Abstract
Matrix metalloproteinases (MMPs) have been implicated in wound healing. To analyze the roles of MMP-9 and MMP-13 in wound healing, we generated full-thickness cutaneous wounds in MMP-9 knockout (KO), MMP-13 KO, MMP-9/13 double KO, and wild-type mice. Macroscopic wound closure was delayed in all of the KO mice, as compared with wild-type mice. The rate of re-epithelialization was significantly delayed in MMP-9 KO and MMP-13 KO mice and remarkably delayed in MMP-9/13 double KO mice, as compared with wild-type mice. Both MMP-9 and MMP-13 were expressed by the leading edges of epidermal cells in wild-type mice, and the migration of keratinocytes was suppressed by treatment with an MMP inhibitor or transfection of small interfering RNAs for MMP-9 or MMP-13, as compared with controls. The vascular density in wound granulation was significantly lower in both MMP-13 KO and MMP-9/13 double KO mice than in wild-type mice. Degradation of connective tissue growth factor in wound tissue was transiently prevented in MMP-13 KO mice. Morphometric analyses demonstrated a reduction in both wound contraction and myofibroblast formation in both MMP-13 KO and MMP-9/13 double KO mice. Proliferation and transforming growth factor-β1-induced myofibroblast differentiation of dermal fibroblasts from MMP-13 KO mice were decreased, as compared with wild-type dermal fibroblasts. These data suggest that MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in wound healing, while MMP-9 functions in keratinocyte migration.
Wound healing is a complex process that includes an acute inflammatory reaction, regeneration of parenchyma cells, cell migration and proliferation, angiogenesis, extracellular matrix (ECM) synthesis, contraction, and tissue remodeling.1,2,3 Cutaneous wound healing by second intention is characterized by the following three continuous and overlapping processes: an inflammatory phase, a proliferative phase, and a contraction and remodeling phase.2 In the inflammatory phase, tissue injury causes the loss of cells and tissue, disruption of blood vessels, extravasation of blood constituents and infiltration of inflammatory cells, composed mainly of neutrophils and macrophages, and provides a provisional ECM for keratinocyte migration. The major events during the proliferative phase are re-epithelialization and angiogenesis, both of which require cell proliferation and migration of keratinocytes and endothelial cells, respectively. In the contraction and remodeling phase, myofibroblasts differentiated from fibroblasts play a key role in wound contraction and controlled synthesis and degradation of ECM proteins, especially collagens, leading to increased wound strength. All of these events occurring during wound healing require the collaborative efforts of many different tissues and cell types.
Accumulated lines of evidence have demonstrated that members of the matrix metalloproteinase (MMP) gene family are essential to the degradation of ECM macromolecules and non-ECM molecules such as growth factors and cytokines under various pathophysiological conditions.4,5 Enhanced expression of MMPs 1, 2, 3, 8, 9, 10, 13, 14, 19, and 26 in wound tissues has been reported in experimental animals and humans.6,7,8,9,10,11,12,13 It is clear that MMP activity is required in wound closure by keratinocyte re-epithelialization and migration and angiogenesis, since broad-spectrum MMP inhibitors inhibit the processes.14,15,16,17,18 However, since many MMPs are expressed by re-epithelializing keratinocytes, inflammatory cells, fibroblasts, and endothelial cells, it is uncertain which MMP species plays a central role in the process of wound healing and how these MMPs function in wounded tissues.
One of the most powerful methods to directly address these questions is to analyze wound healing in MMP knockout (KO) mice. Indeed, wound healing of the skin has been studied in mice deficient for the MMP-3, MMP-8, MMP-9, MMP-13, or MMP-14 genes.13,19,20,21,22 However, all of these KO mice, except for the MMP-8 KO mice, had negative results. In the MMP-8 KO mice, wound closure and re-epithelialization were inhibited mainly due to impaired infiltration of neutrophils,20 which are responsible for MMP-8 production. The study suggested that the effect of MMP-8 on the wound healing is secondary to persistent inflammation at the later time point and alterations in transforming growth factor-β (TGF-β) signaling.20 Keratinocytes at the leading edge of the cutaneous wound express MMP-9 and MMP-13.13,21 Induction of MMP-9 at the wound site in vitro23 and in vivo24 promotes migration of keratinocytes probably because of its effect on detachment of basal keratinocytes from the basal membrane. Keratinocyte migration over the wound bed is known to be dependent on the attachment of keratinocytes to fibrillar type I collagen through integrins α1β1 and α2β1 and subsequent degradation of the collagen by collagenolytic MMPs.25,26 These studies suggest that both MMP-9 and MMP-13 are involved in re-epithelialization in wound healing in mice, a species that lacks the gene for MMP-1.27 However, previous studies on wound healing in MMP-9 KO and MMP-13 KO mice failed to demonstrate significant difference or showed even acceleration of wound closure and re-epithelialization, as compared with that in wild-type mice.13,21 These studies did not provide the reasonable explanations for the unexpected findings except for redundancy among MMPs.13 MMP-9 and MMP-13 synergize with each other during endochondral ossification at the growth plates.28 Thus, MMP-9/13 double KO mice could identify additive effects of MMP-9 and MMP-13 on wound healing, although no such studies have been reported.
In the present study, we developed the MMP-9/13 double KO mice by crossing the MMP-9 KO mice with the MMP-13 KO mice, and evaluated the influence of targeted deletion of the MMP-9 and/or MMP-13 genes on healing by secondary intention by generating large skin wounds in MMP-9 KO, MMP-13 KO, MMP-9/13 double KO, and wild-type mice. Our study provides the first evidence that MMP-13 plays a key role in keratinocyte migration, angiogenesis, and contraction in wound healing, and that MMP-9 is implicated in keratinocyte migration.
Materials and Methods
Animals
MMP-9 KO mice originated on the 129SvEv/CD-1 mixed background were purchased from the Jackson Laboratory29 and MMP-13 KO mice on a 129/Sv genetic background were generated by microinjection of embryonic stem cells into C57BL/6J blastocytes as described previously.30 These mice were backcrossed six times into the C57BL/6J background. MMP-9 KO and MMP-13 KO mice were obtained by breeding heterozygous mice. MMP-9/13 double KO mice were generated by interbreeding heterozygous MMP-9+/−/13+/− mice, which were produced by breeding homozygous MMP-9 KO mice with homozygous MMP-13 KO mice. Genotyping of animals was performed by PCR of DNA obtained from tail biopsies. Primers for wild-type alleles were located in exon 3 (5′-AGGCCTTCAGAAAAGCCTTC-3′) and exon 4 (5′-GCAGTTCCAAAG-3′) of the MMP-13 gene and primers for mutated alleles were located in d2EGFP (5′-GCAAGCTGACCCTGAAGTTCATCTG-3′ and 5′-CAGAAGAACGGCATCAAGGTGA-3′). Primers for wild-type alleles of the MMP-9 gene (5′-GTGGGACCATCATAACATCACA-3′ and 5′-CTCGCGGCAAGTCTTCAGAGTA-3′) and mutated alleles (5′-CTGAATGAACTGCAGGACGA-3′ and 5′-ATACTTTCTCGGCAGGAGCA-3′) were created according to the genotyping protocols from the Jackson Laboratory (Bar Harbor, Maine). MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice exhibited a normal lifespan with sufficient fertility and did not show gross phenotype after maturation, although they had growth retardation because of defects in the growth plate during development (unpublished data for MMP-9/13 double KO mice).29,30 Matched control littermates of MMP-13 KO mice were used as wild-type mice for experiments.
Wound Creation and Macroscopic Examination
Full-thickness wounds were made with a sterile biopsy punch with a diameter of 8 mm (Keisei Medical Industrial Co., LTD) on the dorsal region of wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice, 9 to 11 weeks old. The wounds were left open and the animals were housed in individual cages. Wound healing was macroscopically monitored by taking digital photographs at the indicated time points. The wound areas (percentage of wound areas to initial ones) were calculated from the photographs using PhotoShop (Adobe Photoshop Element 2.0, Adobe Systems) (n = 6 per group). Mice were sacrificed by intraperitoneal injection of overdosed Nembutal, and skin with the wound was removed for further experiments. All procedures were performed according to the guidelines for the Care and Use of Laboratory Animals of Keio University School of Medicine.
Histology and Immunohistochemistry
Excised skin specimens were fixed with Tris-buffered zinc fixative,31 and paraffin sections (4 μm) were stained with H&E and Elastica-van-Gieson. For immunohistochemistry, sections were reacted with anti-CD68 antibody (1:100 dilution; FA-11; Serotec), anti-neutrophil antibody (1:100 dilution; MCA771GA; Serotec), anti-CD3 antibody (1:100 dilution; MCA1477; Serotec), anti-CD31 antibody (1:50 dilution; MEC13.3; BD Biosciences), anti-proliferating cell nuclear antigen (PCNA) antibody (1:200 dilution; PC10; DakoCytomation), anti-α-smooth muscle actin (SMA) antibody (1:200 dilution; 1A4; DakoCytomation), anti-MMP-9 antibody (1:1000 dilution; G-Tonline), or anti-MMP-13 antibody (1:100 dilution; D-17; Santa Cruz Biotechnology) after blocking endogenous peroxidase and nonspecific binding according to our methods.32 Then, they were incubated with peroxidase-conjugated secondary antibodies (1:200 dilution; Vector).32 As a control, sections were treated with non-immune IgG by replacing the first antibodies.
Analyses of Re-Epithelialization, Inflammatory Cell Infiltration, Cell Proliferation, and Angiogenesis
Width of the wound and distance of the traversed epithelium were measured on H&E-stained sections, and percent re-epithelialization was calculated according to the following formula: [distance covered by epithelium]/[distance between wound bed] × 100 (n = 6 per group). Infiltration of neutrophils, macrophages, and T-lymphocytes was evaluated by counting the cells immunostained with anti-neutrophil antibody, anti-CD68 antibody, and anti-CD3 antibody, respectively (n = 3 per group).32 To analyze keratinocyte proliferative activity in migration tongue (mitosis zone), PCNA-positive cell index (percentage of positive cells to whole keratinocytes) was calculated by counting the immunoreactive cells and total keratinocytes in the mitosis zone (n = 3 per group). The vascular density in the granulation tissue of six random fields was determined by counting numbers of CD31-positive vessels per square millimeter (n = 5 for the samples on days 0, 3, 5, 7 and 10 and n = 3 for those on days 14 and 28).33
Reverse Transcription-PCR for MMP-9, MMP-13, and β-actin
Total RNA was extracted from skin wounds in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice, and cDNA was prepared from 1 μg of total RNA with SuperScript II reverse transcriptase (Life Technologies Inc.) according to our methods.34 Reaction products were subjected to RT-PCR analysis at 28, 30, and 25 cycles for the expression of MMP-9, MMP-13, and β-actin, respectively. Sequences of the primers were as follows: for MMP-9 5′-GCGCCACCACAGCCAACTATG-3′ (forward), 5′-TGGATGCCGTCTATGTCGTCTTTA-3′ (reverse); for MMP-13 5′-GGTCCCAAACGAACTTAACTTACA-3′ (forward), 5′-CCTTGAACGTCATCAGGAAGC-3′ (reverse); for β-actin 5′-TTCTACAATGAGCTGCGTGTGGC-3′ (forward), 5′-CTCATAGCTCTTCTCCAGGGAGGA-3′ (reverse). The nucleotide sequence of the amplified fragments was confirmed by cycle sequencing using a DYEnamic ET dye terminator cycle sequencing kit (MegaBACE; Amersham Pharmacia Biotech) and MegaBACE 1000 DNA sequencer (Amersham Pharmacia Biotech).34
In Vitro Keratinocyte Migration Assay
Mouse keratinocytes (PAM212 cells; a gift from Dr. Takashi Hashimoto, in Department of Dermatology, Kurume University)35 were grown to confluence on type I collagen-coated 6-well plates (BD Biosciences) in Spinner’s modified minimum essential medium (Gibco) containing 10% fetal bovine serum, 1% antibiotic solution, 2 mmol/L l-glutamine and 100 μmol/L CaCl2 and then scratch-wounded with a blue pipette tip according to the previous methods.36 The keratinocytes were allowed to heal the wounds in the presence and absence of BB94 (British Biotech) in the medium containing 100 μmol/L hydroxyurea (Sigma-Aldrich), an inhibitor of cell proliferation. The marked areas of the wound were photographed, and cell migration areas were determined using the public domain Scion Image program.
A pool of small interfering (si)RNAs for MMP-9 or MMP-13 and control non-silencing oligonucleotide (AATTCTCGGAACGTGTCAXGT) were purchased from Dharmacon SMART Pool and QIAGEN, Inc, respectively. Transfection of these siRNAs to keratinocytes was performed using the Human Keratinocyte Nucleofetor Kit (Amaxa Inc.). As a positive control, green fluorescent protein vector siRNA was transfected in a similar way. Specific silencing of the MMP-9 and MMP-13 genes was examined by RT-PCR and immunoblotting analyses.
Analysis of Connective Tissue Growth Factor in Wound Tissues
Wound tissues were obtained from wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. Proteins were separated by SDS-polyacrylamide gel electrophoresis and subjected to immunoblotting using anti-connective tissue growth factor (CTGF) antibody (1:1000 dilution)37 or anti-β-actin antibody (1:1000 dilution; AC-74; Sigma-Aldrich). Degradation of CTGF in the wound tissues was monitored by densitometrical analysis of the bands of intact CTGF and its degradation fragments using Scion Image. The expression of vascular endothelial growth factor (VEGF) was also monitored by RT-PCR and immunoblotting according to the previous methods.38,39
Morphometrical Analysis of Granulation Tissue
When the wounds of each group were completely re-epithelialized, the height of dermis (HD) in the center of the wounds and the width of middle dermis (WD) in the wounds were determined on Elastica-van-Gieson-stained sections according to the previous methods40 (n = 3 per group). Myofibroblasts were identified by immunostaining of α-SMA in the granulation tissue, and percentage of the area with myofibroblasts to total granulation area was determined by planimetric image analysis using PhotoShop (Adobe Photoshop Element 2.0, Adobe Systems) according to the following formula: [α-SMA-positive cell area]/[dermal area] × 100 (n = 3 per group).
Proliferative Activity and α-SMA Expression of Primary Mouse Dermal Fibroblasts
Fibroblasts were cultured from dermal explants of adult wild-type, MMP-9 KO, MMP-13 KO, or MMP-9/13 double KO mice in Dulbecco’s Modified Eagle’s medium containing 10% fetal bovine serum. Mitogenic activity of fibroblasts was measured by 5-bromo-2′-deoxy-uridine (BrdU) Labeling and Detection Kit III (Roche Molecular Biochemicals).34 After serum-starved to synchronize their growth arrest, they were treated with or without recombinant active TGF-β1 (0.2 ng/ml; R&D systems) or latent TGF-β1 (2 ng/ml; R&D systems) in DMEM containing 1% fetal bovine serum. To examine the effects of MMP-13 and TGF-β1, neutralizing anti-MMP-13 antibody (1 μg/ml; Chemicon), non-immune IgG (1 μg/ml; R&D systems), neutralizing anti-TGF-β antibody (1 μg/ml; R&D systems), or non-immune IgG (1 μg/ml; Santa Cruz Biotechnology) was added to the culture media before TGF-β1 treatment. In addition, fibroblasts from MMP-13 KO mice were cultured in the presence of recombinant active MMP-13 (500 ng/ml; Calbiochem) or buffer alone together with latent TGF-β1. After 6-hour incubation, 10 mmol/L BrdU was added to the media and cultured for the next 42 hours.
Concentrations of active TGF-β (ng/ml) in culture media from wild-type or MMP-13 KO mouse fibroblasts treated with or without latent TGF-β1 for 24 hours were measured by a specific and sensitive bioassay using minc lung epithelial cells, which were stably transfected with a luciferase reporter under the control of a TGF-β-responsive truncated plasminogen activator inhibitor promoter (a gift from Dr. Mayumi Abe in Department of Nanomedicine, Graduate School, Tokyo Medical and Dental University).41 Similarly, concentrations of active TGF-β1 in the conditioned culture media of granulation tissues from cutaneous wounds were determined by the bioassay. The granulation tissues (∼10 mg each in 2 ml culture media), which were obtained from skin wounds in wild-type and MMP-13 KO mice on day 5, were cultured for 10 hours to wash out endogenous TGF-β, and then the conditioned media for the assay were prepared by culturing for 24 hours in the presence or absence of latent TGF-β1 (2 ng/ml). Effects of TGF-β1 on α-SMA expression in cultured dermal fibroblasts from wild-type or MMP-13 KO mice were examined by immunofluorescent microscopy using a laser-scanning confocal microscope (Olympus Fluoview) and immunoblotting.
Treatment of Wounds with Recombinant MMP-9, MMP-13, or Basic Fibroblast Growth Factor
Wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice underwent an operation for full-thickness wounds on both sides of the dorsal region, and wounds on each side received recombinant basic fibroblast growth factor (bFGF) in saline (20 μg/wound; a gift from Kaken Seiyaku) or saline alone at 0, 1, 3, and 5 days after wounding. Similarly, recombinant human MMP-9 in 50 mmol/L Tris-HCl buffer, pH 7.4, 150 mmol/L NaCl, 10 mmol/L CaCl2 (2 μg/wound; Calbiochem) or the buffer alone was applied to the wounds in MMP-9 KO mice, human MMP-13 (2 μg/wound; Calbiochem) or the buffer alone to those in MMP-13 KO mice, and a mixture of MMP-9 (2 μg/wound) and MMP-13 (2 μg/wound) or the buffer alone to MMP-9/13 double KO mice at 1 and 3 days after wounding. Wound healing was macroscopically and histologically examined up to 7 days after wounding.
Statistical Analyses
Statistical differences were determined using the Mann-Whitney U-test or student’s t-test. P values less than 0.05 were considered significant.
Results
Macroscopic Findings of Skin Wounds
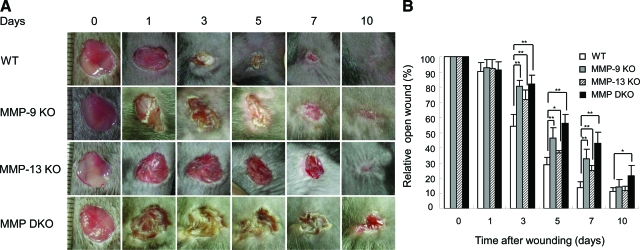
Wounds on the dorsal skin of wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice exhibited a similar size on day 1, but wound closure was delayed in all groups of the MMP KO mice when compared with mice in the wild-type group (Figure 1A). Wound areas on day 3 were significantly larger in MMP-9 KO (80.3 ± 4.1%), MMP-13 KO (71.7 ± 6.5%), and MMP-9/13 double KO (81.8 ± 5.8%) mice when compared with the wound area of the wild-type (54.1 ± 7.9%) mice (Figure 1B). The delay in wound healing in the MMP KO groups was observed up to day 7, but on day 10, only the MMP-9/13 double KO mice exhibited a significant delay (Figure 1B).
Figure 1.
Skin wound healing in wild-type (WT), MMP-9 KO, MMP-13 KO and MMP-9/13 double KO mice. A: Representative macroscopic views of skin wounds on days 0, 1, 3, 5, 7, and 10 after wounding. Full-thickness wounds (8 mm in diameter) were made with a punch biopsy instrument and wound healing was monitored by taking digital photographs. Note the delay of wound healing in the KO mice. MMP DKO, MMP-9/13 double KO mice. B: Evaluation of wound closure by morphometrical analysis of the wound areas. Percentage of the wound areas to the initial areas was calculated from the photographs (n = 6). Bars = mean ± SE. *P < 0.05; **P < 0.01.
Histological Evaluation of Wound Re-Epithelialization
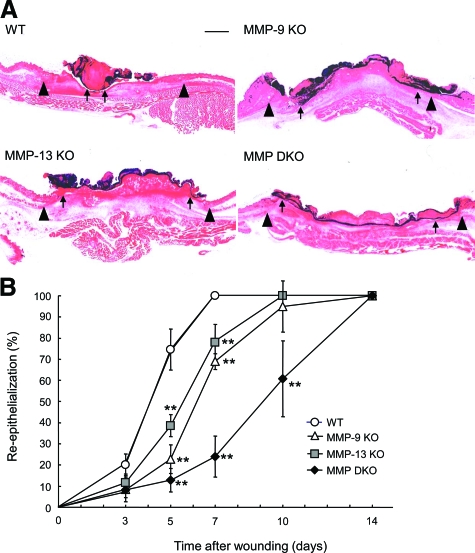
The architecture of skin was histologically identical in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice before initiating cutaneous wounds (data not shown). However, re-epithelialization after wounding appeared to be delayed in MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice, as compared with wild-type mice (Figure 2A). When the time-course changes in the re-epithelialization rate, which was determined by measuring the distance between the leading edges of the epidermis and the width of the wound bed, were compared, it was significantly delayed on day 5 in the MMP-13 KO, MMP-9 KO, and MMP-9/13 double KO mice compared with the wild-type mice, and the delay was more definite on day 7: MMP-13 KO (77.9 ± 8.7%), MMP-9 KO (68.9 ± 3.7%), and MMP-9/13 double KO (24.0 ± 9.6%) mice versus wild-type mice, which exhibited complete re-epithelialization (100 ± 0%) (Figure 2B). Complete covering of the epidermis by re-epithelialization was observed on day 10 in MMP-9 KO and MMP-13 KO mice, and on day 14 in MMP-9/13 double KO mice (Figure 2B).
Figure 2.
Histological evaluation of skin wounds in wild-type (WT), MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. A: Representative histological views of skin wounds on day 5. Arrowheads and arrows indicate original wound edges and re-epithelialized leading edges, respectively. Scale bar = 1 mm. B: Time-course changes of re-epithelialization ratio after wounding in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. Percent re-epithelialization was calculated by measuring the distance between the leading edges and the width of wound bed (n = 6) as described in Materials and Methods. Bars = mean ± SE. **P < 0.01.
Analyses of Inflammatory Cell Infiltration
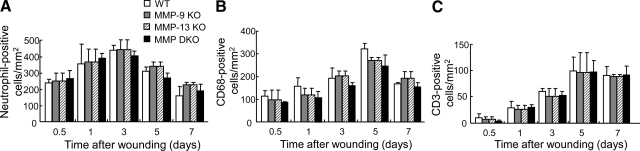
In all experimental groups, inflammatory cells started to infiltrate the wounds 12 hours after wounding, increased with time until day 5, and decreased on day 7. Neutrophils, macrophages, and T-lymphocytes were identified by immunohistochemistry with anti-neutrophil antibody, anti-CD68 antibody, and anti-CD3 antibody, respectively. As shown in Figure 3A, neutrophils infiltrated the wounds at 12 hours after injury, peaked on day 3 and decreased to a low level on day 7. Both macrophages and T-lymphocytes were detected at 12 hours after injury and peaked on day 5 (Figure 3, B and C). These patterns of inflammatory cell infiltration were not different among wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mouse groups, indicating that MMP-9 and MMP-13 do not affect the infiltration of neutrophils, macrophages, and T-lymphocytes during wound healing.
Figure 3.
Time course changes of inflammatory cell infiltration in skin wounds in wild-type (WT), MMP-9 KO, MMP-13, and MMP-9/13 double KO mice. Neutrophils (A), macrophages (B), and T lymphocytes (C) were identified by immunohistochemistry with anti-neutrophil antibody, anti-CD68 antibody and anti-CD3 antibody, respectively. Immunoreactive inflammatory cells/mm2 were determined by counting immunostained cells in 6 different areas in the wounds on days 0.5, 1, 3, 5, and 7 (n = 3). Bars = mean ± SE.
MMP-9 and MMP-13 Expression in Wounds
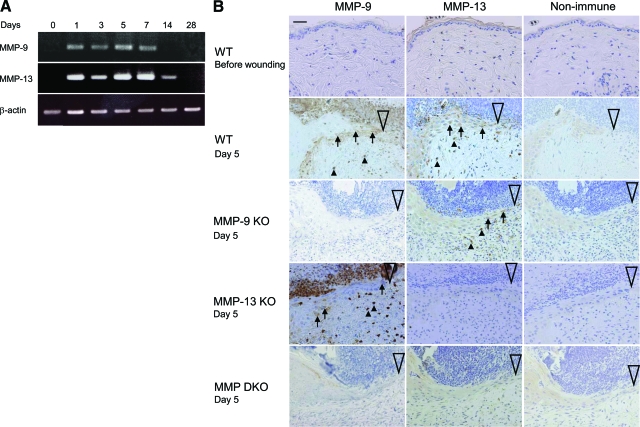
RT-PCR analyses in wild-type mice showed that the expression of MMP-9 and MMP-13 is negligible in the normal unwounded skin (day 0), but it increases on days 1 to 7, diminishes on day 14 and disappears on day 28 (Figure 4A). The unwounded or wounded skin of MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice exhibited no expression of corresponding MMP(s) (data not shown).
Figure 4.
The expression of MMP-9 and MMP-13 in skin wounds. A: The mRNA expression of MMP-9 and MMP-13 in skin wounds of wild-type (WT) mice. RT-PCR was performed for MMP-9 and MMP-13 using total RNA isolated from wounds on days 0, 1, 3, 5, 7, 14, and 28. β-actin was used as a loading control. B: Immunohistochemistry of MMP-9 and MMP-13 in normal skin and skin wounds in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. Paraffin sections of skin tissues were subjected to immunohistochemistry. Arrows indicate immunoreactive epidermal cells and arrowheads show immunoreactive inflammatory cells or fibroblasts. Large open arrowheads show the leading edges of epidermis. Scale bar = 20 μm.
Protein expression of MMP-9 and MMP-13 in normal skin and skin wounds in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice was evaluated by immunohistochemistry. As shown in Figure 4B, negligible staining of MMP-9 and MMP-13 was obtained in the wild-type mouse skin before wounding, but MMP-9 was immunolocalized to the epidermal cells at the leading edge and inflammatory cells including neutrophils and macrophages in wild-type and MMP-13 KO mice. On the other hand, MMP-13 was immunolocalized to the epidermal cells at the leading edge and stromal fibroblasts in the wild-type and MMP-9 KO mice (Figure 4B). A few endothelial cells in the granulation tissue also immunostained with the anti-MMP-13 antibody (data not shown). As expected, however, the expression of MMP-9 and MMP-13 was lacking in the corresponding MMP KO mice and MMP-9/13 double KO mice (Figure 4B). No immunostaining was observed in the tissue samples treated with non-immune IgG (Figure 4B).
Analysis of Keratinocyte Proliferation at the Epidermal Leading Edges
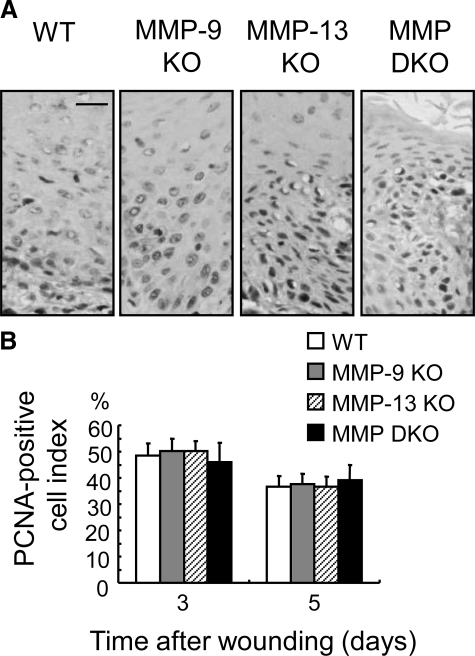
Keratinocytes in the mitosis zone of the leading edges were positively immunostained with anti-PCNA antibody (Figure 5A). When the PCNA-positive cell index was compared on days 3 and 5, there were no significant differences among the four experimental groups (Figure 5B).
Figure 5.
Analysis of keratinocyte proliferative activity at the re-epithelialized leading edges in wild-type (WT), MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. A: Immunohistochemistry of PCNA in epidermal cells at migration tongue (mitosis area). Paraffin sections of skin wounds on day 5 were immunostained using anti-PCNA antibody. Scale bar = 20 μm. B: PCNA-positive cell index (percentage of positive cells to whole keratinocytes) in the mitosis area on days 3 and 5 were calculated (n = 3). No significant differences are seen among the groups. Bars = mean ± SE.
Effect of MMP Activity on Keratinocyte Migration In Vitro
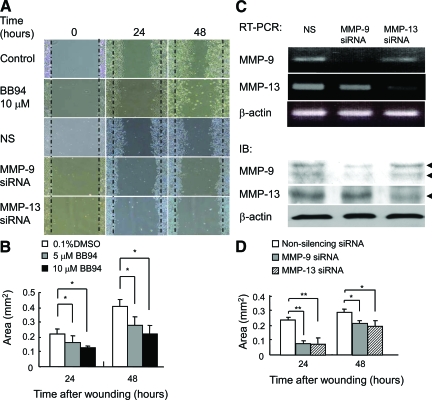
An in vitro wound healing assay on type I collagen-coated plates was performed using mouse keratinocytes, in which expression of MMP-9 and MMP-13 was demonstrated by RT-PCR and immunoblotting. When the keratinocyte monolayers were wounded, they began to migrate over the wound and covered more than half of the wound area by 48 hours (Figure 6A). BB94, a broad-spectrum MMP inhibitor, significantly inhibited migration (Figure 6, A and B), indicating that MMP activity is involved in the migration. Keratinocytes transfected with siRNA for MMP-9 or MMP-13 exhibited down-regulation of mRNA and protein expression of the corresponding MMP (Figure 6C). When the transfected keratinocytes were subjected to the wound healing assay, migration was significantly inhibited in the cells transfected with siRNA for MMP-9 or MMP-13, as compared with those transfected with control siRNA (Figure 6, A and D).
Figure 6.
Involvement of MMP-9 and MMP-13 in keratinocyte migration in vitro. A: Effects of BB94 and siRNA for MMP-9 or MMP-13 on migration of PAM212 keratinocytes. Keratinocyte monolayers were wounded and cultured for up to 48 hours in the presence of 0.1% dimethyl sulfoxide (Control) or 10 μmol/L BB94 in 0.1% dimethyl sulfoxide. Similarly, keratinocytes were transfected with non-silencing (NS), MMP-9, or MMP-13 siRNA and subjected to in vitro migration assay. B: Inhibition of wound healing by treatment with BB94. Migration areas of keratinocytes treated with 0.1% dimethyl sulfoxide or 5 μmol/L and 10 μmol/L BB94 in 0.1% dimethyl sulfoxide were determined by measuring the cell migration areas (n = 3). Bars = mean ± SE. *P < 0.05. C: Down-regulation of the expression of MMP-9 and MMP-13 in keratinocytes transfected with siRNA for MMP-9 or MMP-13. Keratinocytes were treated with non-silencing (NS), MMP-9, or MMP-13 siRNA, and the mRNA and protein expression of MMP-9 and MMP-13 was evaluated by RT-PCR and immunoblotting (IB). Arrowheads indicate latent and active forms of MMP-9 (93 kDa and 87 kDa) or active form of MMP-13 (50 kDa). D: Inhibition of wound healing by treatment with siRNA for MMP-9 or MMP-13. Migration areas of keratinocytes treated with non-silencing, MMP-9, or MMP-13 siRNA were determined by measuring the cell migration areas (n = 3). Bars = mean ± SE. *P < 0.05; **P < 0.01.
Angiogenesis in the Wound Granulation Tissue
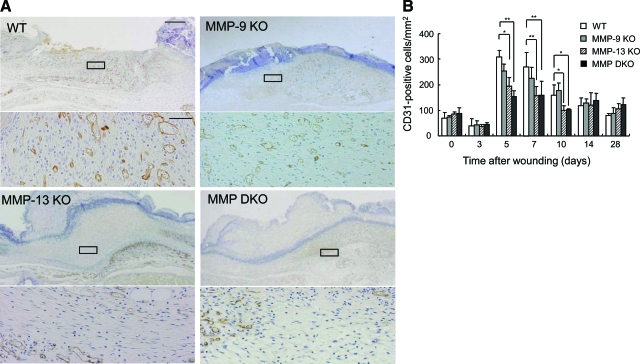
The time-course changes of angiogenesis in the wound tissues in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice were analyzed by evaluating the vascular density in the CD31-immunostained sections. Angiogenesis in the wound bed started at 5 days after wounding (Figure 7A) and continued for 10 days in all of the experimental groups. Although no difference was observed in the vascular density of the unwounded dermis (day 0) and wounds on day 3 in the experimental groups, the density was significantly lower in the MMP-13 KO and MMP-9/13 double KO mouse groups on days 5, 7, and 10 than in the wild-type mouse group, and recovered by day 14 (Figure 7B). These data suggest the involvement of MMP-13 in angiogenesis in the wound tissues.
Figure 7.
Time-course changes of angiogenesis in skin wounds. A: Immunohistochemistry of CD31 in skin wounds in wild-type (WT), MMP-9 KO, MMP-13, and MMP-9/13 double KO mice. Paraffin sections of skin wounds on day 5 were subjected to immunohistochemistry. Scale bar = 500 μm. Higher magnifications of the boxed areas in the wounds of wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice are shown in the lower panels. Scale bar = 50 μm. B: Time-course changes of vascular density/mm2 on days 0, 3, 5, 7, 10, 14, and 28 determined by counting numbers of CD31-positive vessels within six visual fields (n = 3). *P < 0.05; **P < 0.01.
Degradation of CTGF during Wound Healing
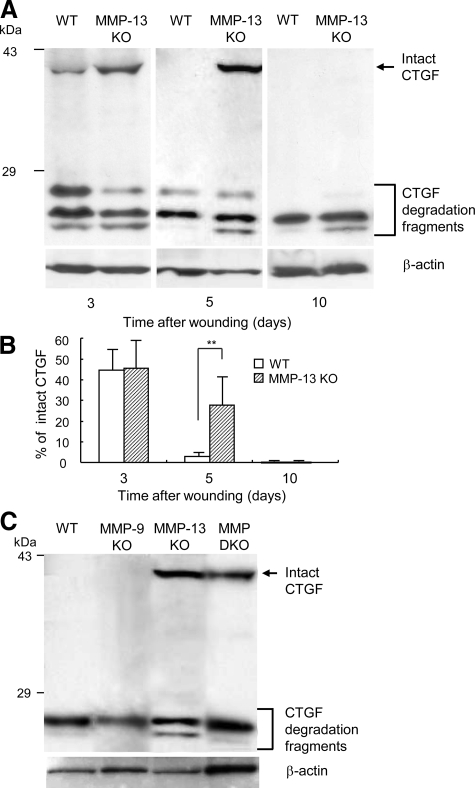
Our previous studies demonstrated that CTGF blocks angiogenic activity of VEGF by complex formation42 and MMP-1, 3, 7, and 13, especially MMP-13, re-activate VEGF activity through selective cleavage of CTGF in the complex.37 Thus, we examined the possible involvement of this pathway in the wound healing by immunoblotting analyses of CTGF in the granulation tissues obtained from wild-type and MMP-13 KO mice. As shown in Figure 8A, intact CTGF in the samples from wild-type mice started to be degraded into smaller fragments 3 days after injury and disappeared on days 5 and 10, but this degradation was delayed in the MMP-13 KO-mouse samples. Densitometric analysis indicated that the percentage of intact CTGF to total CTGF on day 5 is significantly higher in MMP-13 KO mice than in wild-type mice (Figure 8B). Importantly, in the samples on day 5, intact CTGF completely disappeared in wild-type and MMP-9 KO mice, but remained in MMP-13 KO and MMP-9/13 double KO mice (Figure 8C). On the other hand, VEGF expression analyzed by RT-PCR and immunoblotting was not different in the groups (data not shown).
Figure 8.
Analysis of CTGF degradation by immunoblotting. A: Homogenate supernatants were obtained from wound samples in wild-type (WT) and MMP-13 KO mice on days 3, 5, and 10 and subjected to immunoblotting. Intact CTGF and its degradation fragments are indicated. β-actin is used as a loading control. B: Ratio of intact CTGF to total CTGF. The ratio was determined by densitometrical analysis of the bands of intact and its degradation fragments (n = 3). Bars = mean ± SE. **P < 0.01. C: Homogenate supernatants of the wound samples from wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice on day 5 were subjected to immunoblotting for CTGF and β-actin.
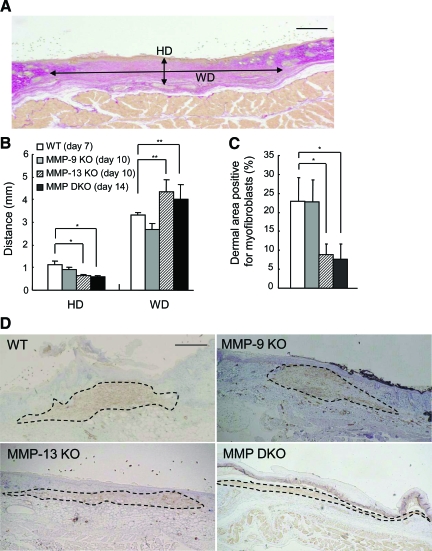
Morphometrical Analyses of Wound Granulation and Myofibroblasts
To study the effects of MMP-9 and MMP-13 on wound contraction and scar formation, we morphometrically analyzed the Elastica-van-Gieson-stained sections when the wounds were completely re-epithelialized: on day 7 in the wild-type mice, on day 10 in the MMP-9 KO and MMP-13 KO mice, and on day 14 in the MMP-9/13 double KO mice. When HD and WD were compared (Figure 9A), HD significantly decreased and WD increased in MMP-13 KO and MMP-9/13 double KO mice, as compared with wild-type mice (Figure 9B), suggesting the impeded wound contraction in these MMP KO mice.
Figure 9.
Analyses of granulation tissue and dermal area with myofibroblasts in wild-type (WT), MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. A: Representative histology of skin wound showing the height of dermis in the center of the wound (HD) and the width of middle dermis in the wound (WD). A section of the skin wound on day 7 in a wild-type mouse was stained with Elastica-van-Gieson. Scale bar = 1 mm. B: Evaluation of HD and WD in skin wounds in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. The parameters were evaluated in wild-type mice (day 7), MMP-9 KO mice (day 10), MMP-13 KO mice (day 10), and MMP-9/13 double KO mice (day 14) (n = 3). Bars = mean ± SE. *P < 0.05; **P < 0.01. C: Planimetric analysis of dermal area with myofibroblasts in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. Percentages of dermal area with myofibroblasts were determined by planimetric image analysis of the α-SMA-immunostained sections (n = 3). Bars = mean ± SE. *P < 0.05. D: Immunohistochemistry of α-SMA for detection of myofibroblasts in the granulation tissue. Immunohistochemistry for α-SMA was performed on the sections obtained at the aforementioned time points. Dotted lines show the areas with myofibroblasts. Hematoxylin was used for counterstaining. Scale bar = 1 mm.
Myofibroblasts were identified by immunohistochemistry of α-SMA on the sections obtained at the aforementioned time points. α-SMA-positive myofibroblasts formed elongated masses that located in the dermal granulation beneath the re-epithelialized epidermis and the areas with myofibroblasts appeared to be thinner in MMP-13 KO and MMP-9/13 double KO mice than wild-type and MMP-9 KO mice (Figure 9D). Planimetric image analysis showed that % α-SMA-positive area is significantly smaller in MMP-13 KO and MMP-9/13 double KO mice than in wild-type and MMP-9 KO mice (Figure 9C).
Effects of TGF-β1 on Dermal Fibroblasts and Activation of Latent TGF-β1 in Wound Skin Tissues under Culture Conditions
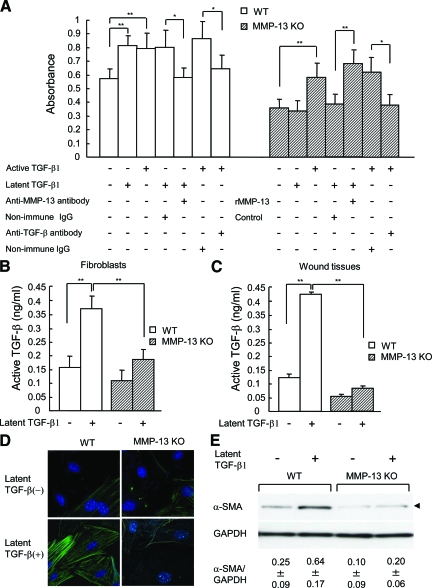
When BrdU incorporation of primary fibroblasts isolated from the dermis of the skin in wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice was compared, it was significantly lower in unstimulated fibroblasts from MMP-13 KO and MMP-9/13 double KO mice, as compared with those from wild-type and MMP-9 KO mice (P < 0.05) (Figure 10A and data not shown for MMP-9 KO and MMP-9/13 double KO mice). After stimulation with latent TGF-β1, the incorporation was significantly increased in fibroblasts from wild-type and MMP-9 KO mice (P < 0.01), but such a stimulatory effect was not observed with fibroblasts from MMP-13 KO or MMP-9/13 double KO mice (Figure 10A and data not shown for MMP-9 KO and MMP-9/13 double KO mice). On the other hand, active TGF-β1 could enhance the incorporation in fibroblasts from wild-type or MMP-13 KO mice (Figure 10A). The increased BrdU incorporation in wild-type fibroblasts treated with latent or active TGF-β1 was significantly inhibited with neutralizing anti-MMP-13 antibody or anti-TGF-β antibody compared with non-immune IgG (Figure 10A). Similarly, active TGF-β1-induced enhancement in fibroblasts from MMP-13 KO mice was significantly reduced by treatment with anti-TGF-β antibody (Figure 10A). Moreover, the BrdU incorporation in fibroblasts from MMP-13 KO mice was significantly increased by treatments with latent TGF-β1 and recombinant active MMP-13 (Figure 10A). These data demonstrate that the activity of TGF-β1 is responsible for the enhanced BrdU incorporation in the fibroblasts and suggest the possible involvement of MMP-13 in the conversion of latent TGF-β to active protein in wild-type mice.
Figure 10.
Proliferative activity of fibroblasts from wild-type (WT) and MMP-13 KO mice and their reactions to TGF-β1 treatment. Primary fibroblasts were cultured from dermal explants of adult wild-type and MMP-13 KO mice. A: Proliferative activity of the fibroblasts treated with or without active or latent TGF-β1 and effects of anti-MMP-13 antibody, anti-TGF-β antibody, or recombinant active MMP-13 on the proliferation. After the cells were treated with these agents, BrdU incorporation was determined (n = 3). Bars = mean ± SE. *P < 0.05; **P < 0.01. B: Conversion of added latent TGF-β1 to active one in culture media of fibroblasts from wild-type and MMP-13 KO mice. Activity of TGF-β1 was assessed by a bioassay as described in Materials and Methods (n = 3). Bars = mean ± SE. **P < 0.01. C: Conversion of latent TGF-β1 in culture media of the granulation tissues from the skin wounds in wild-type and MMP-13 KO mice on day 5. Activity of TGF-β1 was assessed by a bioassay as described above (n = 3). Bars = mean ± SE. **P < 0.01. D: Immunohistochemistry of α-SMA in wild-type and MMP-13 KO fibroblasts treated with or without latent TGF-β1. Fibroblasts were immunostained with anti-α-SMA antibody and observed by a laser-scanning confocal microscope. E: Immunoblotting analysis of α-SMA in wild-type and MMP-13 KO fibroblasts treated with or without latent TGF-β1. Arrowhead indicates α-SMA-immunoreactive band of 43 kDa. Glyceraldehyde-3-phosphate dehydrogenase is used as a loading control. Ratios of α-SMA to glyceraldehyde-3-phosphate dehydrogenase (mean ± SE) were obtained by image analysis of the immunoreactive bands (n = 3).
Since MMP-13 has been reported to activate latent TGF-β by cleaving latent TGF-β,43 we measured the concentration of active TGF-β by a bioassay in culture media of fibroblasts from wild-type and MMP-13 KO mice treated with or without latent TGF-β1. As shown in Figure 10B, the concentration in the culture media from wild-type fibroblasts was significantly increased after treatment with latent TGF-β1, and it was also significantly higher in wild-type fibroblasts than in MMP-13 KO fibroblasts, indicating active conversion of latent TGF-β1 in wild-type fibroblasts. To further examine the changes of latent TGF-β1 in wound skin tissues, we cultured granulation tissues, which were obtained from skin wounds in wild-type and MMP-13 KO mice on day 5, in the presence or absence of latent TGF-β1, and measured the concentrations of active TGF-β1 by a bioassay. The results demonstrated the active conversion of latent TGF-β1 only in wild-type mouse tissue (Figure 10C), supporting the aforementioned data of fibroblasts.
Activity of TGF-β1 is known to provide dermal fibroblasts with a contractile phenotype of myofibroblasts by stimulating α-SMA expression.44 Immunohistochemistry of α-SMA found that fibroblasts from wild-type mice exhibit strong staining after treatment with latent TGF-β1, whereas only weak staining was observed in wild-type fibroblasts without TGF-β1 treatment or those from MMP-13 KO mice treated with or without latent TGF-β1 (Figure 10D). Confirming the immunohistochemical data, the expression ratio of α-SMA to glyceraldehyde-3-phosphate dehydrogenase in wild-type fibroblasts (0.25 ± 0.09) was significantly increased after treatment with latent TGF-β1 (0.64 ± 0.17) (P < 0.01), whereas such an increase was not observed in fibroblasts from MMP-13 KO mice (0.10 ± 0.09 and 0.20 ± 0.06, before and after the TGF-β1 treatment, respectively) (Figure 10E). Under the stimulation with latent TGF-β1, the ratio was significantly higher in wild-type fibroblasts than in MMP-13 KO fibroblasts (P < 0.01).
Topical Treatment of Wounds with Recombinant MMP-9, MMP-13, or bFGF
We tested the supplementary effects of recombinant MMP-9 and/or MMP-13 on the delay of wound healing in MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice. As shown in Table 1, wound healing in these KO mice was macroscopically promoted by topical supplement of MMP-9 to the skin wound of the MMP-9 KO mice, MMP-13 to the wound of the MMP-13 KO mice, or a mixture of MMP-9 and MMP-13 to the wound of the MMP-9/13 double KO mice on day 3. Similar effects of MMP-9 and MMP-13 on histological re-epithelialization were observed in the wounds on day 5 (Table 1). When the wounds were topically treated with bFGF, which is known to induce MMPs including MMP-9 and MMP-1345,46 and widely used to treat pressure ulcers,47 the treatment significantly improved the macroscopic wound healing and histological re-epithelialization in wild-type, MMP-9 KO, and MMP-13 KO mice, as compared with the vehicle-treated wounds of the corresponding mice (Table 1). Interestingly, however, bFGF treatment of the wounds did not show an improvement in the MMP-9/13 double KO mice (Table 1).
Table 1.
Effects of recombinant(r) MMP-9, MMP-13 and bFGF on skin wounds in wild-type, MMP-9 KO, MMP-13 KO and MMP-9/13 double KO mice
| Macroscopic wound area (%) on day 3
|
Re-epithelialization rate (%) on day 5
|
|||||
|---|---|---|---|---|---|---|
| Control | rMMP | rbFGF | Control | rMMP | rbFGF | |
| Wild-type | 61.1 ± 1.8 | 37.0 ± 7.9** | 64.2 ± 2.6 | 84.9 ± 7.1* | ||
| MMP-9 KO | 77.0 ± 5.2 | 45.3 ± 12.2* | 19.0 ± 10.0 | 43.8 ± 9.3* | ||
| 82.7 ± 1.1 | 60.4 ± 3.7** | 14.0 ± 1.7 | 28.4 ± 7.7* | |||
| MMP-13 KO | 72.2 ± 5.2 | 54.4 ± 4.3* | 36.4 ± 11.8 | 67.0 ± 12.3* | ||
| 67.0 ± 6.0 | 52.4 ± 3.8** | 32.9 ± 10.7 | 54.7 ± 5.8* | |||
| MMP DKO | 74.9 ± 3.1 | 73.4 ± 11.1 | 4.5 ± 6.5 | 9.4 ± 1.6 | ||
| 80.2 ± 2.8 | 73.3 ± 2.5* | 8.7 ± 1.2 | 12.4 ± 1.9* | |||
Macroscopic wound area and re-epithelialization rate represent percent of the wound that remains open and closed, respectively. Statistical analyses were carried out between the vehicle control and the rMMP- or rbFGF-treated wounds.
P < 0.05;
P < 0.01.
Discussion
In the present study we have provided the first evidence of the importance of MMP-9 and MMP-13 on cutaneous wound healing by demonstrating that MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice exhibit a significant delay in macroscopic wound closure and histological re-epithelialization. Previous studies showing that various MMPs including MMPs 1, 2, 3, 8, 9, 10, 13, 14, 19, and 26 are expressed at wound sites in experimental animals and humans,6,7,8,9,10,11,12,13 and that broad-spectrum MMP inhibitors retard wound closure,15,18 have demonstrated the involvement of MMPs in wound healing. MMP-3, 9, and 13 were expected to play a key role in mouse wound healing of the skin because of the expression in keratinocytes at the migrating border.8 However, previous studies on MMP-3 KO19 and MMP-13 KO mice13 showed no delays in wound healing. The data in MMP-9 KO mice were rather puzzling, since wound healing of the skin and cornea was accelerated in the mice, although the MMP inhibitor treatment reduced healing in the ex vivo experiments.21 Contrary to the previous studies, our study has demonstrated significant delays of wound healing in the MMP-13 KO and MMP-9 KO mice. The discrepancy between the previous13 and present studies on the MMP-13 KO mice might be explained by differences in the genetic background (a mixed background of C57BL/6;129/Sv for the previous study versus C57BL/6J for our study), the targeting constructs (Cre/LoxP system versus homologous recombination targeting system) and/or the age of mice used for the experiments (7 to 8 weeks old vs 9 to 11 weeks old). However, another plausible explanation is that the size of the wounds (4 mm vs 8 mm in diameter for the previous and current studies, respectively) was a critical factor for the time-course changes in wound healing. Before the present experiments, we performed a preliminary study by making full-thickness, excisional skin wounds, 4, 6, or 8 mm in diameter, in wild-type, MMP-9 KO, and MMP-13 KO mice, and found that only 8 mm-wounds exhibited delays of wound closure in the MMP-9 KO and MMP-13 KO mice. Such a delay was not detectable in the 4-mm wounds, since they were quickly re-epithelialized within 3 days after wounding, as described previously.13 The cutaneous wound healing reported by the previous study21 on the MMP-9 KO mice appeared to be unusual, since wound closure in their wild-type mice required 21 days, which were ∼threefold longer than ours, and a significant enhancement in wound closure was reported only in the late stages. The slow healing in their mice may have been caused by the treatment of skin wounds with transparent Op-Site dressing,21 and acceleration of the wound healing could be obtained only under this condition. In the current study, re-epithelialization was not only significantly delayed in the MMP-9 KO and MMP-13 KO mice, but also remarkably retarded in an additive manner in the MMP-9/13 double KO mice, and topical treatment of the wounds with recombinant MMP-9 and/or MMP-13 for the corresponding MMP KO and double KO mice recovered the delays. Therefore, this study strongly suggests that both MMP-9 and MMP-13 play essential roles in wound closure and re-epithelialization in the wound healing of mouse skin. Previous study on corneal wound healing in MMP-9 KO mice showed the acceleration of re-epithelialization after injury compared with wild-type mice.21 The data appear to contradict our data on skin wound healing in MMP-9 KO mice. However, since corneal wounds differ from skin wounds in that the process of corneal wound healing is avascular and impaired resorption of ECM proteins including laminin-5 and fibronectin is prominent in MMP-9 KO mice,21 the difference may be explained by the tissue specificity.
Re-epithelialization in the wound healing of the skin is dependent on proliferation and migration of keratinocytes.1,2,3 Since proliferation of keratinocytes in the mitosis zone did not differ between wild-type, MMP-9 KO, MMP-13 KO, and MMP-9/13 double KO mice, the delayed re-epithelialization in our MMP KO and double KO mice is ascribed to impaired migration of keratinocytes. Previous studies using broad-spectrum MMP inhibitors or neutralizing anti-MMP-9 antibody showed the inhibition of keratinocyte migration.14,24 In the present experiments using siRNA transfection and MMP inhibition, we have provided direct evidence that MMP-13, as well as MMP-9, is involved in the migration of mouse keratinocyte during wound healing.
One of the intriguing findings of the current study is the delay in angiogenesis in the granulation tissue in the MMP-13 KO and MMP-9/13 double KO mice. Angiogenesis was not significantly inhibited in the MMP-9 KO mice and the inhibition rate of angiogenesis in MMP-9/13 double KO mice was similar to that in MMP-13 KO mice. These data indicate the possibility that MMP-13 alone, and not MMP-9, plays a central role in angiogenesis during skin wound healing. Although angiogenesis in various conditions such as tumor angiogenesis, bFGF-induced cornea angiogenesis and angiogenesis in the developing growth plate is impaired in mice lacking the MMP-2,48,49 MMP-9,29 or MMP-14 gene,50 there have been no reports describing the inhibition of angiogenesis during cutaneous wound healing in mice lacking the MMP-3,19 MMP-8,20 MMP-9,21 MMP-13,13 or MMP-14 genes.22 In the previous wound healing study in MMP-13 KO mice, the authors described no changes in angiogenesis before wounding and after re-epithelialization.13 Since the delay in angiogenesis in the granulation tissue occurred before re-epithelialization, the contradictory findings between the previous and current studies are likely due to a difference in the timing of the observation. Vascular vessel formation is a complex process, which is regulated by the balance between angiogenic factors such as VEGF and anti-angiogenic factors.51 We have previously discovered that the angiogenic activity of VEGF is blocked by complex formation with CTGF42 and several MMPs, including MMP-1, 3, 7, and 13, especially MMP-13, have the ability to release VEGF from the VEGF/CTGF complex by selective cleavage of CTGF.37 A recent study also showed that MMP-2 exerts a similar activity on CTGF.52 In our current study, we have demonstrated that CTGF digestion within the wound tissue is transiently, but significantly, prevented in the MMP-13 KO mice without changing the expression of CTGF and VEGF. Thus, it is possible to speculate that an imbalance in favor of an angiogenic pathway by the action of fibroblast-derived MMP-13 contributes to angiogenesis in wound healing of the mouse skin. Ito et al53 recently reported that angiogenesis in the tumor xenografts is induced through VEGF activation by selective CTGF digestion of the VEGF/CTGF complex with carcinoma cell-derived MMP-7. Although the MMP species (ie, MMP-13 and MMP-7) in the two experiments were different, these data support our original hypothesis that MMPs regulate angiogenesis through activation of CTGF-sequestered VEGF.37 However, since MMP-13 is also expressed by endothelial cells as shown in the present and previous studies,54 the possibility that ECM degradation by endothelial cell-derived MMP-13 directly controls angiogenesis remains to be elucidated by future work.
In the maturation phase of wound healing, the size of wounds is reduced by contraction of the granulation tissue grown beneath the re-epithelialized epidermis, which is an important step to complete the healing of large cutaneous wounds.1,2,3 It is generally accepted that wound contraction is ascribed mainly to the action of myofibroblasts proliferating in granulation tissue. Our morphometrical analyses demonstrated a retardation of wound contraction and a decrease in the myofibroblast formation in the MMP-13 KO and MMP-9/13 double KO mice. TGF-β1 is a pluripotent growth factor, which promotes proliferation, myofibroblast differentiation, and migration of fibroblasts.44 The biologically active dimer is released from the latent TGF-β1 component by proteolysis with various proteinases, which include MMP-13.43 In the present study, primary dermal fibroblasts from wild-type mice, which produced active MMP-13 under the culture conditions, proliferated faster than those from MMP-13 KO and MMP-9/13 double KO mice in the presence and absence of latent TGF-β1 and expressed a larger amount of α-SMA after treatment with latent TGF-β1, whereas fibroblasts from MMP-13 KO mice did not exhibit this phenotype. In addition, conversion of latent TGF-β1 to its active form was observed in the culture media of fibroblasts from wild-type mice, but not MMP-13 KO mice. Thus, all of the data suggest that MMP-13 is involved in wound contraction through fibroblast proliferation and myofibroblast differentiation by activation of latent TGF-β1.
Our data, that the topical application of MMP-9 and MMP-13 to wounds in the corresponding MMP KO mice and MMP-9/13 double KO mice improved the delay of wound healing, and bFGF, a strong inducer of MMPs including MMP-9 and MMP-13,16,33 also exhibited a similar effect in the MMP KO mice, not only support the involvement of both MMP-9 and MMP-13 in re-epithelialization of wound healing, but also suggest the possible treatment of delayed wound healing by the application of the MMPs or inducers of the MMPs. In humans, MMP-1, but not MMP-13, is reported to play an essential role in re-epithelialization55 and MMP-13 is suggested to be responsible for minimal scarring observed in wound healing of fetal skin.9 However, it was impossible to analyze the role of MMP-1 in the present study, since the MMP-1 gene is lacking in mice,27 and our study did not provide the positive data supporting the latter notion of MMP-13 in wound healing with minimal scaring. bFGF has been used with some success to treat chronic pressure sores and ulcers in patients with poor nutrition, diabetes mellitus, or corticosteroid treatment, all of which are systemic factors affecting wound healing.1,3 This effect of bFGF is generally thought to be due to enhanced angiogenesis. However, since bFGF is known to stimulate migration of epidermal keratinocytes,56 our data suggest the possibility that the migration-promoting activity of bFGF results from the induction or stimulation of MMP-9 and MMP-13 expression. Our study also provides experimental evidence supporting the clinical usage of bFGF to accelerate wound closure in such patients, who might have impaired MMP expression in the epidermal keratinocytes after wounding.
Acknowledgments
We thank Dr. Kimura for his advice for immunohistochemical experiments and Ms. Mayumi Kishi for her technical assistance. We are also grateful to Dr. Edward D. Harris, Jr. for reviewing the manuscript.
Footnotes
Address reprint requests to Yasunori Okada, M.D., Ph.D., Department of Pathology, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-0016, Japan. E-mail: okada@sc.itc.keio.ac.jp.
Supported by Grant-in Aid from the Ministry of Education, Science and Culture of Japan (19109004) to Y.O.
References
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Wound healing. Philadelphia: W.B. Saunders Company,; Robbins Pathologic Basis of Disease. (Sixth edition) 1999:pp 107–111. [Google Scholar]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Overall CM, McQuibban GA, Clark-Lewis I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol Chem. 2002;383:1059–1066. doi: 10.1515/BC.2002.114. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–134. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Toriseva M, Penttinen R, Crombleholme T, Foschi M, Han J, Kahari VM. Expression of human collagenase-3 (MMP-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J. 2001;15:1098–1100. [PubMed] [Google Scholar]
- Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol. 2003;121:997–1004. doi: 10.1046/j.1523-1747.2003.12533.x. [DOI] [PubMed] [Google Scholar]
- Beare AH, O'Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–163. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- Ahokas K, Skoog T, Suomela S, Jeskanen L, Impola U, Isaka K, Saarialho-Kere U. Matrilysin-2 (matrix metalloproteinase-26) is upregulated in keratinocytes during wound repair and early skin carcinogenesis. J Invest Dermatol. 2005;124:849–856. doi: 10.1111/j.0022-202X.2005.23640.x. [DOI] [PubMed] [Google Scholar]
- Hartenstein B, Dittrich BT, Stickens D, Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z, Angel P. Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J Invest Dermatol. 2006;126:486–496. doi: 10.1038/sj.jid.5700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela M, Larjava H, Pirila E, Maisi P, Salo T, Sorsa T, Uitto VJ. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res. 1999;251:67–78. doi: 10.1006/excr.1999.4564. [DOI] [PubMed] [Google Scholar]
- Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, Dano K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–4656. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge MF, Coge F, Galizzi JP, Boutin JA, West DC, Tucker GC. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis. 2002;5:215–226. doi: 10.1023/a:1023889805133. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Impola U, Karsdal MA, Saarialho-Kere U, Agren MS. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol. 2002;118:55–64. doi: 10.1046/j.0022-202x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2007;21:2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U, Zhou Z, Rollman O, Tryggvason K, Agren MS. Wound healing in membrane-type-1 matrix metalloproteinase-deficient mice. J Invest Dermatol. 2004;123:600–602. doi: 10.1111/j.0022-202X.2004.23230.x. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, O'Brien P, Hudson LG. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J Cell Physiol. 1998;176:255–265. doi: 10.1002/(SICI)1097-4652(199808)176:2<255::AID-JCP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Scott KA, Arnott CH, Robinson SC, Moore RJ, Thompson RG, Marshall JF, Balkwill FR. TNF-alpha regulates epithelial expression of MMP-9 and integrin alphavbeta6 during tumour promotion. A role for TNF-alpha in keratinocyte migration? Oncogene. 2004;23:6954–6966. doi: 10.1038/sj.onc.1207915. [DOI] [PubMed] [Google Scholar]
- Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Beare AH, Krane SM, Ferguson MW. Variable impairment of wound healing in the heterozygous collagenase-resistant mouse. Wound Repair Regen. 2005;13:27–40. doi: 10.1111/j.1067-1927.2005.130105.x. [DOI] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi H, Kimura T, Dalal S, Okada Y, D'Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994;42:1127–1134. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya K, Enomoto H, Inoki I, Okazaki S, Fujita Y, Ikeda E, Ohuchi E, Matsumoto H, Toyama Y, Okada Y. Expression of ADAM15 in rheumatoid synovium: up-regulation by vascular endothelial growth factor and possible implications for angiogenesis. Arthritis Res Ther. 2005;7:R1158–R1173. doi: 10.1186/ar1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui Y, Mochizuki S, Kodama T, Shimoda M, Ohtsuka T, Shiomi T, Chijiiwa M, Ikeda T, Kitajima M, Okada Y. ADAM28 is overexpressed in human breast carcinomas: implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res. 2006;66:9913–9920. doi: 10.1158/0008-5472.CAN-06-0377. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Sitailo LA, Qin JZ, Bodner B, Denning MF, Curry J, Zhang W, Brash D, Nickoloff BJ. Knockdown of p53 levels in human keratinocytes accelerates Mcl-1 and Bcl-x(L) reduction thereby enhancing UV-light induced apoptosis. Oncogene. 2005;24:5299–5312. doi: 10.1038/sj.onc.1208650. [DOI] [PubMed] [Google Scholar]
- Daniel RJ, Groves RW. Increased migration of murine keratinocytes under hypoxia is mediated by induction of urokinase plasminogen activator. J Invest Dermatol. 2002;119:1304–1309. doi: 10.1046/j.1523-1747.2002.19533.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- Claffey KP, Wilkison WO, Spiegelman BM. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992;267:16317–16322. [PubMed] [Google Scholar]
- Vandervelde S, van Luyn MJ, Rozenbaum MH, Petersen AH, Tio RA, Harmsen MC. Stem cell-related cardiac gene expression early after murine myocardial infarction. Cardiovasc Res. 2007;73:783–793. doi: 10.1016/j.cardiores.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T. Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol. 2004;203:710–720. doi: 10.1002/path.1574. [DOI] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- D'Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). A role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–932. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- Payne WG, Ochs DE, Meltzer DD, Hill DP, Mannari RJ, Robson LE, Robson MC. Long-term outcome study of growth factor-treated pressure ulcers. Am J Surg. 2001;181:81–86. doi: 10.1016/s0002-9610(00)00536-5. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Kato T, Kure T, Chang JH, Gabison EE, Itoh T, Itohara S, Azar DT. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, Overall CM. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–7203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nerusu KC, Bhagavathula N, Brennan M, Hattori N, Murphy HS, Su LD, Wang TS, Johnson TM, Varani J. Vascular expression of matrix metalloproteinase-13 (collagenase-3) in basal cell carcinoma. Exp Mol Pathol. 2003;74:230–237. doi: 10.1016/s0014-4800(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Vaalamo M, Mattila L, Johansson N, Kariniemi AL, Karjalainen-Lindsberg ML, Kahari VM, Saarialho-Kere U. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109:96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- Sogabe Y, Abe M, Yokoyama Y, Ishikawa O. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen. 2006;14:457–462. doi: 10.1111/j.1743-6109.2006.00143.x. [DOI] [PubMed] [Google Scholar]