Abstract
Epithelial-mesenchymal transition (EMT) plays an important role in organ fibrosis, including that of the kidney. Loss of E-cadherin expression is a hallmark of EMT; however, whether the loss of E-cadherin is a consequence or a cause of EMT remains unknown, especially in the renal system. In this study, we show that transforming growth factor (TGF)-β1-induced EMT in renal tubular epithelial cells is dependent on proteolysis. Matrix metalloproteinase-mediated E-cadherin disruption led directly to tubular epithelial cell EMT via Slug. TGF-β1 induced the proteolytic shedding of E-cadherin, which caused the nuclear translocation of β-catenin, the transcriptional induction of Slug, and the repression of E-cadherin transcription in tubular epithelial cells. These findings reveal a direct role for E-cadherin and for matrix metalloproteinases in causing EMT downstream of TGF-β1 in fibrotic disease. Specific inhibition rather than activation of matrix metalloproteinases may offer a novel approach for treatment of fibrotic disease.
Epithelial-mesenchymal transition (EMT) is an important process in embryonic development and cancer metastasis.1,2,3 Emerging evidence has shown that EMT is also a key mechanism for organ fibrosis including that of kidney.4,5,6 One third or more of interstitial myofibroblasts, the main effector cells contributing to kidney fibrosis, originate from renal tubular epithelial cells via EMT.7
A variety of cytokines and growth factors [transforming growth factor (TGF)-β1, epidermal growth factor, FGF-2, interleukin -1, CTGF] acting through multiple signaling pathways including Src, Ras, Ets, integrin, Wnt/β-catenin, Smads, and Snail/Slug have been reported to induce EMT in embryonic development as well as tumor progression.2,4,8,9,10 In the Wnt signaling pathway, inhibition of GSK-3β with consequent stabilization of cytosolic pool β-catenin and its binding to TCF/LEF family transcription factors induced EMT in both mesoderm formation during development and in invading cancer cells.2,8,11,12 Cross-talk has been reported among different signaling pathways.13,14 However, questions remain about sequential events in and among individual pathways, particularly those of TGF-β1-induced EMT.
A hallmark of EMT is down-regulation of E-cadherin, which has been considered as an epithelial marker in most EMT studies. However, re-expression of E-cadherin in vitro blocked invasiveness and inhibited growth of E-cadherin negative tumor cells.15,16,17 Moreover, E-cadherin binding prevented β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation,18 indicating a possible role for E-cadherin in signal transduction. While the Snail/Slug transcription factor family is considered to induce EMT directly in cancer cells,9,19 in human colon cancer cells Slug is induced by β-catenin signaling following disruption of E-cadherin-mediated cell-cell contact.20 Thus, at least in cancer cells, loss of E-cadherin functions as a mediator of EMT, not merely as an epithelial marker.
Traditionally, matrix metalloproteinases (MMPs) have been considered to be antifibrogenic factors due to their proteolytic degradation of extracellular matrix. However, MMP-3 was found capable of inducing EMT in tumor cells through its shedding of E-cadherin and the consequent nuclear localization of β-catenin.21,22 Counterintuitively, other proteolytic enzymes including tissue plasminogen activator and plasmin have been found not to be protective in kidney fibrosis.23,24 Moreover, MMP-2 was shown to induce tubular EMT independently of TGF-β1.25 Of perhaps greater interest, TGF-β1 was unable to induce EMT without disrupting the integrity of cell-cell contact, indicating involvement of E-cadherin in TGF-β1-mediated EMT.26 In the context of inflammation or cancer progression, certain MMPs are up-regulated and may serve to disrupt cell-cell contact through E-cadherin. Our current study has examined the hypotheses that E-cadherin is a key mediator, and not a mere bystander, in EMT of tissue fibrosis, and that MMPs can be profibrotic by disrupting E-cadherin and thereby inducing EMT downstream of TGF-β1.
Materials and Methods
Cell Culture
NRK52e kidney tubular epithelial cells were cultured in Dulbecco’s modified Eagle’s medium /low modified (JRH Bioscience, Brooklyn, Australia) supplemented with 5% fetal calf serum (Invitrogen, Carlsbad, CA) at 37°C, 5% CO2. NRK49f kidney fibroblast cells were cultured in Dulbecco’s modified Eagle’s medium/high modified (JRH Bioscience) supplemented with 7.5% fetal calf serum at 37°C, 5% CO2. For treatment of either NRK52e or NRK49f cells, the corresponding serum-containing media were replaced with respective serum-free Dulbecco’s modified Eagle’s medium supplemented with 0.2% bovine serum albumin.
Treatments
Subconfluent cultures of NRK52e cells were washed three times with phosphate-buffered saline, then treated with recombinant human TGF-β1 (rhTGF-β1) (Biosource, Camarillo, CA), recombinant human proteolytic domain MMP-3 (rhMMP-3) (ALEXIS Biochemical, San Diego, CA), recombinant MMP-9 (Biomol, Plymouth Meeting, PA) or E-cadherin N terminus antibody (N-20, Santa Cruz Biotechnology, Santa Cruz, CA) of indicated concentrations in Dulbecco’s modified Eagle’s medium/low modified supplemented with 0.2% bovine serum albumin, in presence or absence of the broad spectrum MMP inhibitor GM6001 (Calbiochem, Darnstadt, Germany) or anti-TGF-β1 monoclonal Ab 1D11 (R&D Systems, Minneapolis, MN) or anti-MMP-9 neutralizing Ab (Calbiochem). The dosages of MMP-3, MMP-9, and E-cadherin N-terminus antibody used are those reported capable of inducing EMT in cancer cells, whereas GM6001, 1D11, and anti-MMP-9 neutralizing Ab were used at concentrations causing maximal inhibition.
Plasmids
Plasmids were prepared using the EndoFree plasmid kits (Qiagen, Hilden, Germany). E-cadherin expression construct pmoEcad/IRESneoGL2 was made by cutting mouse E-cadherin ORF from plasmid moEcad/BIISK+ (BglII) with EcoR V/NotI and inserting into the corresponding sites of pIRESneoGL2. Wild-type (WT) and mutated β-catenin constructs: huWTβ-cat Flag/pcDNA3, huS33Yβ-cat Flag/pcDNA3 (containing a mis-sense mutation of tyrosine for serine at codon 33) were kindly donated by Dr. Eric Fearon.27 E-cadherin promoter luciferase reporter constructs, pmoEcad (−201 to +131)/GL3 (with mouse E-cadherin promoter −201 to +131 driving firefly luciferase reporter gene) and pRL-RSV (with RSV promoter driving Renilla luciferase as a transfection control).
Stable and Transient Transfection
Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. One microgram of PvuI linerized pmoEcad/IRESneoGL2 or vector control were used for stable transfection of NRK49f or NRK52e cells in each well of a 24-well plate. Stable transfections were obtained after selection by G418 at 100 μg ml−1 for NRK49f cells and 500 μg ml−1 for NRK52e cells. Transient transfection was performed as described below in the section on luciferase assay.
RNA Interference
Validated stealth siRNA oligonucleotides were purchased from Invitrogen. Three stealth siRNAs targeting different sequences of Slug mRNA were selected to silence rat Slug. Stealth RNAi negative control: medium GC content control 12935-300 and low GC content control 12935-200 (Invitrogen), were used as controls. Among the three stealth siRNA oligonucleotides tested at different titrations (100, 50, and 10 pmol) on NRK52e cells, RSS332712 was the most efficient siRNA for Slug by reverse transcription-polymerase chain reaction and Western blot (see Supplemental Figure S1 at http://ajp.amjpathol.org). NRK52e cells were reverse transfected with 50 pmol Slug or control stealth siRNA oligonucleotides using Lipofectamine 2000 (Invitrogen) in 24-well plates. At 12 hours after transfection, the transfected NRK52e cells were treated with MMP-3 for 72 hours, then processed for immunofluorescence staining or Western blot.
Luciferase Assay
NRK52e cells were transiently transfected with E-cadherin promoter Firefly luciferase reporter construct pmoEcad (−201 to +131)/GL3, and a control Renilla luciferase construct pRL-RSV, with or without co-transfection of WT or mutant (S33Y) β-catenin constructs. 12 hours after transfection, cells were treated with indicated treatments, or refreshed with respective serum containing medium. The Firefly luciferase and Renilla luciferase activities were measured using Dual-Glo luciferase assay system (Promega, Madison, WI) by a luminometer (Victor2 multilabel counter) (Perkin Elmer Life Sciences) according to the manufacturer’s instructions. E-cadherin promoter activity was calculated using the Firefly luciferase activity normalized by Renilla luciferase activity as transfection control. Data are reported as the mean ± SD relative promoter activity (to non-treatment or transfection control) obtained from three independent experiments.
Immunofluorescence and Western Blot
Cells cultured in glass chamber slides (Nunc, Rochester, NY) were fixed with 3.7% paraformaldehyde and permeabilized with 0.2% Triton X-100 in phosphate-buffered saline. Anti-E-cadherin antibody (BD Transduction Lab, Lexington, KY), anti-α-smooth muscle actin (SMA) (Chemicon, Billerica, MA), anti-β-catenin antibody (BD Transduction Lab), anti-Slug antibody (Santa Cruz Biotechnology, St Louis, MO), anti-β-actin antibody (Sigma), anti-MMP-9 antibody (Calbiochem) and anti-HSP47 antibody (Santa Cruz Biotechnology) were used with respective secondary antibodies: fluorescein isothiocyanate-conjugated rat anti-mouse IgG2a/b (BD Biosciences PharMingen, San Jose, CA), Texas Red conjugated goat anti-rabbit IgGH/L (Calbiochem),, or biotin conjugated rabbit anti-mouse IgG1 (Zymed, San Francisco, CA) together with fluorescein-conjugated streptavidin (eBioscience, San Diego, CA). Images were acquired using Spot Advanced version 3.4 (Diagnostic Instruments, Inc.) with fluorescence microscope (Olympus BX51, Olympus Optical Co. Ltd.) and Spot RT Slider camera, and using 40×/0.75 Ph2 UPlanFI objective. Adobe Photoshop 6.0 was used for processing of images. Western blot analyses were performed on cell lysates or immunoprecipitation of conditioned medium using indicated antibodies as described.28 Total protein staining using Ponceau S has been used to verify equality of protein loading for conditioned medium samples and β-actin was used as a loading control for cell lysate samples. Densitometric measurement was performed using ImageJ software to quantify the relative expression of target proteins versus β-actin.
Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was isolated from NRK52e cells in culture using Absolutely RNA Microprepare kit (Stratagene, La Jolla, CA). Reverse transcription-polymerase chain reaction was performed using SuperScript III first-strand synthesis system (Invitrogen) for cDNA synthesis and Red Hot Taq (ABgene, Rockford, IL) for amplification with primers: Slug, forward: 5′-AACACACACTGGGGAAAAGC-3′; reverse: 5′-ACAGCAGCCAGACTCCTCAT-3′ (product size 181 bp), Snail, forward: 5′-GAGGACAGTGGCAAAAGCTC-3′; reverse: 5′-CGGATGTGCATCTTCAGAG-3′ (product size 243 bp), GAPDH, forward: 5′-TGCACCACCAACTGCTTAGC-3′; reverse: 5′-GGAAGGCCATGCCAGTGA-3′ (product size 247 bp). PCR reactions were performed as follows: 94°C for 2 minutes; 35 cycles of 94°C for 15 seconds; 54°C for 30 seconds; and 72°C for 60 seconds.
Immunohistochemistry
Immunohistochemistry was performed as described previously.28 The antibodies used included: goat polyclonal anti-MMP-2, anti-MMP-3 and anti-MMP-9 antibodies (Santa Cruz Biotechnology), mouse monoclonal anti-E-cadherin (BD Transduction Lab), anti-α-SMA (Chemicon) antibodies and rabbit anti-Slug (Santa Cruz Biotechnology) antibody, together with respective secondary antibodies: donkey anti-goat IgG-HRP (Santa Cruz Biotechnology) or Discovery universal secondary antibody (Ventana Medical Systems, Tucson, AZ).
Animals and Adriamycin Nephropathy (AN)
Male Wistar rats approximately 4 to 5 weeks old weighing at 90 to 110 g were purchased from the Australian Research Council and maintained under clean conditions in the Department of Animal Care at Westmead Hospital. Experiments were performed in accordance with protocols approved by Animal Ethics Committee of Western Sydney Area Health Service. AN was induced by a single tail vein injection of adriamycin (ADR 5 mg/kg; David Bull Labs, Victoria, Australia). Kidney tissues were harvested at 4 weeks after adriamycin.
Statistical Analysis
Results from three independent experiments are expressed as means ± SD. P values were calculated by one way analysis of variance followed by post hoc Fisher PLSD test and by Student’s t-test where applicable.
Results
TGF-β1-Induced EMT in NRK52e Cells Is Abrogated by MMP Inhibitor GM6001
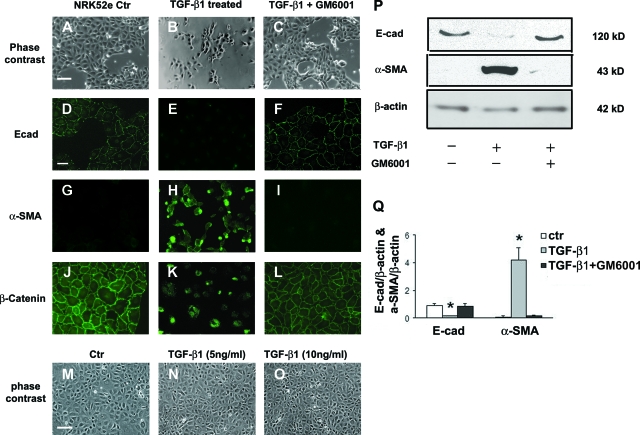
Rat tubular epithelial cells (NRK52e) were treated with recombinant human TGF-β1 (5 ng ml−1), which induced EMT in NRK52e cells under subconfluent conditions, as evidenced by morphological change from cuboid clustered epithelial cells to spindle-shaped scattered fibroblast-like cells (Figure 1, A and B), and reduced E-cadherin expression 72 hours after treatment (Figure 1, D, E, P, Q) and de novo α-SMA expression (Figure 1, G, H, P, Q). During this TGF-β1-induced EMT, β-catenin staining shifted from the cell membrane to cytoplasm and nucleus (Figure 1, J and K) where it may be involved in signal transduction. A broad spectrum MMP inhibitor GM6001 (25 μmol/L) abrogated all features of TGF-β1-induced NRK52e EMT (Figure 1, C, F, I, L, P, Q), suggesting involvement of proteolytic activity. However, TGF-β1 was unable to induce EMT in confluent NRK52e cells (Figure 1, M–O), indicating the importance of cell-cell contact in the TGF-β1-mediated EMT.
Figure 1.
MMP inhibitor GM6001 blocks TGF-β1-induced EMT in NRK52e cells. A–C: Phase-contrast images of subconfluent NRK52e cells untreated (Ctr) (A: Scale bar = 25 μm), or treated with TGF-β1 5 ng ml−1 for 72 hours in the absence (B) or presence (C) of GM6001 (25 μmol/L). D–L: Immunofluorescence images showing E-cadherin (D–F: Scale bar = 15 μm), α-SMA (G–I), and β-catenin (J–L) in NRK52e cells with corresponding treatments. M–O: Phase-contrast images of confluent NRK52e cells untreated (Ctr) (M: Scale bar = 25 μm), or treated with TGF-β1 5 ng ml−1 (N) and 10 ng ml−1 (O) for 72 hours. P and Q: Western blot analysis of E-cadherin and α-SMA versus β-actin control in lysate of NRK52e cells treated with TGF-β1 in absence or presence of GM6001. *P < 0.05.
MMP Induces EMT in NRK52e Cells through Its Proteolytic Action
To investigate why TGF-β1-induced EMT was abrogated by GM6001, NRK52e cells were treated with the recombinant proteolytic domain of MMP-3 (rMMP-3). rMMP-3 (2 μg ml−1) induced EMT in subconfluent NRK52e cells after 72 hours. NRK52e cell morphology changed from that of a cuboid shaped and clustered growing pattern to a spindle shaped and scattered growing pattern (Figure 2, A and B). Immunofluorescence staining and Western blot showed loss of E-cadherin expression (Figure 2, D, E, T, U), de novo α-smooth muscle cell actin (α-SMA) expression (Figure 2, G, H, T, U), and shift of β-catenin from membrane to cytoplasm and nucleus (Figure 2, J and K). HSP47 (which binds specifically to collagen7) staining indicated collagen synthesis by NRK52e cells induced to undergo EMT by rMMP-3 (Figure 2, M and N). rMMP-3-induced EMT in NRK52e cells was also abrogated by GM6001 (25 μmol/L) (Figure 2, C, F, I, L, O, T, U), indicating that the induction of EMT by rMMP-3 was mediated through its proteolytic action. Examination of the time course of E-cadherin and α-SMA protein levels by Western blotting indicated that the loss of E-cadherin preceded the appearance of α-SMA (Figure 2, V and W). Consistent with the proteolytic action of rMMP-3, an 80-kd proteolytic E-cadherin ectodomain sE-cad (soluble E-cadherin) was detected in the medium of NRK52e cells treated with rMMP-3 (Figure 2S), and was absent in the presence of GM6001. In contrast to TGF-β1 treatment, rMMP-3 was able to induce EMT in confluent NRK52e cells at higher concentration (5 μg ml−1) (Figure 2, P–R).
Figure 2.
rMMP-3 proteolytic domain induces EMT in NRK52e cells and GM6001 blocks this induction. A–C: Phase-contrast images of NRK52e cells untreated (ctr) (A: Scale bar = 25 μm) or treated with rMMP-3 (2 μg ml−1) for 72 hours in absence (B) or presence (C) of GM6001 (25 μmol/L). D–O: Immunofluorescence images showing E-cadherin (D–F: Scale bar = 15 μm), α-SMA (G–I), β-catenin (J–L) and HSP47 (a collagen-binding stress protein that always coexists with collagen) (M–O) of NRK52e cells with corresponding treatments. P–R: Phase contrast images of confluent NRK52e cells untreated (Ctr) (P: Scale bar = 25 μm), or treated with rMMP-3 2 μg ml−1 (Q) and 5 μg ml−1 (R) for 72 hours. S: Western blot using E-cadherin N-terminal Ab (N-20) of 80-kd proteolytic E-cadherin ectodomain present in the medium of NRK52e cells treated with rMMP-3 in absence or presence of GM6001 (25 μmol/L). Equal amounts of protein were loaded in each lane and checked by Ponceau S staining. T and U: Western blot analysis of E-cadherin and α-SMA versus β-actin in lysates of NRK52e cells treated with rMMP-3 in absence or presence of GM6001. V and W: Time course of E-cadherin and α-SMA protein levels in NRK52e cells during treatment with rMMP-3. *P < 0.05.
E-Cadherin N Terminus Antibody Treatment Induces EMT in NRK52e Cells
To test the hypothesis that E-cadherin is the molecule involved in rMMP-3-induced EMT, NRK52e cells were treated next with an antibody (N-20, 2 μg ml−1) that targets the N-terminus of E-cadherin at its extracellular domain to disrupt cell-cell adhesion. After 72 hours NRK52e cells changed morphologically from cuboid-shaped and clustered cells to spindle-shaped and scattered fibroblast-like cells (Figure 3, A and B), accompanied by reduced E-cadherin expression (Figure 3, C, D, I, J), de novo α-SMA expression (Figure 3, E, F, I, J), and cytoplasmic and nuclear localization of β-catenin (Figure 3, G and H), similar to those of EMT induced by TGF-β1 or rMMP-3.
Figure 3.
E-cadherin N-terminal Ab (N-20) induces EMT in NRK52e cells. A and B: Phase-contrast images of NRK52e cells untreated (ctr) (A: Scale bar = 25 μm), or treated with E-cadherin N-terminal Ab (N-20 2 μg ml−1) for 72 hours (B). C–H: Immunofluorescence images showing E-cadherin (C and D: Scale bar = 10 μm), α-SMA (E and F) and β-catenin (G and H) of NRK52e cell untreated (C, E, G) or treated with E-cadherin N-terminal Ab (N-20) (D, F, H). I and J: Western blot analysis of E-cadherin and α-SMA versus β-actin in lysates of NRK52e cells untreated or treated with N-20. *P < 0.05.
Forced Expression of E-Cadherin Causes Mesenchymal-Epithelial Transition in Rat Kidney Fibroblast NRK49f Cells and Prevents EMT in NRK52e cells
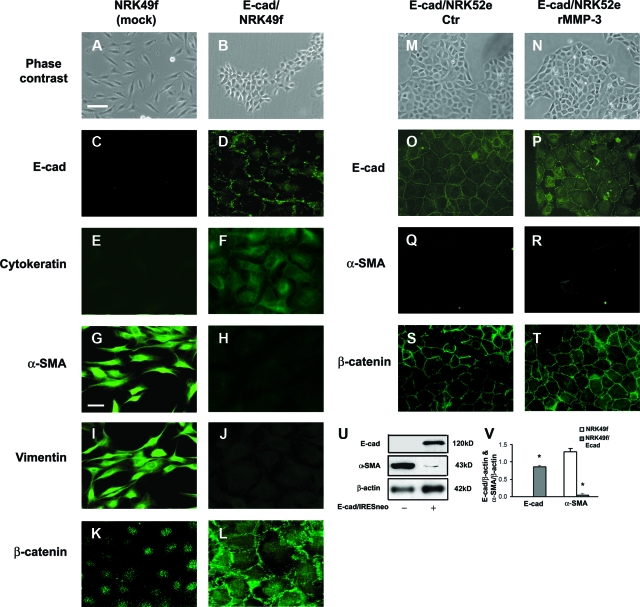
The role of E-cadherin in EMT was further investigated by forced expression of E-cadherin in rat kidney fibroblast (NRK49f) and tubular epithelial (NRK52e) cells. NRK49f cells with ectopic expression of E-cadherin displayed an epithelial-like cuboid-shaped and clustered growing pattern, replacing the spindle-shaped scattered growing pattern of native NRK49f fibroblast cells (Figure 4, A and B). Transfected NRK49f cells express epithelial marker E-cadherin (Figure 4, C, D, U, V) and cytokeratin (Figure 4, E and F) with reduced α-SMA (Figure 4, G, H, U, V) and vimentin expression (Figure 4, I and J) and redistribution of β-catenin from cytoplasm and nucleus to cell membrane (Figure 4, K and L), consistent with mesenchymal-epithelial transition. Exogenous E-cadherin expression in NRK52e cells prevented rMMP-3-mediated EMT induction (Figure 4, M–T), suggesting a key role for E-cadherin in EMT.
Figure 4.
Forced expression of E-cadherin causes mesenchymal-epithelial transition in rat kidney fibroblast NRK49f cells and prevents rMMP-3-induced EMT in NRK52e cells. A and B: Phase-contrast images of NRK49f cells transfected with vector control (mock) (A: Scale bar = 25 μm) or E-cadherin (B). C–L: Immunofluorescence images showing E-cadherin (C and D), cytokeratin (E and F), α-SMA (G and H: Scale bar = 10 μm), vimentin (I and J), and β-catenin (K and L) of NRK49f cells transfected with vector control (mock) (C, E, G, I, K) or E-cadherin (D, F, H, J, L). M–T: Phase-contrast (M and N) and immunofluorescence images (O–T) for E-cadherin (O and α-SMA) (Q and R), and β-catenin (S and T) of NRK52e cells stably transfected with E-cadherin construct with or without rMMP-3 (2 μg ml−1) treatment. U and V: Western blot analysis of E-cadherin and α-SMA versus β-actin in lysates of mock or E-cadherin transfected NRK49f cells. *P < 0.05.
MMP Lies Downstream of TGF-β1 in Repressing E-Cadherin Transcription
To investigate the mechanism by which TGF-β1 and rMMP-3 induced EMT in tubular epithelial cells, a luciferase assay for E-cadherin promoter activity was performed in NRK52e cells. Treatment with rMMP-3 or TGF-β1 for 24 hours repressed the E-cadherin promoter activity (Figure 5A). GM6001 abolished E-cadherin promoter repression by rMMP-3 or TGF-β1, indicating that the induction of EMT by either rMMP-3 or TGF-β1 was likely mediated via proteolytic action. An anti-TGF-β1 antibody 1D11 (30 μg ml−1) neutralized the effect of TGF-β1 on E-cadherin promoter repression, but not that of rMMP-3 repression of the E-cadherin promoter, showing that the MMP-mediated repression of E-cadherin promoter in NRK52e does not require proteolytic release and activation of latent TGF-β1,25 and works downstream of TGF-β1 (Figure 5A).
Figure 5.
MMPs lie downstream of TGF-β1 in repressing E-cadherin transcription by β-catenin. A: Relative E-cadherin promoter activity (firefly versus Renilla luciferase activities) in NRK52e cells transiently co-transfected with mouse E-cadherin promoter (−201 to +131) Firefly luciferase reporter construct and control Renilla luciferase construct, and treated with rMMP-3 proteolytic domain or TGF-β1 alone or with GM6001 (25 μmol/L) or anti-TGF-β1 Ab 1D11 (30 μg ml−1). B: Relative E-cadherin promoter activity by co-transfection with WT β-catenin or vector control. Data are expressed as mean ± SD (n = 3). P values represent statistical significance assessed by one way analysis of variance followed by post hoc Fisher PLSD test in comparison with control.
β-Catenin Represses E-Cadherin Transcription
To test the role of β-catenin in the proteolytic disruption by MMPs of E-cadherin and consequent tubular cell EMT, we co-transfected the E-cadherin promoter reporter construct with a β-catenin expression construct in the NRK52e cells. Both WT and mutant β-catenin construct (S33Y) that was stabilized by mutation of its phosphorylation site repressed the E-cadherin promoter (Figure 5B). This implicates that free β-catenin, released from E-cadherin either by shedding of its extracellular domain or by its N-terminal Ab, is capable of signaling E-cadherin repression and EMT in NRK52e cells.
Slug Operates Downstream of MMP, Induced through β-Catenin by Proteolytic Action
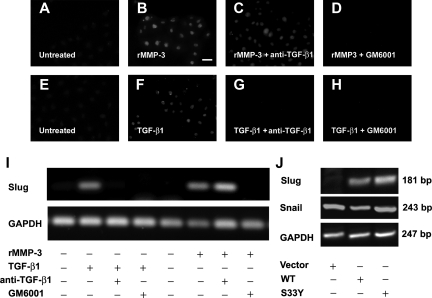
Slug, a repressor of E-cadherin promoter, was induced by rMMP-3 or TGF-β1 (Figure 6, A–H) at 24 hours. Slug transcripts appeared within 12 hours of treatment with rMMP-3 and TGF-β1 (Figure 6I), whereas Snail transcripts were not changed by the treatment (data not shown). The induction of Slug transcription by both rMMP-3 and TGF-β1 was prevented by GM6001. While anti-TGF-β1 monoclonal Ab 1D11 neutralized TGF-β1 induction of Slug transcription, it had no effect on rMMP-3 induction of Slug. Transfection of NRK52e cells by WT or mutant β-catenin (S33Y, stabilized form of β-catenin) recapitulated the induction of Slug by rMMP-3 or TGF-β1 (Figure 6J). However, Snail transcription was not affected by either WT or mutant β-catenin (S33Y) (Figure 6J).
Figure 6.
Slug operates downstream of MMPs. A–H: Immunofluorescence images showing Slug expression in NRK52e cells treated with rMMP-3 proteolytic domain (2 μg ml−1) (B: Scale bar = 10 μm) or TGF-β1(5 ng ml−1) (F) alone or with anti-TGF-β1 Ab 1D11 (30 μg ml−1) (C and G) or GM6001 (25 μmol/L) (D and H). I and J: Slug (I and J), Snail (J), and GAPDH transcripts (I and J) were analyzed by reverse transcription-polymerase chain reaction from NRK52e cells with treatments or transfection by WT or stabilized (S33Y) β-catenin constructs as indicated.
MMP Induces EMT in Tubular Epithelial NRK52e Cells via Slug
To prove that Slug is a transcription factor downstream of E-cadherin and β-catenin dominantly involved in rMMP-3 induction of EMT in NRK52e cells, we performed RNAi to silence Slug expression. Slug siRNA prevented rMMP-3-induced EMT in NRK52e cells (Figure 7). E-cadherin expression was maintained in Slug silenced NRK52e cells treated with rMMP-3 (Figure 7 B, C, J, K), but not in negative control siRNA transfected cells (Figure 7, A, J, K). rMMP-3 treatment resulted in nuclear staining of β-catenin, but cytoplasm membrane staining remained in Slug silenced NRK52e cells treated with rMMP-3 (Figure 7, G–I). De novo expression of α-SMA by rMMP-3 (Figure 7, D and F) was abrogated by Slug siRNA (Figure 7E). Gaps developed at cell-cell adhesion junctions in Slug silenced NRK52e cells treated with rMMP-3 and stained for E-cadherin (Figure 7B). This indicates that cell-cell adhesion junction was disrupted to some extent. However, E-cadherin expression was maintained when Slug was silenced. These results demonstrate that rMMP-3-induced EMT in NRK52e cells is Slug-dependent.
Figure 7.
rMMP-3 proteolytic domain-induced EMT in NRK52e cells is Slug dependent. A–I: Immunofluorescence images of NRK52e cells transfected with stealth negative control (ctr) siRNA (100 pmol) (A, D, G) or Slug siRNA oligonucleotides (50 pmol) (B, E, H) after treatment with rMMP-3 proteolytic domain (2 μg ml−1) for 72 hours. Compared with untreated cells (C, F, I), EMT was induced in NRK52e cells transfected with stealth negative control siRNA after rMMP-3 proteolytic domain treatment, as demonstrated by loss of E-cadherin (A), de novo expression of α-SMA (D: Scale bar = 10 μm), and cytoplasmic and nuclear staining of β-catenin (G), but not in NRK52e cells transfected with Slug siRNA where E-cadherin (B) and β-catenin cytoplasm membrane staining (H) remained, and no α-SMA staining was observed (E). J and K: Western blot analysis of E-cadherin, α-SMA and Slug in lysates of NRK52e cells transfected with negative control (ctr) or Slug siRNA or no oligonucleotides after treatment with rMMP-3 proteolytic domain for 3 days. *P < 0.05.
MMP-9 Mediates Slug-Dependent EMT in NRK52e Cells Downstream of TGF-β1
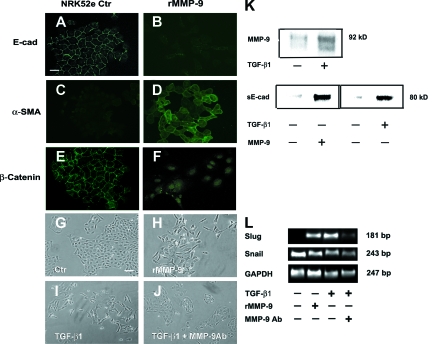
MMP-9 but not MMP-3 was up-regulated by TGF-β1 in NRK52e cells (Figure 8K), and was reported recently to be capable of proteolytic shedding of E-cadherin.29 We found that rMMP-9 shed E-cadherin (Figure 8K) and induced Slug (Figure 8L) and EMT (Figure 8, A–H) in NRK52e cells. 80-kd sE-cad was also detected from medium of NRK52e cells treated with TGF-β1 (Figure 8K), suggesting a role for MMP-9 downstream of TGF-β1. Anti-MMP-9 neutralizing Ab (10 μg ml−1) greatly reduced TGF-β1-induced EMT and induction of Slug in NRK52e cells (Figure 8, I, J, L). These data indicate that MMP-9 works downstream of TGF-β1 to mediate EMT in NRK52e cells.
Figure 8.
MMP-9 mediates Slug-dependent EMT in NRK52 cells downstream of TGF-β1. A–F: Immunofluorescence images showing E-cadherin (A and B: Scale bar = 15 μm), α-SMA (C and D) and β-catenin (E and F) of NRK52e cells treated with rMMP-9 (2 μg ml−1) for 72 hours. G–J: Phase-contrast images of NRK52e cells untreated (Ctr) (G: Scale bar = 25 μm), treated with rMMP-9 (2 μg ml−1) for 72 hours (H), or treated with TGF-β1 5 ng ml−1 for 72 hours in the absence (I) or presence (J) of MMP-9 neutralizing Ab (10 μg ml−1). K: Western blot of MMP-9 and 80-kd proteolytic ectodomain of E-cadherin (sE-cad) from medium of NRK52e cells treated by either TGF-β1 (5 ng ml−1) or rMMP-9 (2 μg ml−1) for 72 hours. Equal loading was confirmed by total protein staining with Ponceau S. L: Slug, Snail, and GAPDH transcripts from NRK52e cells with corresponding treatments were analyzed by reverse transcription-polymerase chain reaction.
MMP Up-Regulation Correlates with EMT in Kidney of Rat AN
In rat AN, a model of chronic proteinuric renal injury with marked tubulointerstitial inflammation and fibrosis, certain MMPs were up-regulated (Figure 9A). Immunohistochemical staining revealed tubular or tubular and interstitial staining of MMP-2, −3 and −9 at 4 weeks after adriamycin. In vivo evidence for EMT in tubular epithelial cells was shown by reduced E-cadherin staining, de novo staining of α-SMA and the EMT inducer Slug (Figure 9, B and C) and co-staining of MMP-9 with α-SMA in tubule cells of AN kidney (Figure 9C). It is noted that the intracellular immunofluorescence pattern for MMP-9 was different to that of α-SMA. However, those tubular epithelial cells that were positive for MMP-9 co-stained for α-SMA. Interestingly, some tubular epithelial cells negative for MMP-9 stained positive for α-SMA, suggesting that other MMPs such as MMP-3 might be expressed in those cells or MMP-9 secreted by adjacent cells might contribute to EMT induction. Interstitial cells positive for MMP-9 only could be infiltrating macrophages, which were found by us to be capable of secreting MMP-9 on activation (unpublished observation).
Figure 9.
MMPs up-regulation and EMT in diseased kidney. A: Up-regulation of MMP-2, MMP-3 and MMP-9 in kidney of rats with AN at 4 weeks by immunohistochemistry. Arrows indicate the tubular and interstitial staining (dark brown) of corresponding MMPs. B: Immunohistochemical staining (dark brown indicated by arrows) of E-cadherin, α-SMA, and Slug in kidney of AN at 4 weeks. C: Immunofluorescence double staining for MMP-9 (red) and α-SMA (green) with nuclear counterstain (Dapi) in kidney of rat with AN at 4 weeks. Arrow indicates co-staining of MMP-9 and α-SMA in tubule cells. Arrowhead indicates interstitial staining of MMP-9. Asterisk indicate tubule cells stained positive for α-SMA only. Scale bar = 50 μm.
Discussion
EMT has been recognized recently as an important source of interstitial myofibroblasts, which contribute to kidney fibrosis. However, the processes underlying conversion between tubular epithelial cells and myofibroblasts and interactions among different EMT pathways are poorly understood. In the current study, we made the novel observation that proteolytic disruption of E-cadherin by MMPs plays a role in TGF-β1-induced EMT through β-catenin signaling and Slug induction in kidney tubular epithelial cells.
Loss of E-cadherin expression has been widely recognized as an early feature of EMT. However, it has remained unresolved whether the loss of E-cadherin is merely a marker and consequence or a cause of EMT. Conacci-Sorrell et al have recently identified in colon cancer cells that E-cadherin-mediated cell-cell adhesion autoregulated E-cadherin expression by β-catenin/TCF signaling.20 We demonstrated in this study that E-cadherin autoregulation is also true of TGF-β1-induced EMT in nonmalignant tubular epithelial cells and that disruption of E-cadherin is in fact a cause of EMT.
Both Cheng et al25 and Masszi et al26 have reported that TGF-β1 could not induce EMT in the presence of cell-cell contact. Consistent with Masszi et al, we found that TGF-β1 was unable to induce EMT in confluent tubular epithelial cells. Masszi et al concluded that loss of E-cadherin-mediated cell-cell contact was permissive for EMT induction. However, their approach to disrupt cell-cell contact by removing calcium caused only partial disruption, whereas our use of specific antibody or proteolytic shedding of E-cadherin was much more potent. This difference in potency may explain the contrasting conclusions. Moreover, Mazzi et al provided no explanation for their TGF-β1-induced EMT in subconfluent epithelial cells from which calcium was not removed. Our current study has provided a plausible explanation for disruption of E-cadherin mediated cell-cell contact by MMPs downstream of TGF-β1. We found that TGF-β1 even at higher dosages could not induce EMT in confluent NRK52e cells, while rMMP-3 could do so in higher concentration. In an in vivo context of profibrotic inflammation, with the presence of multiple inflammatory cytokines and growth factors that are all capable of up-regulating MMPs, it is likely that the concentration of MMPs, ie, MMP-3 and MMP-9 in AN in the current study, would be higher than the MMP-9 induced by TGF-β1 alone in vitro.
In agreement with studies in cancer,15,30 we also demonstrated that forced expression of E-cadherin in a kidney fibroblast caused mesenchymal-epithelial transition and more importantly prevented EMT in tubular epithelial cells, further supporting an active role for E-cadherin in these processes. Ohkubo et al found that ectopic E-cadherin expression could not reverse EMT morphology in MDCK cells expressing Snail constitutively,31 indicating that Snail induces EMT downstream of E-cadherin. Li et al reported loss of E-cadherin by overexpression of Id1 may be necessary but not sufficient to induce EMT.32 It is also known that Id1 is a dominant negative antagonist of the EMT inducers Snail/Slug.33 Therefore, it is possible in Li’s study that EMT was not induced because Id1 both repressed E-cadherin and inhibited Snail/Slug. Compelling evidence for the causative role of E-cadherin in EMT has also been provided by Lehembre et al who investigated the consequences of loss of E-cadherin by shRNA knockdown of E-cadherin in MCF-7 cells or genetic ablation of E-cadherin using transgenic mice.34
β-catenin/TCF/LEF of the Wnt signaling pathway has been shown to be responsible for EMT in development and carcinogenesis.2,20,35,36 Wnt-independent β-catenin transactivation was observed due to loss of E-cadherin and consequent release of free β-catenin, mimicking Wnt signaling.10,18,20 In our study, nuclear translocation of β-catenin was observed after TGF-β1, MMP or E-cadherin antibody treatment. Repression of E-cadherin promoter by β-catenin of wild-type or stabilized mutant, together with their induction of Slug indicated a role of β-catenin in TGF-β1-induced EMT. A body of evidence including our own has shown that MMPs (MMP-3 and MMP-9 in the current study) are capable of dissociating β-catenin from E-cadherin through proteolytic shedding of the extracellular domain of E-cadherin.21,37,38
Slug (Snail 2) has a well established role as an E-cadherin repressor inducing EMT during development and in cancer.9,20,39,40,41 Our observation that Slug was induced by β-catenin was substantiated by the presence of β-catenin/LEF binding site within Slug promoter.42 Here we demonstrated for the first time that Slug was induced in rat NRK52e cells by MMP (rMMP-9 or rMMP-3) or TGF-β1, and is the downstream factor responsible for EMT induction in rat NRK52e cells. Slug is considered to be functionally equivalent to Snail both within and across species.43 However, involvement of Snail in TGF-β1-induced EMT as well as in renal fibrosis in mice44 could not be excluded by our study.
TGF-β1 is considered prototypical in EMT induction, with proposed mechanisms including β-integrin transduction,45 integrin-linked kinase,46 Phosphatidylinositol 3′-kinase /Akt,8 Smad3-dependent p38MAP kinase activation and GTPase-mediated signaling.4,47 Transcription factors identified downstream of TGF-β signaling include Snail/Slug, Twist, ZEB1 and ZEB2/Sip1, and Smads. One explanation among others for the superficial redundancy in transcription factors in TGF-β1-mediated EMT is that none of them orchestrates EMT on its own but may act synergistically in combination.48 In a highly orchestrated multistep event such as EMT, it would be undesirable if loss of E-cadherin were an isolated early event not linked to later events such as transactivation of mesenchymal genes. Smads-dependent β-catenin nuclear translocation13 and LEF/TCF signaling14 and reduction of TGF-β1-induced α-SMA expression in β-catenin null cells49 strongly suggested interaction between TGF-β1 and Wnt/β-catenin signaling. Our study demonstrated that proteolytic disruption of E-cadherin by MMPs link through E-cadherin the two distinct signaling pathways of EMT. However, the direct interaction between Smads and β-catenin although demonstrated by others13 was not investigated in the current study. Cross-talk between different pathways and transcription factors involved in sequential events of TGF-β1-induced EMT remain to be elucidated. For example, it would be relevant to investigate whether phosphatidylinositol 3′-kinase/Akt signaling, which may cause an immediate redistribution of E-cadherin away from cell-cell junctions8 and has been shown responsible for TGF-β1-induced EMT in NRK52e cells,50 is also involved in MMP-mediated E-cadherin disruption.
Radisky et al showed recently that MMP-3-induced EMT involves Rac1b and reactive oxygen species induction of Snail.51 The induction of EMT by proteolytic disruption of E-cadherin in tubular epithelial cells via Slug may or may not involve Rac1b and reactive oxygen species, which may be more relevant to carcinoma cells in which genomic instability is an important feature.
Cheng et al convincingly demonstrated that MMP-2 was sufficient to induce EMT.25 The suggested underlying mechanisms were the generation of specific biologically active extracellular matrix cleavage products or proteolytic generation of active TGF-β1,25 which could be true in vivo. However, we found by application of TGF-β1 neutralizing antibody that TGF-β1 was not involved in rMMP-3-induced EMT in NRK52e cells in vitro. In addition to the role of MMP-2 in TGF-β1-induced EMT, we showed the involvement of MMP-9 downstream of TGF-β1 through shedding of E-cadherin.
In agreement with our in vitro findings, up-regulation of relevant MMPs together with evidence of tubular cell EMT were found in the chronic interstitial fibrosis model of AN. These findings suggest a likely central role for MMPs in EMT and kidney fibrosis. However, non-specific inhibition of MMPs could be problematic as a therapeutic option to prevent kidney fibrosis, as suggested by the partial failure of MMP inhibitors in anticancer clinical trials.52 MMPs are dysregulated and are involved in virtually every aspect of inflammation and tissue repair. A detailed understanding of in vivo expression patterns of individual MMPs is necessary if they are to be targeted optimally to treat EMT and fibrosis.
In conclusion, MMP-mediated E-cadherin disruption is a key step in tubular cell EMT where E-cadherin serves as a pivotal molecule passing on signals to epithelial cells through β-catenin and Slug. Our observations suggest that specific inhibition rather than promotion of proteolytic actions of particular MMPs may offer a novel approach to treating fibrotic diseases.
Supplementary Material
Acknowledgments
We thank Dr. Eric Fearon for kindly providing huWTβ-cat Flag/pcDNA3 and huS33Yβ-cat Flag/pcDNA3 plasmids via Dr. Manisha Sharma.
Footnotes
Address reprint requests to Guoping Zheng, Centre for Transplantation and Renal Research, the University of Sydney at Westmead Millennium Institute, Westmead, NSW 2145 Australia. E-mail: guoping_zheng@wmi.usyd.edu.au.
Supported by project grant 402435 from the National Health and Medical Research Council of Australia.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Cano A P-MM, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle. 2004;3:571–573. [PubMed] [Google Scholar]
- Bienz M. Beta-catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–674. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S31–S39. [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- Batlle ESE, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgtton KL, Gow RM, Kelly DJ, Carmeliet P, Kitching AR. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int. 2004;66:68–76. doi: 10.1111/j.1523-1755.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol. 2004;165:1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Wang Y, Xiang SH, Tay YC, Wu H, Watson D, Coombes J, Rangan GK, Alexander SI, Harris DC. DNA vaccination with CCL2 DNA modified by the addition of an adjuvant epitope protects against “nonimmune” toxic renal injury. J Am Soc Nephrol. 2006;17:465–474. doi: 10.1681/ASN.2005020164. [DOI] [PubMed] [Google Scholar]
- Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Luo JH, Dedhar S, Liu Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol. 2007;18:449–460. doi: 10.1681/ASN.2006030236. [DOI] [PubMed] [Google Scholar]
- Rice R, Thesleff I, Rice DP. Regulation of Twist. Snail, and Id1 is conserved between the developing murine palate and tooth. Dev Dyn. 2005;234:28–35. doi: 10.1002/dvdy.20501. [DOI] [PubMed] [Google Scholar]
- Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, Jonkers J, Christofori G. NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 2008;27:2603–2615. doi: 10.1038/emboj.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Mei JM, Borchert GL, Donald SP, Phang JM. Matrix metalloproteinase(s) mediate(s) NO-induced dissociation of beta-catenin from membrane bound E-cadherin and formation of nuclear beta-catenin/LEF-1 complex. Carcinogenesis. 2002;23:2119–2122. doi: 10.1093/carcin/23.12.2119. [DOI] [PubMed] [Google Scholar]
- Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol. 2008;10:1021–1023. doi: 10.1038/ncb0908-1021. [DOI] [PubMed] [Google Scholar]
- Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattla JJ, Carew RM, Heljic M, Godson C, Brazil DP. Protein kinase B/Akt activity is involved in renal TGF-beta1-driven epithelial-mesenchymal transition in vitro and in vivo. Am J Physiol Renal Physiol. 2008;295:F215–F225. doi: 10.1152/ajprenal.00548.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.