carries the human HO-1 gene. Overexpression of HO-1 protected against tumor necrosis factor (TNF)-α-mediated airway inflammation via the down-regulation of oxidative stress, adhesion molecules, and interleukin-6 in both cultured human tracheal smooth muscle cells and the airways of mice. In addition, HO-1 overexpression inhibited TNF-α-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression, adherence of THP-1 cells, generation of interleukin-6, p47phox translocation, and nuclear factor-κB activation. HO-1 overexpression also attenuated TNF-α-induced oxidative stress, which was abrogated in the presence of both the HO-1 inhibitor, zinc protoporphyrin IX, as well as a carbon monoxide scavenger. In addition, HO-1 overexpression reduced the formation of a TNFR1/c-Src/p47phox complex. These results suggest that HO-1 functions as a suppressor of TNF-α signaling, not only by inhibiting the expression of adhesion molecules and generation of interleukin-6, but also by diminishing intracellular reactive oxygen species production and nuclear factor-κB activation in both cultured human tracheal smooth muscle cells and the airways of mice.
Airway smooth muscle is considered as an end-response effector, regulating regional differences in ventilation by contracting in response to various pro-inflammatory mediators and exogenous substances released under homeostatic or pathological conditions, such as asthma.1 When airway cells and tissues are exposed to oxidative stress elicited by inflammatory reactions, elevated levels of reactive oxygen species (ROS) can trigger a variety of deleterious effects within the airways.
The multifunctional cytokine tumor necrosis factor (TNF)-α is capable of activating multiple signal transduction pathways and downstream processes in mammalian cells, such as human tracheal smooth muscle cells (HTSMCs).2 TNF-α has been shown to increase airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility.3 Cellular adhesion molecules are key players in initiating inflammatory responses of the respiratory system.4 Certain cellular adhesion molecules, such as vascular cellular adhesion molecule (VCAM)-1 and intracellular adhesion molecule (ICAM)-1, are expressed on smooth muscle cells where they contribute to leukocyte recruitment. The cellular adhesion molecule-induced accumulation of activated leukocytes and macrophages inevitably leads to oxidative stress associated with tissue damage. Several studies have indicated that the expression of VCAM-1 and ICAM-1 are mediated by the activation of c-Src, phosphatidylinositol 3-kinase /Akt, mitogen-activated protein kinases, or nuclear factor (NF)-κB.2,5,6,7,8 In addition, ROS also have been shown to mediate NF−κB activation and the expression of VCAM-1 and ICAM-1.8 TNF-α may stimulate ROS production by several sources, such as mitochondria, but recent studies have strongly suggested that a major source of ROS is a phagocyte-type NADPH oxidase.9 Activated NADPH oxidase is a multimeric protein complex consisting of at least three cytosolic subunits of p47phox, p67phox, and p40phox. The p47phox regulatory subunit plays a critical role in acute activation of NADPH oxidase; phosphorylation of p47phox is thought to relieve inhibitory intracellular interactions and permit the binding of p47phox to p22phox, thereby increasing oxidase activation.9
Heme oxygenase (HO)-1 is the key enzyme responsible for the degradation of heme to carbon monoxide (CO), free iron, and biliverdin-IXα.10 In mammals, biliverdin-IXα is further converted to bilirubin-IXα, an endogenous radical scavenger, with recently recognized anti-inflammatory properties.10 However, the release of free iron is rapidly sequestered into the iron storage protein ferritin, leading to additional antioxidant and anti-apoptotic effects.10 CO exerts several biological functions, including anti-apoptotic and anti-inflammatory properties.11 HO-1 expression is up-regulated in the airways of patients with asthma.12 Airway smooth muscle contractility, which can be increased through ROS formation, is inhibited by HO-1 induction.13 Moreover, the 5′-untranslated region of the human HO-1 gene contains many stress-activated response elements, such as antioxidant response elements. NF-E2-related factor 2 (Nrf2) is an important transcription factor that regulates expression of antioxidant defense genes through binding to antioxidant response elements in the promoter region, such as HO-1.14
HO-1 has been reported to play a critical role in protection against oxidative tissue injury following ischemia and inflammation,15 however, the mechanism is not clear. Therefore, we determined the role of HO-1 in TNF-α-mediated adhesion molecules expression and airway inflammation in vitro and in vivo. Although a role of HO-1 as an antioxidant has been reported in many studies, most of these antioxidant properties were associated with the HO-1 product bilirubin. Here, we first show that overexpression of HO-1 in HTSMCs or in the airways of mice is strongly associated with resistance to airway inflammation induced by TNF-α. Our findings also indicated that HO-1-attenuated the activation of p47phox and NF-κB provide a new insight into the mechanisms to explain HO-1 antioxidant and anti-inflammation properties.
Materials and Methods
Materials
4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP1) and diphenylene iodonium chloride (DPI) were from Biomol (Plymouth Meeting, PA). Metafectene transfection reagent was from Biontex (Munich, Germany). Anti-TNF receptor 1 (TNFR1), Anti-HO-1, anti-Nrf2, anti-ICAM-1, anti-VCAM-1, anti-c-Src, anti-p47phox, and anti-p65 Abs were from Santa Cruz (Santa Cruz, CA). Anti-TNFR1 neutralizing Ab was from R&D System (Minneapolis, MN). Anti-phospho-Src, anti-phospho-p65, and anti-phospho-IκBα Abs were from Cell Signaling (Danver, MA). 2′,7′-bis-(z-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethylester (BCECF/AM), dihydroethidium (DHE), and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) were from Molecular Probes (Eugene, OR). Cobalt protoporphyrin IX (CoPP IX), zinc protoporphyrin IX (ZnPP IX), and N-acetyl-l-cysteine (NAC) were from Sigma (St. Louis, MO). Apocynin was purchased from ChromaDex (Santa Ana, CA).
Cell Culture
HTSMCs were purchased from ScienCell Research Lab (San Diego, CA) and grown as previously described.16 Experiments were performed with cells from passages 4 to 7.
Animal Care and Experimental Procedures
Male imprinting control region mice aged 6 to 8 weeks were purchased from the National Laboratory Animal Centre (Taipei, Taiwan) and handled according to the guidelines of Animal Care Committee of Chang Gung University and NIH Guides for the Care and Use of Laboratory Animals. ICR mice were anesthetized with ethyl ether and placed individually on a board in a near vertical position and the tongues were withdrawn with a lined forceps. TNF-α (0.75 mg/kg body weight) was placed posterior in the throat and aspirated into lungs. Control mice were administrated sterile 0.1% bovine serum albumin. Mice regained consciousness after 15 minutes. Mice were given one dose of CoPP IX or ZnPP IX (1.637 μg/kg body weight) for 16 hours or 1 hour, respectively, before TNF-α treatment, and sacrificed after 24 hours. To examine the cellular expression and localization of the ICAM-1 and VCAM-1 proteins, immunohistochemical staining was performed on the first and second serial sections of the airways, which were deparaffinized, rehydrated, and washed with PBS. Non-specific binding was blocked by preincubation with PBS containing 5 mg/ml of bovine serum albumin for 1 hour at room temperature. The sections were incubated with an anti-ICAM-1 or anti-VCAM-1 Ab at 37°C for 1 hour and then with an anti-goat or anti-rabbit horseradish peroxidase Ab at room temperature for 1 hour. Bound Abs were detected by incubation in 0.5 mg/ml of 3,3′-diaminobenzidine/0.01% hydrogen peroxide in 0.1 M/L Tris-HCl buffer, as chromogen (Vector Lab). The third section was incubated with an anti-α-smooth muscle actin Ab for the positive localization and identification of smooth muscle cells, and then incubated with a fluorescein isothiocyanate-conjugated goat anti-mouse secondary Ab and observed by a fluorescence microscope.
Isolation of Bronchoalveolar Lavage Fluid
Mice were injected with TNF-α at a dose of 0.75 mg/kg and sacrificed 24 hours later. Bronchoalveolar lavage (BAL) fluid was performed through a tracheal cannula using 1 ml aliquots of ice-cold PBS medium. BAL fluid was centrifuged at 500 × g at 4°C, and cell pellets were washed and re-suspended in PBS. Leukocyte count was determined by a hemocytometer.
Transient Transfection with Small Interfering RNA
SMARTpool RNA duplexes corresponding to human HO-1, p47phox, Nrf2, and scrambled #2 small interfering (si)RNA were from Dharmacon Research Inc (Lafayette, CO). Transient transfection of siRNAs was performed using Metafectene transfection reagent. siRNA (100 nmol/L) was formulated with Metafectene transfection reagent according to the manufacturer’s instruction.
Preparation of Recombinant Adenovirus
A recombinant adenovirus containing human HO-1 (Adv-HO-1) was kindly provided by Dr. L. Y. Chau (Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan). Recombinant adenovirus was generated by homologous recombination and amplified in 293 cells. Large scales of viral vectors were purified by CsCl ultracentrifugation and stored in 10 mmol/L Tris-HCl (pH 7.4), 1 mmol/L MgCl2, and 10% (v/v) glycerol at −80°C until used for experiments. Virus titers were determined by a plaque assay on a 293 cell monolayer.
Western Blot Analysis
After the incubation, cells were washed, scraped, collected, and centrifuged at 45,000 × g at 4°C for 1 hour to yield the whole cell extract, as previously described.16 Samples were denatured, subjected to SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane. Membranes were incubated with an anti-ICAM-1, anti-VCAM-1, or anti-HO-1 Ab for 24 hours, and then incubated with an anti-rabbit, anti-mouse, or anti-goat horseradish peroxidase Ab for 1 hour. The immunoreactive bands detected by enhanced chemiluminescence reagents were developed by Hyperfilm-ECL.
Reverse Transcription-PCR Analysis
Total RNA was isolated from HTSMCs with Trizol according to the protocol of the manufacturer. The cDNA obtained from 0.5 μg total RNA was used as a template for PCR amplification as previously described.17 The primers used were as follows: 5′-GGAACCTTGCAGCTTACAGTGACAGAGCTCCC-3′ (sense) and 5′-CAAGT- CTACATATCACCCAAG-3′ (anti-sense) for VCAM-1; 5′-CAAGGGGAGGTC- ACCCGCGAGGTG-3′ (sense) and 5′-TGCAGTGCCCATTATGACTG-3′ (anti-sense) for ICAM-1.
Measurement of ICAM-1 and VCAM-1 Luciferase Activity
The human ICAM-1 (pIC-339/0)/firefly luciferase was kindly provided by Dr. P. T. van der Saag (Hubrecht Laboratory, Utrecht, The Netherlands). For construction of the VCAM-1-luc plasmid, human VCAM-1 promoter, a region spanning −1716 to −119 bp (kindly provided by Dr. W.C. Aird, Department of Molecular Medicine, Beth Israel Deaconess Medical Center, Boston, MA) was cloned into pGL3-basic vector (Promega, Madison, WI). ICAM-1-luc or VCAM-1-luc activity was determined as previously described using a luciferase assay system (Promega, Madison, WI).17
Adhesion Assay
HTSMCs were grown to confluence in 6-well plates, incubated with TNF-α for 24 hours, and then adhesion assays were performed. Briefly, THP-1 cells (human acute monocytic leukemia cell line) were labeled with a fluorescent dye, 10 μmol/L BCECF/AM, at 37°C for 1 hour in RPMI-1640 medium (Gibco BRL, Grand Island, NY) and subsequently washed by centrifugation. Confluent HTSMCs in 6-well plates were incubated with THP-1 cells (2 × 106 cells/ml) at 37°C for 1 hour. Non-adherent THP-1 cells were removed and plates were gently washed twice with PBS. The numbers of adherent THP-1 cells were determined by counting four fields per ×200 high-power field well using a fluorescence microscope (Zeiss, Axiovert 200M). Experiments were performed in triplicate and repeated at least three times.
Measurement of Interleukin-6 Generation
Interleukin (IL)-6 released into the medium of HTSMCs cultures was detected using an enzyme-linked immunosorbent assay kit (R&D System, Minneapolis, MN) according to the manufacturer’s instructions.
Measurement of Intracellular ROS Accumulation
The intracellular H2O2 levels were determined by measuring fluorescence of dichlorofluorscein diacetate (DCFH-DA), and the O2•− levels were determined by measuring the level of DHE. The fluorescence for dichlorofluorscein (DCF) and DHE staining was detected at 495/529 and 518/605 nm, respectively, using a fluorescence microscope (Zeiss, Axiovert 200M). For the purpose of these experiments, HTSMCs were washed with warm HBSS and incubated in HBSS or cell medium containing 10 μmol/L DCFH-DA or DHE at 37°C for 45 minutes. Subsequently, HBSS or medium containing DCFH-DA or DHE was removed and replaced with fresh medium. HTSMCs were then incubated with various concentrations of TNF-α. Cells were washed twice with PBS and detached with trypsin/EDTA, and the fluorescence intensity of the cells was analyzed using a FACScan flow cytometer (BD Biosciences, San Jose, CA) at 518 nm excitation and 605 nm emission for DHE and at 495 nm excitation and 529 nm emission for DCF.
Determination of NADPH Oxidase Activity by Chemiluminescence assay
After incubation, cells were gently scraped and centrifuged at 400 × g for 10 minutes at 4°C. The cell pellet was resuspended with 35 μl/per well of ice-cold RPMI-1640 medium, and the cell suspension was kept on ice. To a final 200 μl volume of pre-warmed (37°C) RPMI-1640 medium containing either NADPH (1 μmol/L) or lucigenin (20 μmol/L), 5 μl of cell suspension (0.2 × 105 cells) were added to initiate the reaction followed by immediate measurement of chemiluminescence in an Appliskan luminometer (Thermo) in out-of-coincidence mode. Appropriate blanks and controls were established, and chemiluminescence was recorded. Neither NADPH nor NADH enhanced the background chemiluminescence of lucigenin alone (30 to 40 counts per min). Chemiluminescence was continuously measured for 12 minutes, and the activity of NADPH oxidase was expressed as counts per million cells.
Isolation of Cell Fractions
Cells were harvested, sonicated for 10 seconds at output 4 with a sonicator (Ultrasonics Inc. NY), and centrifuged at 8000 rpm for 15 minutes at 4°C. The pellet was collected as the nuclear fraction. The supernatant was centrifuged at 14,000 rpm at 4°C for 60 minutes to yield the pellet (membrane fraction) and the supernatant (cytosolic fraction).
Co-Immunoprecipitation Assay
Cell lysates containing 1 mg of protein were incubated with 2 μg of an anti-TNFR1, anti-c-Src, or anti-p47phox Ab at 4°C for 24 hours, and then 10 μl of 50% protein A-agarose beads was added and mixed for 24 hours at 4°C. The immunoprecipitates were collected and washed three times with a lysis buffer without Triton X-100; 5× Laemmli buffer was added and subjected to electrophoresis on SDS-polyacrylamide gel electrophoresis, and then blotted using an anti-TNFR1, anti-c-Src, anti-p47phox, or anti-phospho-tyrosine Ab.
Immunofluorescence Staining
Growth-arrested HTSMCs were incubated with TNF-α or CoPP IX for the indicated time intervals. After washing twice with ice-cold PBS, cells were fixed, permeabilized, and stained using an anti-p65 or anti-Nrf2 Ab as previously described.18 The images were observed using a fluorescence microscope (Zeiss, Axiovert 200M).
HO-1 Activity Assay
The method used for the determination of HO-1 activity via bilirubin formation follows the protocol published by Motterlini and co-workers.19 HO-1 activity was measured as picomoles of bilirubin formed per milligram of HTSMCs protein per hour.
Analysis of Data
Concentration-effect curves were fitted and EC50 values were estimated using the GraphPad Prism Program (GraphPad, San Diego, CA). Data were expressed as the mean ± SEM and analyzed by one-way analysis of variance followed with Tukey’s posthoc test. P < 0.05 was considered significant.
Results
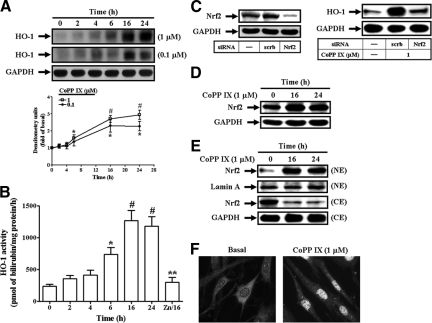
CoPP IX Induces HO-1 Expression and Activity through Nrf2 in HTSMCs
It has been established that HO-1 could be up-regulated by cobalt protoporphyrin (CoPP).20 Moreover, as expected, CoPP IX also induced HO-1 protein expression (Figure 1A) and HO-1 activity (Figure 1B) in a time-dependent manner, with a maximal response within 16 to 24 hours in HTSMCs. ZnPP IX, an inhibitor of HO-1 activity,21 blocked CoPP IX-induced HO-1 activity (Figure 1B). To further determine the role of Nrf2 in CoPP IX-mediated HO-1 induction, cells were transfected with Nrf2 siRNA. As shown in Figure 1C, transfection with Nrf2 siRNA down-regulated the expression of Nrf2 protein and attenuated HO-1 expression induced by CoPP IX. Moreover, CoPP IX also directly enhanced Nrf2 protein expression in HTSMCs (Figure 1D). Translocation of Nrf2 from the cytosol into the nucleus was seen in CoPP IX-treated HTSMCs, determined by Western blot (Figure 1E). To further ensure this result, nuclear translocation of Nrf2 was confirmed by using an immunofluorescence staining (Figure 1F). Altogether, these results indicate that CoPP IX-induced HO-1 expression was mediated through Nrf2 in HTSMCs.
Figure 1.
CoPP IX induces Nrf2-dependent HO-1 expression and activity in HTSMCs. A: Cells were incubated with CoPP IX for the indicated time intervals and HO-1 protein expression was determined by Western blot. B: Cells were incubated with 1 μmol/L CoPP IX in the absence or presence of 1 μmol/L ZnPP IX for the indicated time intervals and HO-1 activity was determined. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01 as compared with the basal level. **P < 0.05 as compared with the cells exposed to CoPP IX alone for 16 hours. C: Cells were transfected with siRNA of scramble or Nrf2, and then treated with CoPP IX for 16 hours. The expression of Nrf2 or HO-1 was examined. D, E: Cells were incubated with CoPP IX for 16 hours or 24 hours, and then (D) the expression of Nrf2 was examined or (E) cytosolic (CE) and nuclear (NE) extracts were prepared and subjected to Western blot using an anti-Nrf2 Ab. Lamin A and GAPDH were used as marker proteins for nuclear and cytosolic extracts, respectively. F: Cells were treated with CoPP IX for 16 hours. Cells were fixed, and then labeled using an anti-Nrf2 Ab and fluorescein isothiocyanate-conjugated secondary Ab. Individual cells were imaged.
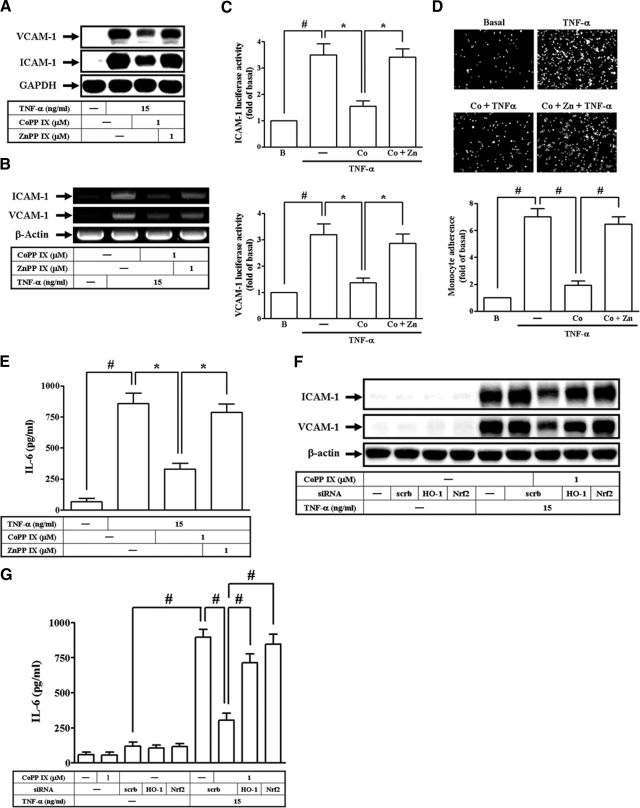
CoPP IX Suppresses TNF-α-Induced Airway Inflammatory Response via HO-1-Dependent Pathway
In our study, TNF-α has been shown to stimulate the expression of various “pro-asthmatic” mediators, including IL-6 as well as adhesion molecules in vitro and in vivo (see Supplemental Figure S1 at http://ajp.amjpathol.org). We next investigated whether CoPP IX modulated TNF-α-induced adhesion molecules expression and IL-6 production in HTSMCs. Pretreatment of HTSMCs with CoPP IX significantly abrogated TNF-α-induced ICAM-1 and VCAM-1 protein levels, mRNA expression, promoter activity, THP-1 cell adherence, and IL-6 production (Figure 2, A–E). Moreover, these observed inhibitory effects by CoPP IX were abrogated in the presence of ZnPP IX (Figure 2, A–E). To further investigate whether these inhibitory effects of CoPP IX on adhesion molecule expression and IL-6 production were mediated through an Nrf2-dependent HO-1 expression, cells were pretreated with CoPP IX, or transfected with either HO-1 siRNA or Nrf2 siRNA, and then incubated with TNF-α for 24 hours. As shown in Figure 2, F and G, the observed inhibition of ICAM-1 and VCAM-1 protein levels and IL-6 generation by CoPP IX was abrogated by transfection with either HO-1 siRNA or Nrf2 siRNA.
Figure 2.
Overexpression of Nrf2-dependent HO-1 inhibits VCAM-1 and ICAM-1 expression in HTSMCs. Cells were pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX, and then treated with TNF-α for (A) 24 hours or (B) 5 hours. The protein levels and mRNA expression of VCAM-1 and ICAM-1 were determined. C: Cells were transiently transfected with an ICAM-1- or VCAM-1-luc reporter gene, pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX, and then treated with TNF-α for 4 hours. The luciferase activity of ICAM-1 or VCAM-1 was measured. D, E: HTSMCs were pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX, and then treated with TNF-α for 24 hours. The (D) THP-1 cells adherence and (E) IL-6 generation were measured. F, G: Cells were pretreated with CoPP IX for 16 hours, transfected with siRNA of HO-1, Nrf2, or scrambled, and then incubated with TNF-α for 24 hours. The (F) levels of VCAM-1 and ICAM-1 expression and (G) IL-6 generation were measured. Data are expressed as mean ± SEM of three independent experiments. Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01.
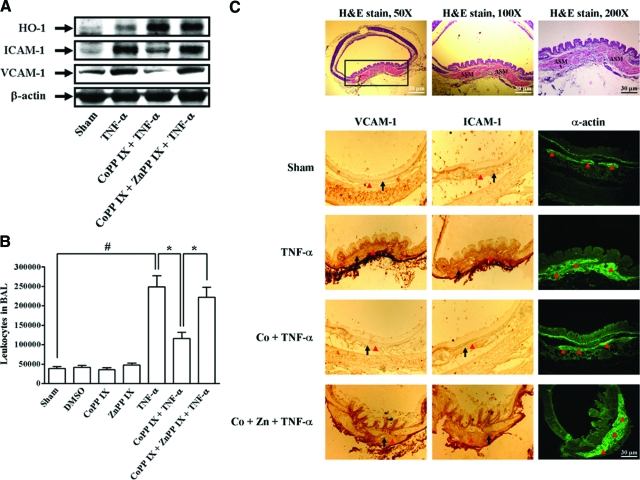
To confirm these results, in an in vivo study, mice were intratracheally administered with CoPP IX for 16 hours, and then followed with TNF-α for 24 hours. BAL fluid was acquired and airway tissues were homogenized to extract proteins. As shown in Figure 3, A and B, administration with CoPP IX significantly inhibited TNF-α-induced ICAM-1 and VCAM-1 expression and leukocyte count in BAL fluid. Moreover, immunohistochemical staining with Abs against VCAM-1 and ICAM-1 or anti-α-actin Ab (positive staining for smooth muscle cells) was performed on serial sections. In TNF-α-treated mice, ASM strongly expressed VCAM-1 or ICAM-1 (Figure 3C). In CoPP IX-pretreated mice, CoPP IX markedly reduced the expression of VCAM-1 and ICAM-1. The observed suppression of TNF-α-mediated ICAM-1 and VCAM-1 expression and leukocyte count in BAL fluid by CoPP IX was abrogated in the presence of ZnPP IX (Figure 3A-C). These data demonstrated that overexpression of HO-1 suppressed TNF-α-induced ICAM-1 and VCAM-1 expression in vitro and in vivo, which was dependent on Nrf2 activation.
Figure 3.
Overexpression of HO-1 inhibits VCAM-1 and ICAM-1 expression in vivo. Mice were intratracheally injected with CoPP IX for 16 hours in the presence or absence of ZnPP IX, and then injected with TNF-α for 24 hours. A: Airway tissues were homogenized to extract protein. The levels of ICAM-1 and VCAM-1 expression were determined. B: BAL fluid was acquired and leukocyte count was determined by a hemocytometer. Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01. C: Immunohistochemical staining for VCAM-1, ICAM-1, or α-actin in serial sections of the airways from bovine serum albumin-treated mice (Sham), TNF-α-injected mice (TNF-α), CoPP IX-pretreated mice (CoPP IX + TNF-α), and CoPP IX-pretreated in the presence of ZnPP IX mice (CoPP IX + ZnPP IX + TNF-α). The airway smooth muscle (ASM) is indicated by an arrowhead. The arrow indicates smooth muscle cells overlapping with VCAM-1 and ICAM-1 expression. H&E stain for the airway from untreated mice was observed (top).
TNF-α-Induced Adhesion Molecules Expression and ROS Generation Are Mediated by the Formation of a TNFR1/c-Src/p47phox Complex
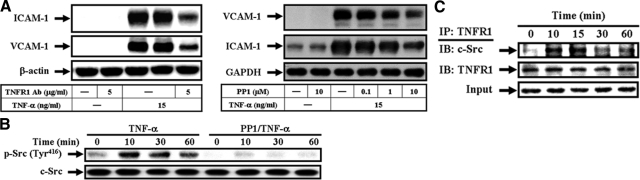
ROS produced by cytokines contribute to the intracellular signaling cascades associated with inflammatory responses.9 NADPH oxidase is an important source for the production of ROS under various pathological conditions.9 To determine whether ROS generated by NADPH oxidase involved in TNF-α-induced responses, pretreatment of HTSMCs with a ROS scavenger (NAC) and NADPH oxidase inhibitors (DPI and apocynin, APO), or transfection with p47phox siRNA significantly abrogated TNF-α-induced ICAM-1 and VCAM-1 protein levels, mRNA expression, promoter activity, or THP-1 cell adherence (see Supplemental Figure S2 at http://ajp.amjpathol.org). In addition, TNF-α induced a significant increase in superoxide and H2O2 levels, which were inhibited by pretreatment with NAC, DPI, or APO (see Supplemental Figure S2 at http://ajp.amjpathol.org). TNF-α also induced a significant increase in NADPH oxidase activity and translocation of p47phox from the cytosol to the membrane (see Supplemental Figure S2 at http://ajp.amjpathol.org). These data demonstrated the involvement of NADPH oxidase in supporting ROS generation induced by TNF-α in HTSMCs.
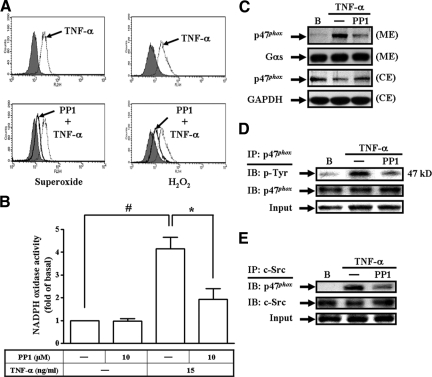
Most of TNF-α actions are elicited through TNFR1.22 The Src family kinases have been shown to regulate ICAM-1 and VCAM-1 expression, NADPH oxidase activation, and ROS generation.5,6,7,23 Therefore, we investigated whether TNFR1 and c-Src involved in adhesion molecules expression, p47phox translocation, and ROS production. Pretreatment with an anti-TNFR1 neutralizing Ab or a c-Src kinase inhibitor (PP1) attenuated TNF-α-induced ICAM-1 and VCAM-1 protein levels (Figure 4A). TNF-α-induced ICAM-1 and VCAM-1 mRNA expression, promoter activity, and THP-1 cell adherence were also inhibited by pretreatment with PP1 (see Supplemental Figure S3 at http://ajp.amjpathol.org). Moreover, the major phosphorylation site of c-Src at the Tyr416 residue results in c-Src autophosphorylation.24 Thus, we further examined c-Src phosphorylation at Tyr 416 stimulated by TNF-α in HTSMCs using an anti-phospho-c-Src Ab at Tyr416 In our study, TNF-α stimulated a time-dependent Tyr416 phosphorylation of c-Src, with a maximal response within 10 to 30 minutes, which was attenuated by pretreatment with PP1 during the period of observation (Figure 4B). To further investigate the physical association between TNFR1 and c-Src in TNF-α-induced responses, cells were treated with TNF-α for the indicated time intervals and the cell lysates were subjected to immunoprecipitation using an anti-TNFR1 Ab. The immunoprecipitates were analyzed by Western blot using an anti-c-Src Ab. The protein levels of c-Src were time-dependently increased in TNFR1-immunoprecipitated complexes (Figure 4C).
Figure 4.
TNF-α-enhanced ICAM-1 and VCAM-1 expression are mediated by the formation of a TNFR1/c-Src complex. A: Cells were pretreated with an anti-TNFR1 neutralizing Ab or PP1, and then incubated with TNF-α for 24 hours. The levels of VCAM-1 and ICAM-1 expression were determined. B: Cells were pretreated with or without PP1 (10 μmol/L), and then stimulated with TNF-α for the indicated time intervals. The cell lysates were subjected to Western blot using an anti-phospho-c-Src (Tyr 416) Ab. C: Cells were treated with TNF-α for the indicated time intervals and the cell lysates were subjected to immunoprecipitation using an anti-TNFR1 Ab, and then the immunoprecipitates were analyzed by Western blot using an anti-c-Src or anti-TNFR1 Ab.
We next investigated the role of c-Src in the activation of NADPH oxidase induced by TNF-α. As illustrated in Figure 5, A–C, pretreatment with PP1 attenuated TNF-α-induced ROS generation, NADPH oxidase activity, and p47phox translocation. Src has been shown to mediate tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of ROS in lung endothelial cells.23 Thus, we also investigated whether TNF-α stimulated tyrosine phosphorylation of p47phox and the involvement of c-Src in tyrosine phosphorylation of p47phox. Cells were pretreated with PP1 and then stimulated with TNF-α for 10 minutes. The cell lysates were subjected to immunoprecipitation with an anti-p47phox Ab and analyzed by Western blot using an anti-phosphotyrosine Ab. The results revealed that TNF-α-induced tyrosine phosphorylation of p47phox was inhibited by pretreatment with PP1 (Figure 5D). We further investigated the physical association between c-Src and p47phox in TNF-α-induced NADPH oxidase activation. As shown in Figure 5E, cells were pretreated without or with PP1, and then stimulated with TNF-α for 10 minutes. The cell lysates were subjected to immunoprecipitation using an anti-c-Src Ab, and then analyzed by Western blot using an anti-p47phox Ab. The protein level of p47phox was increased in a c-Src-immunoprecipitated complex. Pretreatment with PP1 decreased the association of c-Src/p47phox. These results demonstrated that TNF-α induced adhesion molecules expression and NADPH oxidase activation via the formation of a TNFR1/c-Src/p47phox complex.
Figure 5.
c-Src is involved in TNF-α-mediated NADPH oxidase activation. A: DHE and DCF fluorescence intensities after stimulation with TNF-α for 1 hour in the presence or absence of PP1 (10 μmol/L) were measured by a flow cytometer. B, C: Cells were pretreated with PP1 for 1 hour, and then treated with TNF-α for 1 hour. B: NADPH oxidase activity was measured. Data are expressed as mean ± SEM of three independent experiments. Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01. C: The membrane and cytosolic extracts were prepared and subjected to Western blot using an anti-p47phox Ab. D: Cells were pretreated without or with PP1 for 1 hour, and then stimulated with TNF-α for 10 minutes. Total cell lysates were subjected to immunoprecipitation with anti-p47phox Ab and the immunoprecipitates were analyzed by Western blot using an anti-phosphotyrosine Ab. E: Cells were pretreated without or with PP1, and then stimulated with TNF-α for 10 minutes. The cell lysates were subjected to immunoprecipitation using an anti-c-Src Ab, and then the immunoprecipitates were analyzed by Western blot using an anti-p47phox or anti-c-Src Ab.
HO-1 Overexpression Abrogates TNF-α-Induced Oxidative Stress in HTSMCs
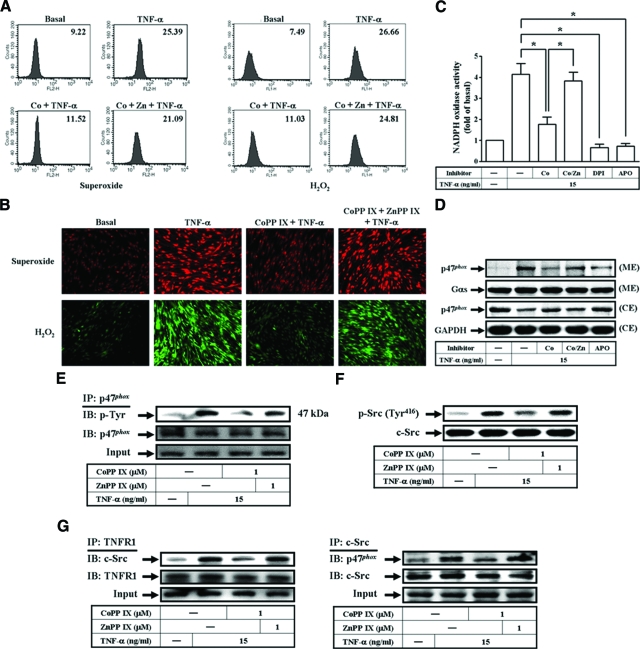
HO-1 has been reported to play a critical role in protection against oxidative tissue injury following inflammation.25 Thus, we investigated whether CoPP IX inhibited ROS generation through up-regulation of HO-1 expression in HTSMCs. As illustrated in Figure 6A, pretreatment with CoPP IX for 16 hours significantly inhibited intracellular ROS generation, including superoxide and H2O2. To further confirm these results, the fluorescence for DCF and DHE staining were observed using a fluorescence microscope (Figure 6B). Pretreatment with CoPP IX also inhibited TNF-α-induced NADPH oxidase activity and p47phox activation (Figure 6, C and D). We next determined the effects of CoPP IX on tyrosine phosphorylation of p47phox and c-Src, or the association of TNFR1/c-Src/p47phox. It was found that pretreatment with CoPP IX reduced phosphorylation of p47phox and c-Src, and the formation of a TNFR1/c-Src/p47phox complex induced by TNF-α (Figure 6, E–G). The observed suppression of TNF-α-mediated ROS generation, NADPH oxidase activity, p47phox translocation, p47phox and c-Src phosphorylation, or the association of TNFR1/c-Src/p47phox by CoPP IX was abrogated in the presence of ZnPP IX (Figure 6H). These results indicated that HO-1 overexpression suppressed TNF-α-induced ICAM-1 and VCAM-1 expression by down-regulation of NADPH oxidase-dependent ROS production and the formation of a TNFR1/c-Src/p47phox complex.
Figure 6.
Overexpression of HO-1 inhibits TNF-α-induced ROS generation by down-regulation of the association of TNFR1/c-Src/p47phox. A: DHE and DCF fluorescence intensities of HTSMCs after stimulation with TNF-α for 1 hour in the presence or absence of CoPP IX (1 μmol/L) or ZnPP IX (1 μmol/L) were measured. B: DHE or DCF fluorescence image after 1 hour of stimulation with TNF-α in the presence or absence of CoPP IX or ZnPP IX. Images shown are representative of three independent experiments with similar results. C: Cells were pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX or pretreated with DPI or APO, and then treated with TNF-α for 1 hour. NADPH oxidase activity was determined. Data are expressed as mean ± SEM of three independent experiments. Significant differences between the compared groups are indicated: *P < 0.05. D: Cells were pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX or pretreated with APO, and then treated with TNF-α for 1 hour. The membrane and cytosolic extracts were prepared and subjected to Western blot using an anti-p47phox Ab. E, F, G: Cells were pretreated with CoPP IX in the presence or absence of ZnPP IX, and then stimulated with TNF-α for 10 minutes. E: Total cell lysates were subjected to immunoprecipitation with anti-p47phox Ab and the immunoprecipitates were analyzed by Western blot using an anti-phosphotyrosine or anti-p47phox Ab. F: The cell lysates were subjected to Western blot using an anti-phospho-c-Src (Tyr 416) or anti-c-Src Ab. G: The cell lysates were subjected to immunoprecipitation using an anti-TNFR1 or anti-c-Src Ab, and then the immunoprecipitates were analyzed by Western blot using an anti-TNFR1, anti-p47phox, or anti-c-Src Ab.
CoPP IX Suppresses TNF-α-Induced NF-κB Activation via HO-1-Dependent Pathway in HTSMCs
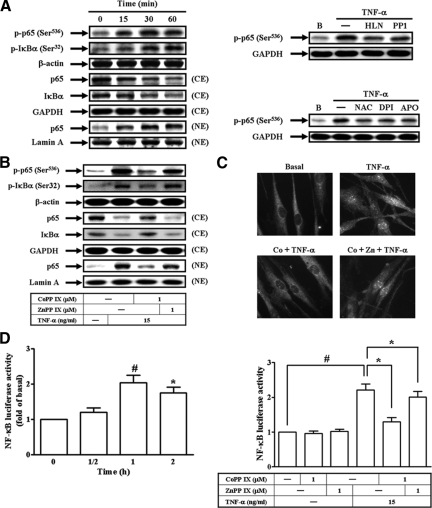
Activation of NF-κB is a central event in the initiation and amplification of inflammatory responses.26 Moreover, it was also found that TNF-α-induced ICAM-1 and VCAM-1 protein levels, mRNA expression, promoter activity, and THP-1 cell adherence were inhibited by pretreatment with a NF-κB inhibitor, helenalin (see Supplemental Figure S4 at http://ajp.amjpathol.org). Recently, c-Src and ROS have been shown to regulate NF-κB activation.27,28 Therefore, we next investigated the roles of c-Src and ROS in TNF-α-mediated NF-κB activation. As shown in Figure 7A (left), TNF-α stimulated NF-κB p65 phosphorylation and translocation, or IκBα phosphorylation and degradation in a time-dependent manner. Moreover, TNF-α-induced NF-κB p65 phosphorylation was inhibited by pretreatment with helenalin, PP1, NAC, DPI, or APO (Figure 7A, right). To investigate whether HO-1 overexpression inhibited TNF-α-induced ICAM-1 and VCAM-1 expression by down-regulation of NF-κB activation, cells were pretreated with CoPP IX for 16 hours, and then stimulated with TNF-α for 1 hour. As shown in Figure 7B, TNF-α-stimulated p65 phosphorylation at Ser536, NF-κB translocation, or IκBα degradation was inhibited by pretreatment with CoPP IX. These results with nuclear translocation of NF-κB (p65) were further ensured by an immunofluorescence staining (Figure 7C). Moreover, pretreatment with CoPP IX also reduced TNF-α-induced NF-κB promoter activity (Figure 7D, right). The observed suppression of TNF-α-stimulated NF-κB phosphorylation, translocation, and promoter activity by CoPP IX was abrogated in the presence of ZnPP IX (Figure 7, B–D). These results indicated that HO-1 overexpression suppressed TNF-α-induced ICAM-1 and VCAM-1 expression through down-regulation of NF-κB activation and translocation.
Figure 7.
Overexpression of Nrf2-dependent HO-1 inhibits NF-κB activation and translocation. A: Cells were stimulated with TNF-α for the indicated time intervals. The cell lysates were subjected to Western blot using an anti-phospho-p65 (Ser536) or anti-phospho-IκBα (Ser32) Ab. The cytosolic and nuclear extracts were prepared and subjected to Western blot using an anti-p65 or anti-IκBα Ab (left). Cells were pretreated with helenalin (1 μmol/L), PP1 (10 μmol/L), NAC (10 mmol/L), DPI (10 μmol/L), or APO (100 μmol/L), and then treated with TNF-α for 1 hour. The cell lysates were subjected to Western blot using an anti-phospho-p65 (Ser536) Ab (right). B, C: Cells were pretreated with CoPP IX for 16 hours in the presence or absence of ZnPP IX, and then treated with TNF-α for 1 hour. B: The cell lysates were subjected to Western blot using an anti-phospho-p65 (Ser536) or anti-phospho-IκBα (Ser32) Ab. The cytosolic and nuclear extracts were prepared and subjected to Western blot using an anti-p65 or anti-IκBα Ab. C: Cells were fixed, and then labeled with anti-p65 Ab and fluorescein isothiocyanate-conjugated secondary Ab. Individual cells were imaged as described in the Materials and Methods. D: Cells were transiently transfected with a NF-κB-luc reporter gene, and then treated with TNF-α for the indicated time intervals (left) or for 1 hour in the presence or absence of CoPP IX or ZnPP IX (right). The luciferase activity of NF-κB was measured. Data represent the mean ± SEM from at least three independent experiments. *P < 0.05; #P < 0.01 as compared with the basal level (left). Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01 (right).
Adenovirus-Mediated HO-1 Transduction Suppresses TNF-α-Induced Inflammatory Responses in Vitro
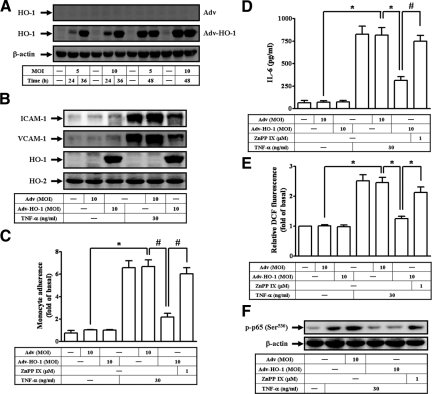
To confirm the role of HO-1 in TNF-α-induced responses, HTSMCs were infected with an empty Adv or Adv-HO-1. As shown in Figure 8A, HO-1 was overexpressed in HTSMCs infected with Adv-HO-1 but not with Adv. In addition, TNF-α-induced ICAM-1 and VCAM-1 protein levels, THP-1 cells adherence, IL-6 production, or ROS generation was abolished in HTSMCs infected with Adv-HO-1 (Figure 8, B–E). We also found that TNF-α-stimulated p65 phosphorylation at Ser 536 was significantly attenuated by infection with Adv-HO-1 (Figure 8F). The suppressive effects of Adv-HO-1 were significantly reversed by treatment with ZnPP IX (Figure 8, C–F).
Figure 8.
Adenovirus-regulated HO-1 transduction reduces TNF-α-induced inflammatory responses. A: HTSMCs were infected with an indicated multiplicity of infection (MOI) of either Adv or Adv-HO-1 for the indicated time intervals. HTSMCs were infected with 10 MOI of Adv or Adv-HO-1 for 48 hours in the presence or absence of ZnPP IX, followed by treatment with TNF-α for (B, C, D) 24 hours or (E, F) 1 hour. The (B) levels of VCAM-1 and ICAM-1 proteins, (C) THP-1 cells adherence, (D) IL-6 generation, (E) DCF fluorescence intensity, and (F) p65 phosphorylation at Ser536 were measured. Data are expressed as mean ± SEM of three independent experiments. Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01.
Carbon Monoxide from HO-1 Suppresses Adhesion Molecules Expression, ROS Generation, and IL-6 Production in HTSMCs
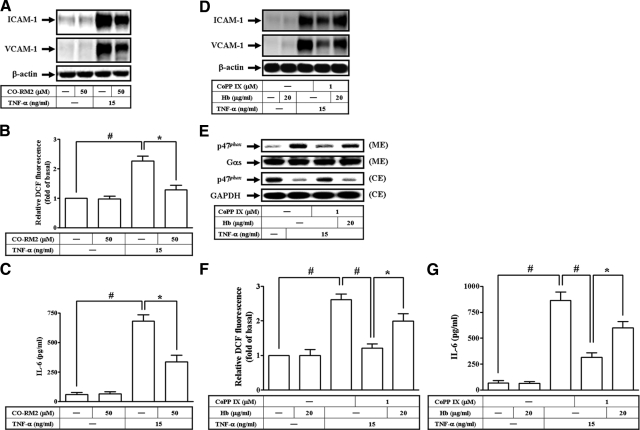
The antioxidant activity of HO-1 has been reported to be due to the elimination of pro-oxidant heme and the biological activities of bilirubin and CO. In addition, CO also exerts several biological functions, including anti-apoptotic and anti-inflammatory properties.11 To further examine the mechanisms by which end metabolites of heme catabolism by HO-1, including CO, contribute to the attenuation of TNF-α-mediated responses in HTSMCs, cells were incubated with the CO donor, CO-RM2 (CO releasing molecule, [Ru(CO)3CI2]2) before the addition of TNF-α (Figure 9, A–C). The results showed that pretreatment with CO-RM2 caused a significant decrease in TNF-α-induced adhesion molecules expression, ROS generation, and IL-6 secretion. We further examined the possibility that endogenous CO production by CoPP IX-mediated Nrf2/HO-1 activation may also account for the attenuation of TNF-α-induced responses by CoPP IX. As shown in Figure 9D-G, the addition of CO scavenger, hemoglobin, significantly reversed the attenuating effects of CoPP IX on adhesion molecules expression, p47phox translocation, ROS generation, and IL-6 secretion induced by TNF-α, indicating that CoPP IX contributes to protection of cells from TNF-α insult through, in part, a CO-dependent attenuation of adhesion molecules expression, ROS generation, and IL-6 secretion.
Figure 9.
Endogenous CO production in CoPP IX-treated HTSMCs and its role on the attenuation of TNF-α-mediated adhesion molecules expression and ROS generation. Cells were pretreated with 50 μmol/L CO-RM2, and then incubated with TNF-α for (A, C) 24 hours or (B) 1 hour. A: The levels of VCAM-1 and ICAM-1 expression were determined. B: The fluorescence intensity (relative DCF fluorescence) was measured. C: IL-6 generation was measured. Cells were pretreated with CoPP IX in the presence or absence of 20 μg/ml hemoglobin, and then incubated with TNF-α for (D, G) 24 hours or (E, F) 1 hour. D: The levels of VCAM-1 and ICAM-1 expression were determined. E: The membrane and cytosolic extracts were prepared and subjected to Western blot using an anti-p47phox Ab. F: The fluorescence intensity (relative DCF fluorescence) was measured. G: IL-6 generation was measured. Data are expressed as mean ± SEM of three independent experiments. Significant differences between the compared groups are indicated: *P < 0.05; #P < 0.01.
Discussion
TNF-α has been implicated in the pathophysiology of many inflammatory lung diseases, including chronic bronchitis, COPD, acute respiratory distress syndrome, and asthma.22 The elevated levels of TNF-α have been detected in the airways, BAL fluid, and nasal lavage from asthmatic and rhinitic patients. Leukocytes and monocytes isolated from BAL fluid of asthmatics were shown to release more TNF-α than those of cells from control subjects. Recent research indicates that TNF-α may be associated with acquired airway hyperresponsiveness, which is a pathophysiological hallmark of asthma.3 It has been suggested that TNF-α up-regulates adhesion molecules and is directly responsible for transendothelial migration of inflammatory cells, which is a central feature underlying the inflammatory responses.4 Oxidative processes are considered to play an important role in the induction of cell adhesion molecules, a key event in inflammatory processes. Moreover, HO-1 possesses antioxidant and anti-inflammatory properties.25 Overexpression of HO-1 has been shown to decrease airway inflammation and airway responsiveness to histamine in ovalbumin-sensitized guinea pigs,29 suggesting that HO-1 plays a critical role in protecting the host during airway inflammation. However, the molecular mechanisms underlying overexpression of HO-1-inhibited inflammatory response in HTSMCs remain unclear. In this study, we found that TNF-α markedly induced ROS generation, adhesion molecules expression, and IL-6 secretion in HTSMCs. In addition, TNF-α enhanced adhesion molecules expression and leukocyte count in BAL fluid in vivo. We also observed that TNF-α (0.75 mg/kg body weight) markedly induced pulmonary hematoma in mice. However, these responses will further lead to airway inflammatory diseases, such as asthma or COPD. Pretreatment with CoPP IX or infection with Adv-HO-1 inhibited TNF-α-induced adhesion molecules expression, THP-1 cell adherence, IL-6 generation, and NF-κB activation. Moreover, HO-1 overexpression inhibited ROS generation by down-regulation of the formation of a TNFR1/c-Src/p47phox complex. Thus, as induction of HO-1 expression may hence hold therapeutic promises, continuous efforts toward identifying novel airway protective anti-oxidant/anti-inflammatory substances that target HO-1 and establishment of well-designed in vivo models properly evaluating the efficacy of these agents will be warranted.
HO-1 catalyzes the rate-limiting step in the catabolism of heme to biliverdin, free iron, and CO.10 HO-1 has been reported to reduce inflammatory cell rolling, adhesion, and migration from the vascular compartment, possibly by down-regulating the function and expression of adhesion molecules on the vessel wall.30 HO-1 has been shown to be up-regulated by a variety of stressful stimuli, such as CoPP.20 We also found that CoPP IX induced HO-1 protein expression and activity in HTSMCs. Nrf2 is a major transcription factor that regulates expression of antioxidant defense genes through binding to antioxidant response elements in the promoter region of antioxidant genes, such as HO-1.14 This is confirmed by our observation that CoPP IX-induced HO-1 expression was mediated through Nrf2 by transfection with Nrf2 siRNA. We further demonstrated that the cytoprotective properties of HO-1 were due to the attenuation of adhesion molecules expression, IL-6 generation, THP-1 cells adherence, and leukocyte count in BAL fluid in vitro and in vivo. We also further ascertained that CoPP IX inhibited adhesion molecules expression and IL-6 production through Nrf2-dependent HO-1 expression by transfection with siRNA of HO-1 or Nrf2. These results suggested that the protective effects of HO-1 are dependent on Nrf2 activation in HTSMCs.
When airway cells and tissues are exposed to oxidative stress, increased levels of ROS exert many deleterious effects within the airways.30 The NADPH oxidase family members are proteins that transfer electrons across biological membranes. In general, the electron acceptor is oxygen and the product of the electron transfer reaction is a superoxide. Therefore, the biological function of NADPH oxidase enzymes might be attributable to the production of ROS.31 ROS have been shown to mediate the expression of VCAM-1 and ICAM-1.8 Most of TNF-α actions are elicited by TNFR1, which contains a death domain that fosters protein-protein interactions, particularly with other death-domain proteins.22 The Src family kinases have been shown to regulate ICAM-1 and VCAM-1 expression, NADPH oxidase activation, and ROS generation.5,6,7,23 In our study, we also established that TNF-α induced adhesion molecules expression and ROS generation through TNFR1 and c-Src by pretreatment with an anti-TNFR1 neutralizing Ab or PP1. In addition, our data also showed that TNF-α induced adhesion molecules expression and NADPH oxidase activation via the formation of a TNFR1/c-Src/p47phox complex in HTSMCs. The domains of TNFR1, c-Src, and p47phox involved in protein–protein interaction caused by TNF-α are important issues needed to be investigated in the future. However, HO-1 has been reported to play a critical role in protection against oxidative tissue injury following ischemia and inflammation.25 Therefore, we also investigated whether CoPP IX inhibited ROS production by up-regulation of HO-1 expression. In our study, we found that overexpression of HO-1 reduced ROS generation, NADPH oxidase activity, and p47phox activation. These results demonstrated that HO-1 overexpression reduced the formation of a TNFR1/c-Src/p47phox complex induced by TNF-α in HTSMCs.
NF-κB has been shown to be an inducible and ubiquitously expressed transcription factor responsible for regulating the expression of genes involved in cell survival, cell adhesion, and inflammation.32 Furthermore, increased levels of ROS have been implicated in initiating inflammatory responses in the lungs through the activation of transcription factors, such as NF-κB and AP-1.32 This is confirmed by our observation that TNF-α-induced NF-κB translocation was inhibited by pretreatment with NAC, DPI, or APO. These results suggested that ROS-dependent NF-κB activation is necessary for adhesion molecules induction by TNF-α in HTSMCs. Thus, ROS may be critical for the inflammatory responses triggered by TNF-α, through the up-regulation of redox-sensitive transcription factors and hence pro-inflammatory gene expression. Moreover, we found that HO-1 overexpression decreased TNF-α-stimulated NF-κB phosphorylation and translocation from the cytosol to the nucleus. IκBα phosphorylation was also reduced by pretreatment with CoPP IX. The observed suppression of TNF-α-regulated IκBα degradation, or NF-κB phosphorylation and translocation by CoPP IX, was abrogated in the presence of ZnPP IX. Thus, NF-κB also appears to be one of the important targets for the action of HO-1, although further studies are required to elucidate the underlying mechanisms.
The signaling properties of CO may contribute to the antioxidant and anti-inflammatory effects of HO-1.11 Thus, we investigated the mechanisms by which end metabolites of heme catabolism by HO-1, including CO, contributed to the attenuation of TNF-α-mediated adhesion molecules expression, ROS generation, and IL-6 secretion in HTSMCs. We found that pretreatment with CO-RM2 caused a significant decrease in TNF-α-induced adhesion molecules expression, ROS generation, and IL-6 secretion. The addition of CO scavenger, hemoglobin, also significantly reversed the attenuating effects of CoPP IX on TNF-α-mediated responses, indicating that CoPP IX contributed to protection of cells from TNF-α insult through, in part, a CO-dependent attenuation of adhesion molecules expression, ROS generation, and IL-6 secretion. In any event, the diverse effects of endogenous CO derived from heme degradation on cellular signaling pathways in HTSMCs warrant further investigation. Thus, the signaling properties of CO may contribute to the anti-inflammatory effects of HO-1. In asthma, a potentially critical role for HO-1-derived CO is its ability to relax airway smooth muscle via the activation of guanylyl cyclase and formation of cGMP. HO-1 and its products may be useful in both diagnostic and therapeutic strategies. Defining not only the regulation of HO-1 during airway inflammation and asthma but also diagnostic and therapeutic strategies of CO and the other components of the HO-1 pathway will remain an important avenue of investigation in the future.
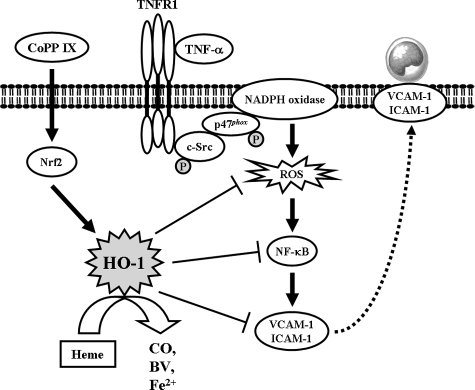
In summary, as depicted in Figure 10, TNF-α induces ROS generation through TNFR1/c-Src/NADPH oxidase, and in turn initiates the activation of NF-κB. Activated NF-κB was recruited to the promoter regions of ICAM-1 and VCAM-1 leading to an increase of ICAM-1 and VCAM-1 promoter activity and the expression of ICAM-1 and VCAM-1 mRNA and protein in HTSMCs. Moreover, CoPP IX is capable of inducing HO-1 expression, activating Nrf2-dependent pathway, resulting in inhibition of c-Src and p47phox activation, ROS generation, IL-6 production, and NF-κB activation, and suppressing adhesion molecules expression and THP-1 cells adherence in HTSMCs. Taken together, these results showed that overexpression of HO-1 might protect against TNF-α-induced airway inflammation by down-regulation of ROS generation and NADPH oxidase activity.
Figure 10.
A proposed pathway for overexpression of Nrf2-dependent HO-1 protects against TNF-α-induced oxidative stress and airway inflammation. TNF-α stimulates ROS production through TNFR1/c-Src/NADPH oxidase, in turn initiates the activation of NF-κB. Activated NF-κB was recruited to the promoter regions of ICAM-1 and VCAM-1 leading to an increase of adhesion molecules expression. Moreover, CoPP IX is capable of inducing HO-1 expression, activating Nrf2-dependent pathway, resulting in inhibition of c-Src and p47phox activation, ROS generation, IL-6 production, and NF-κB activation, and suppressing adhesion molecules expression and THP-1 cells adherence on HTSMCs.
Supplementary Material
Acknowledgments
The authors appreciate Dr. Lee-Young Chau (Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan, Republic of China) for providing a recombinant adenovirus containing human HO-1 (Adv-HO-1). We thank Mr. Li-Der Hsiao for his technical assistance in preparation of this manuscript.
Footnotes
Address reprint requests to Chuen-Mao Yang, Ph.D., Department of Pharmacology, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-San, Tao-Yuan, Taiwan. E-mail: chuenmao@mail.cgu.edu.tw.
Supported by grants from NSC95-2320-B182-010 (C.M.Y.), NSC95-2314-B182-040 (C.C.L.), NSC95-2314-B182-053 (S.F.L.), CMRPD170331 (C.M.Y.), and CMRPG350652 (C.C.L.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, Herszberg B, Lavoie JP, McVicker CG, Moir LM, Nguyen TT, Peng Q, Ramos-Barbon D, Stewart AG. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–S17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Lee CW, Lin CC, Luo SF, Lee HC, Lee IT, Aird WC, Hwang TL, Yang CM. Tumor necrosis factor-α enhances neutrophil adhesiveness: induction of vascular cell adhesion molecule-1 via activation of Akt and CaM kinase II and modifications of histone acetyltransferase and histone deacetylase 4 in human tracheal smooth muscle cells. Mol Pharmacol. 2008;73:1454–1464. doi: 10.1124/mol.107.038091. [DOI] [PubMed] [Google Scholar]
- Thabut G, El-Benna J, Samb A, Corda S, Megret J, Leseche G, Vicaut E, Aubier M, Boczkowski J. Tumor necrosis factor-α increases airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility. J Biol Chem. 2002;277:22814–22821. doi: 10.1074/jbc.M200315200. [DOI] [PubMed] [Google Scholar]
- Hosselet JJ. [Role of adhesion molecules in bronchial inflammation and bronchial hyperreactivity]. Allerg Immunol (Paris) 1994;26:278–282. [PubMed] [Google Scholar]
- Bijli KM, Minhajuddin M, Fazal F, O'Reilly MA, Platanias LC, Rahman A. c-Src interacts with and phosphorylates RelA/p65 to promote thrombin-induced ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L396–L404. doi: 10.1152/ajplung.00163.2006. [DOI] [PubMed] [Google Scholar]
- Lin CC, Lee CW, Chu TH, Cheng CY, Luo SF, Hsiao LD, Yang CM. Transactivation of Src, PDGF receptor, and Akt is involved in IL-1β-induced ICAM-1 expression in A549 cells. J Cell Physiol. 2007;211:771–780. doi: 10.1002/jcp.20987. [DOI] [PubMed] [Google Scholar]
- Lin WN, Luo SF, Wu CB, Lin CC, Yang CM. Lipopolysaccharide induces VCAM-1 expression and neutrophil adhesion to human tracheal smooth muscle cells: involvement of Src/EGFR/PI3-K/Akt pathway. Toxicol Appl Pharmacol. 2008;228:256–268. doi: 10.1016/j.taap.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, Koo TH, Jang HO, Yun I, Kim KW, Kwon YG, Yoo MA, Bae MK. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-κB activation in endothelial cells. Biochim Biophys Acta. 2008;1783:886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007;36:158–165. doi: 10.1165/rcmb.2006-0331TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- Li HY, Wu SY, Shi N. Transcription factor Nrf2 activation by deltamethrin in PC12 cells: involvement of ROS. Toxicol Lett. 2007;171:87–98. doi: 10.1016/j.toxlet.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am J Physiol. 1998;274:H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- Lee CW, Chien CS, Yang CM. Lipoteichoic acid-stimulated p42/p44 MAPK activation via Toll-like receptor 2 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L921–L930. doi: 10.1152/ajplung.00124.2003. [DOI] [PubMed] [Google Scholar]
- Lee CW, Lin WN, Lin CC, Luo SF, Wang JS, Pouyssegur J, Yang CM. Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-α in human tracheal smooth muscle cells: involvement of MAPKs. NF-κB, p300, and histone acetylation. J Cell Physiol. 2006;207:174–186. doi: 10.1002/jcp.20549. [DOI] [PubMed] [Google Scholar]
- Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK. JNK, and NF-κB in IL-1β-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L227–L237. doi: 10.1152/ajplung.00224.2004. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- Shan Y, Pepe J, Lu TH, Elbirt KK, Lambrecht RW, Bonkovsky HL. Induction of the heme oxygenase-1 gene by metalloporphyrins. Arch Biochem Biophys. 2000;380:219–227. doi: 10.1006/abbi.2000.1921. [DOI] [PubMed] [Google Scholar]
- Nowis D, Bugajski M, Winiarska M, Bil J, Szokalska A, Salwa P, Issat T, Was H, Jozkowicz A, Dulak J, Stoklosa T, Golab J. Zinc protoporphyrin IX, a heme oxygenase-1 inhibitor, demonstrates potent antitumor effects but is unable to potentiate antitumor effects of chemotherapeutics in mice. BMC Cancer. 2008;8:197. doi: 10.1186/1471-2407-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincheira R, Castro AF, Ozes ON, Idumalla PS, Donner DB. Type 1 TNF receptor forms a complex with and uses Jak2 and c-Src to selectively engage signaling pathways that regulate transcription factor activity. J Immunol. 2008;181:1288–1298. doi: 10.4049/jimmunol.181.2.1288. [DOI] [PubMed] [Google Scholar]
- Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Fong YC, Lai CH, Hung CH, Hsu HC, Lee TS, Yang RS, Fu WM, Tang CH. Thrombin-induced IL-6 production in human synovial fibroblasts is mediated by PAR1, phospholipase C, protein kinase Cα, c-Src, NF-kappa B, and p300 pathway. Mol Immunol. 2008;45:1587–1599. doi: 10.1016/j.molimm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M, Megret J, Henin D, Aubier M, Boczkowski J. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L26–L34. doi: 10.1152/ajplung.00237.2003. [DOI] [PubMed] [Google Scholar]
- Carter EP, Garat C, Imamura M. Continual emerging roles of HO-1: protection against airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L24–L25. doi: 10.1152/ajplung.00097.2004. [DOI] [PubMed] [Google Scholar]
- Lagente V, Planquois JM, Leclerc O, Schmidlin F, Bertrand CP. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin Exp Pharmacol Physiol. 2008;35:601–605. doi: 10.1111/j.1440-1681.2007.04848.x. [DOI] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation. NF-κB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.