Abstract
Pancreatic cancer is characterized by an intense stromal reaction. Reproducible three-dimensional in vitro systems for exploring interactions of the stroma with pancreatic cancer cells have not previously been available, prompting us to develop such a model. Cancer cells were grown on collagen/Matrigel and embedded with or without stromal cells (hTERT-immortalized human PS-1 stellate cells or MRC-5 fibroblasts) for 7 days. Proliferation and apoptosis, as well as important cell–cell adhesion and cytoskeleton-regulating proteins, were studied. PS-1 cells were confirmed as stellate based on the expression of key cytoskeletal proteins and lipid vesicles. Capan-1, and to a lesser extent PaCa-3, cells differentiated into luminal structures, exhibiting a central apoptotic core with a proliferating peripheral rim and an apico-basal polarity. Presence of either stromal cell type translocated Ezrin from apical (when stromal cells were absent) to basal aspects of cancer cells, where it was associated with invasive activity. Interestingly, the presence of ‘normal’ (not tumor-derived) stromal cells induced total tumor cell number reduction (P < 0.005) associated with a significant decrease in E-cadherin expression (P < 0.005). Conversely, β-catenin expression was up-regulated (P < 0.01) in the presence of stromal cells with predominant cytoplasmic expression. Moreover, patient samples confirmed that these data recapitulated the clinical situation. In conclusion, pancreatic organotypic culture offers a reproducible, bio-mimetic, three-dimensional in vitro model that allows examination of the interactions between stromal elements and pancreatic cancer cells.
Pancreatic cancer, with a continuing dismal prognosis despite considerable progress in understanding underlying genetic and molecular events, is characterized by an intense desmoplastic stroma.1,2,3 It is now appreciated that transformed cells interact with stromal cells, extracellular matrix proteins, and neighboring normal epithelial cells to generate feedback mechanisms essential for tumor progression.4,5 However, few models exist to enable investigators to dissect out these interactions of cancer cells with their surrounding stroma. Recently, an excellent animal model of pancreatic cancer has been created using transgenic mice with conditional pancreatic expression of mutated K-Ras; producing tumors that mimic human pancreatic intraepithelial neoplasia and full-blown cancers.6 However, the long latency period involved makes this model costly and non-amenable to rapid experimental manipulation. For many of the problems needed to be investigated in pancreatic cancer it is possible that organotypic models, where cancer cells are cultured on a “synthetic” stroma comprised of an extracellular matrix gel embedded with stromal cells, can provide a solution.7 To our knowledge, such a three-dimensional (3D) in vitro system has not yet been developed for pancreatic cancer. Therefore we aimed to establish such a model in which we could study the effect of stromal cells (pancreatic stellate cells [PSCs] and fibroblasts) on pancreatic cancer cell behavior. We have isolated a PSC line from normal human pancreas and, additionally, have used non-tumorigenic MRC-5 fibroblasts, derived from human fetal lung, which previously were validated as representative stromal cells in the absence of a pancreatic stromal cell line.8 The effects of co-culture conditions on proliferation and apoptosis, as well as the expression and subcellular distribution of key proteins regulating cell–cell interactions, such as E-cadherin,9 β-catenin,10 and members of the Ezrin-Radixin-Moesin (ERM) family,11 have been studied in pancreatic cancer cells as a means of investigating the utility of this model.
We show here that reproducible quantitative data can be derived from such assays, illuminating the role and mechanisms of epithelial–stromal interactions in modulating pancreatic cancer progression.
Materials and Methods
Isolation of PS-1, Human Telomerase Reverse Transcriptase, Immortalization of MRC-5, and PS-1 Cells
Using the outgrowth method,12 pancreatic stellate cells were isolated from an unused donated human pancreas (donation for transplantation) by the UK Human Tissue Bank (Ethics approval; Trent MREC, 05/MRE04/82). The resulting cell strain, designated PS-1, was verified as being of stellate cell origin (grown in E4:F12 medium).12,13 MRC-5 fibroblasts and PS-1 cells were immortalized by 24 hour incubation with retroviruses containing cDNA encoding human telomerase reverse transcriptase (hTERT) derived from the AM12 packaging cell line (AM12-hTERT) with empty-vector transduced controls and selected with 1 μg/ml puromycin.14 Immortalized cell telomerase activity was ascertained by the TRAP assay (Telomerase Repeat Amplification Protocol, Oncor, Inc.; manufacturer’s instructions).
Proliferation Assay
PS-1 cells were plated (3000 cells per well) in a 96-well plate coated with diluted (1:100 in PBS) collagen type I (BD Bioscience, #354236), fibronectin (Sigma Aldrich, #F0895), Matrigel (BD Bioscience, #354234), or PBS only. Cell proliferation was analyzed at day 1, 2, 4, and 6 with Cell Proliferation Reagent WST-1 (Roche Diagnostics, #5015944; manufacturer’s instructions).
Cancer Cells and Generation of Spheroids
Capan-1 and PaCa-3, well- and poorly differentiated pancreatic cancer cell lines respectively,11 (Cell Services, Cancer Research UK, London) were cultured under standard conditions. Three-dimensional multicellular spheroids of cancer cells were grown by suspending 50,000 cells in standard medium per well in 6-well plates coated overnight with poly-2-hydroxyethylmethacrylate at 6 mg/ml in ethanol (Sigma Aldrich). Established spheroids were harvested after 2 weeks. Capan-1 spheroids usually consisted of about 15 to 20 cells while PaCa-3 spheroids generally were smaller, consisting of about 10 cells.
Culturing Cancer Cells or Spheroids on the Surface of Organotypic Gels
One ml of a mixture of 5.25 volumes of collagen type I, 1.75 of Matrigel, 1 volume of 10 × Roswell Park Memorial Institut (RPMI) medium, 1 volume of filtered fetal bovine serum, and 1 volume of stromal cell suspension (5 × 105 MRC-5 or PS-1 cells) were plated into wells of a 24-well plate coated with diluted collagen type I (1:100 in PBS).7 Next day, medium was aspirated off and 5 × 105 cancer cells, or spheroids (collected by gravity sedimentation on ice), suspended in 1 ml of radio-immunoprecipitation assay medium were added on top of the gels. Experiments were in triplicate and cancer cells and spheroids were cultured on the surface of gels prepared without fibroblasts or stellate cells as a control. Medium was changed on alternate days and gels were harvested after 7 days of culture, fixed in 10% formal saline, bisected, and embedded in paraffin.
Western Blot for E-Cadherin
When medium of the organotypic cultures was changed, cells in the supernatant were collected at day 6 and 7 and analyzed for E-cadherin expression. Cells were collected by centrifugation (5 minutes, 1500 rpm) and lysed using radio-immunoprecipitation assay lysis buffer with additives as described.11 Between 5 and 10 μg of cell lysate was separated on 8% SDS-polyacrylamide electrophoresis gels and transferred to nitrocellulose membranes, which were blocked in 5% skimmed milk in 0.1% Tween20-PBS followed by incubation with primary antibody at 4°C overnight (E-cadherin 1:1000 or HSC-70 1:5000, Table 1) followed by incubation with secondary horseradish peroxidase-conjugated anti-mouse antibody. Densitometric analysis of specific bands (ECL Advance, GE Health care) was done using Image J software (Image J 1.39u).
Table 1.
Antibodies Used for the Experiments
| Primary antibody | Species raised in | Supplier | Dilution for IF | Dilution for WB |
|---|---|---|---|---|
| HSC70 | Mouse | Santacruz (#sc-7298) | N/A | 1:5000 |
| aSMA | Mouse | Dako (Clone 1A4, #M0851) | 1:300 | 1:100 |
| Vimentin | Mouse | Dako (Clone V9, #M0725) | 1:2000 | 1:250 |
| GFAP | Mouse | Sigma-Aldrich (Clone G-A-5, #G3893) | 1:500 | 1:250 |
| Desmin | Mouse | Sigma-Aldrich (Clone DE-U-10, #D1033) | 1:100 | 1:250 |
| Ki-67 | Rabbit | Novocastra (#NCL-Ki67p) | 1:200 | N/A |
| Active Caspase-3 | Mouse | R&D Systems (#AF835) | 1:100 | N/A |
| E-cadherin | Mouse | Abcam (Clone HECD-1, #ab1416) | 1:1000 | 1:200 |
| β-catenin | Mouse | BD Bioscience (Clone 14,#610154) | 1:200 | N/A |
| Ezrin | Mouse | BD Biosciences (Clone 18, #610603) | 1:200 | N/A |
| Cytokeratin | Rabbit | DAKO (#Z0622) | 1:500 | N/A |
| P_ERM | Rabbit | Cell Signalling (#3141S) | 1:100 | N/A |
| Anti-mouse HRP | Goat | Abcam (#ab5879) | N/A | 1:1000 |
| Biotinylated anti-rabbit | Swine | Dako (#E0353) | 1:500 | N/A |
| Anti-mouse-Alexa-488 | Goat | Invitrogen (#A11029) | 1:500 | N/A |
| Anti-mouse-Alexa-488 | Rabbit | Invitrogen (#A11059) | 1:500 | N/A |
| Anti-rabbit-Alexa-488 | Goat | Invitrogen (#A11034) | 1:500 | N/A |
Immunohistochemistry/Fluorescence/Oil Red O
For immunofluorescence, cells were fixed (3.7% formaldehyde), permeabilized (0.1% Triton-X100), blocked (0.1% bovine serum albumin), and incubated with primary antibody (Table 1) followed by appropriate Alexa488-labeled secondary antibody. F-actin was stained with phalloidin-tetramethylrhodamine B isothiocyanate and nuclei were visualized with 4′,6-diamidino-2-phenylindole. Oil Red O staining of fixed PS-1 cells used saturated solution of Oil Red O (Sigma Aldrich, #O-0625) in isopropanol. Vitamin A autofluorescence was detected at an excitation wavelength of 320 to 380 nm.12
For immunohistochemistry of paraffin embedded gels, 4 μm sections were de-waxed and rehydrated. Proliferation and apoptosis were assessed with antibodies to Ki-67 and active caspase-3, respectively, (antigen retrieval [10 mmol/L citrate buffer, pH 6.0]; peroxidase blocked [0.45% H2O2 in methanol]) followed by incubation with biotinylated secondary antibody. Detection was with peroxidase-labeled streptavidin (Dako, #P0397) and 3,3-diaminobenzidine (Sigma Aldrich, #D5637). Counterstain was with Mayer’s hematoxylin. For immunofluorescent staining, sections were dewaxed, rehydrated, permeabilized (0.2% Triton), blocked (2% bovine serum albumin + 0.02% fish skin gelatin + 10% fetal calf serum), and incubated with primary antibodies (Table 1) followed by appropriate Alexa488-labeled secondary antibody (1:500) and phalloidin-tetramethylrhodamine B isothiocyanate as well as 4′,6-diamidino-2-phenylindole. Controls (uniformly negative) were with appropriate isotype-specific immunoglobulins at matching dilution.
Patient Samples
Archived patient samples were obtained with prior Research Ethics Committee approval (East London & the City REC3 07/H0705/87). After quenching endogenous peroxide and blocking (normal serum), incubation was with anti-E-cadherin (1:200, antigen retrieval, 5% urea), anti-β-catenin (1:200), or matched IgG (control) followed by horseradish peroxidase-conjugated anti-mouse secondary antibody and detection of color with Vectastain ABC kit and counterstain (hematoxylin).
Image Acquisition and Rendering
Bright field images of H&E, Ki-67 and caspase-3 stained sections were taken at ×630 magnification. Immunofluorescent-stained cells and gels were visualized (×630 magnification) with a Confocal laser scanning microscope (Zeiss LSM 510, Carl Zeiss Inc.), using Immersol 518 TF oil (Zeiss). Z-stacks were rendered into three-dimensional images with Volocity software (Improvision). For movies, z-stacks of respective sections were played at 1 frame per second.
Quantification of Staining and Statistical Analysis
All cell counts and analyses were done in six random fields per gel and at least three gels from three separate experiments were analyzed. For total cell count, proliferation and apoptosis, cells were counted ×200 magnification (FEMF, TAM, independently). For Ezrin stained sections gels were scanned ×630 magnification (FEMF, HMK, independently) and cellular processes and cells within the matrix (defined as below the line of the epithelial cell mass) were counted. For β-catenin, the percentage of cells with intense membranous or cytoplasmic stain (subjectively assessed on a +, ++, or +++ range) was recorded per field. For E-cadherin, the area of green E-cadherin stain within the region of interest was determined using NIS-Elements Imaging Software, after setting thresholds for pixel intensity and area, which were kept constant to enable comparison (Nikon, NIS-Elements AR 3.0, SP3, Hotfix 4, Build 472). Data were analyzed and groups were compared using the Mann-Whitney U-test (SPSS for Windows, version 14.0.0). All comparisons were two-tailed and significance was defined as P < 0.05. For normally distributed data, Student’s t-test was applied.
Results
Characterization of Isolated Pancreatic Stellate Cells PS-1
The hTERT immortalized normal human pancreatic stellate cells universally expressed the stellate cell markers desmin, glial fibrillary acidic protein (GFAP), vimentin, and α-smooth muscle actin (αSMA), as determined by immunofluorescence, even after nearly 3 years of continuous tissue culture (Figure 1A). They also exhibited fat-containing vesicles in their cytoplasm, especially in response to retinoic acid-enriched culture medium (Figure 1B). These markers together (evident in >95% of PS-1 cells) are considered defining criteria of pancreatic stellate cells.12,13 Immortalized PS-1 cells cultured on different diluted substrates revealed no differences in proliferation or expression profiles (Figure 1C, a and b) and were comparable with primary stellate cells (data not shown).
Figure 1.
Expression of stellate cell markers by immortalized PS-1 cells and their proliferation on different substrates. A: Isolated and immortalized PS-1 cells express the stellate cell markers desmin, GFAP, αSMA, and vimentin (a–d). Scale bar = 20 μm. B: Oil Red O visualized fat storing vesicles (arrowheads) in the cytoplasm of immortalized PS-1 cells (a), which store vitamin A as detected by autofluorescent particles (b, arrows). Scale bar = 5 μm. C: (a) Proliferation of PS-1 cells is unaffected by culture on various substrates, including diluted collagen type I (C), fibronectin (F), Matrigel (M) or PBS alone (P). C (b): Western blot of key stellate cell marker, α-SMA, after culture on various substrates, alongside cell lysates from pancreatic cancer cells (HPAF, PaCa-3) and human pancreatic fibroblasts (HPF).
Morphological and Proliferative Responses of Tumor Cells Cultured on Organotypic Gels
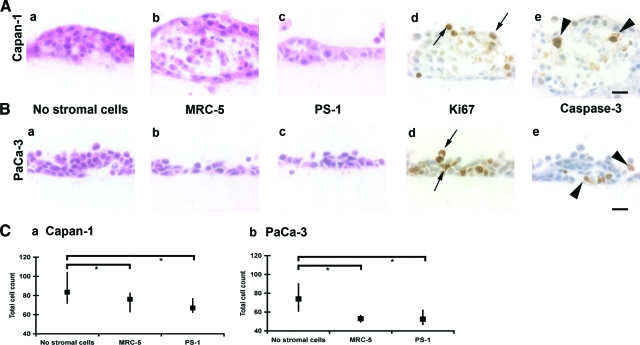
Preliminary experiments with stromal cells cultured in varying ratios of collagen type I and Matrigel and gels harvested at different time points (day 4, 7, 10, and 14) suggested that the most representative and reproducible experiments occur after 7 days of culture on 75:25 collagen I:Matrigel gels (data not shown); hence this ‘culture schedule’ was used throughout for the purpose of comparison across different cell types. Capan-1 cells cultured on gels differentiated into structures with well-defined “lumens” (Figure 2A), while PaCa-3 cells developed multicellular epithelial layers, clusters, and occasional lumen formation (Figure 2B). Ki-67 positivity revealed that proliferating cells were confined to the peripheral rims of these clusters whereas caspase-3 positivity, indicative of apoptosis, was more pronounced within the central luminal region (Figure 2, A and B,d,e). Since multicellular spheroids are thought to provide a good 3D model representing polarized, epithelial morphology,15 we additionally cultured pre-established Capan-1 and PaCa-3 spheroids on top of organotypic gels. Capan-1 spheroids, however, rapidly lost their structures on plating onto organotypic gels and the cells grew as an epithelial palisade monolayer. Unlike Capan-1 spheroids, PaCa-3 spheroids maintained their shape (see Supplemental Figure 1A, http://ajp.amjpathol.org). All these reproducible morphological changes occurred irrespective of whether the gels contained MRC-5 fibroblasts or PS-1 stellate cells.
Figure 2.
Organotypic cultures. A and B: H&E stain demonstrates differences observed in morphology after culturing Capan-1 (A) and PaCa-3 (B) cells on gels with no stromal cells (a), MRC-5 fibroblasts (b), or PS-1 stellate cells (c). Images (d) and (e) demonstrate the different zones of proliferating (Ki-67 immunostain, arrows, d) and apoptotic (active caspase-3 immunostain, arrowheads, e) cells, which leads to lumen formation. Scale bar = 20 μm. C: Total counts per high power field for Capan-1 cell gels (a) and PaCa-3 cell gels (b) at day 7 (median, interquartile range). Mann-Whitney U-test; *P < 0.05.
For both Capan-1 and PaCa-3 cell lines, the total number of cancer cells reduced significantly after seeding as cell suspensions onto gels containing MRC-5 fibroblasts (Capan-1 P < 0.05; PaCa-3 P < 0.001, Mann-Whitney U-test) or onto gels containing PS-1 cells (Capan-1 P < 0.005; PaCa-3 P < 0.001, Mann-Whitney U-test) (Figure 2C). Spheroids were unaffected by the presence of stromal cells in terms of total counts, proliferation or apoptotic indices (see Supplemental Figure 1, B–D, http://ajp.amjpathol.org). These differences in cell numbers could not be explained solely by differences in proliferation or apoptosis (see Supplemental Figure 1E, http://ajp.amjpathol.org). In all organotypic cultures the incidence of apoptosis (range, 0% to 20.2%) was very low as compared with proliferation (range 5.7% to 84.9%). We hypothesized that the changes observed could result from alterations in cell–cell and cell–matrix adhesions and we sought to evaluate this further.
Adhesion Molecule Expression of Tumor Cells on Organotypic Gels
Glandular epithelial cells are characterized by a polarized morphology, with apical poles facing a lumen, and specialized cell–cell contact. Breast cancer cells regain their apico-basal polarity when grown in 3D culture models15,16 and differentiation from single cell suspensions into multicellular luminal structures, on plating on top of organotypic gels, suggested this also was true in our model. Accordingly, we immunostained organotypic cultures for E-cadherin and phosphorylated-ERM (p-ERM).9,10,11
In both Capan-1 and PaCa-3 cultures, the cell–cell junctions were positive for E-cadherin, whereas no expression was evident where there was no cell–cell contact, such as at the cell–matrix interface (Figure 3A). These findings mimicked the breast-cancer model.15,16 In a 3D-reconstructed, cross-sectional image of a layer of Capan-1 cells, we show that, having established typical lumen-containing structures, E-cadherin positivity was located on the plasma membrane of cells forming the peripheral rim. There was no staining when the cells were in contact with the medium or with the matrix (arrowheads, inset, Figure 3A). When these luminal structures were stained for p-ERM proteins, the most positive cells (of the peripheral rim) generally were located at the junction with the lumen or at the interface with the medium (Figure 3B). There also was prominent staining where the cells were lining newly developing small lumens (sL) and where cells were in contact with the underlying matrix (arrowhead, inset). PaCa-3, which formed less obvious luminal structures, also expressed E-cadherin at cell–cell junctions (Figure 3A) and p-ERM at cell–matrix junctions (Figure 3B).
Figure 3.
Expression of E-cadherin and p-ERM. (A) and (B) show 3D reconstruction and rendering of a z-stack of immunofluorescent images of Capan-1 (a) and PaCa-3 (b) cells for E-cadherin (A) and p-ERM (B). E-cadherin is seen primarily at points of cell–cell contact while loss of E-cadherin expression is seen where cells either are in contact with the extracellular matrix (Mx, arrowheads) or with the medium. Insets show E-cadherin and p-ERM expression in an invagination of cells into the extracellular matrix. Note that E-cadherin expression is lost while p-ERM is expressed at the cell–matrix junction both in Capan-1 and PaCa-3 cells (arrow heads). P-ERM also is expressed at the margins of newly forming microlumens (sL = microlumens, L = large lumen). Scale bar = 100 μm.
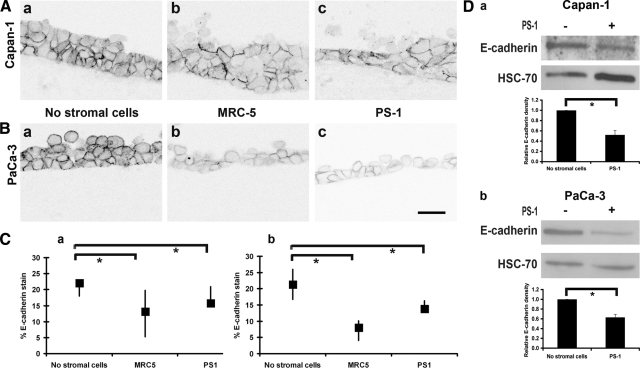
Modification of E-Cadherin and β-Catenin Expression Due to Stromal Cells
E-cadherin expression became focally patchy among the mass of cancer cells, when co-cultured with stromal cells (Figure 4, A and B), with a reduction in the total area of the E-cadherin stain (both cancer cell types) in the presence of the two stromal cell types (Figure 4C). Western blot analyses of the lysates of supernatant cells confirmed that E-cadherin expression was down-regulated in the presence of stellate cells (Figure 4D) and MRC-5 fibroblasts (data not shown). This effect occurred across the full thickness of the transformed cell, as can best be appreciated in the animation of the z-stack of these cells with a fluorescent stain for E-cadherin and nuclei counterstained with 4′,6-diamidino-2-phenylindole (see Supplemental Movie 1, http://ajp.amjpathol.org).
Figure 4.
E-cadherin expression in organotypic cultures. (A) (Capan-1) and (B) (PaCa-3) organotypic gels with no stromal cells (a), MRC-5 (b), and PS-1 (c) cells respectively with E-cadherin immunofluorescent stain rendered gray. Scale bar = 20 μm. C: Quantification of E-cadherin stain is shown in graphs (median and interquartile ranges) for Capan-1 (a) and PaCa-3 (b) cells (Mann-Whitney U-test; *P < 0.005) in response to presence of stromal cells. Supplemental Movie 1 (http://ajp.amjpathol.org) shows the animation of z-stacks across one-cell thickness, along with nuclear stain to illustrate the focal loss of E-cadherin. D: The Western blot of Capan-1 (a) and PaCa-3 (b) cells isolated from the supernatant of organotypic gels shows loss of E-cadherin in the presence of PS-1 stellate cells. Quantification of replicates is shown in respective graphs (mean ± SEM, Student’s t-test; *P < 0.05).
Conversely, β-catenin, which binds to the cytoplasmic domain of E-cadherin, showed more pronounced expression in cancer cells in the presence of stromal cells relative to when stromal cells were absent (Figure 5, A and B). Thus, both membranous and cytoplasmic β-catenin expression was observed more frequently in cancer cells in the vicinity of stromal cells. Moreover, β-catenin positivity also occurred in the cellular processes; as observed at earlier time points (4 days) in previous work where it was driven by Ezrin, a membrane–cytoskeleton linker protein.11
Figure 5.
β-Catenin expression in organotypic gels. (A) (Capan-1) and (B) (PaCa-3) organotypic gels with no stromal cells (a), MRC-5 (b), and PS-1 (c) cells respectively showing β-catenin expression by immunofluorescent stain rendered as a gray image. Quantification of number of cells with β-catenin expression (median and interquartile ranges) in the membrane and cytoplasm is shown in (d) and (e) respectively (Mann-Whitney U-test; *P < 0.005) in response to presence of stromal cells. Scale bar = 20 μm.
Ezrin Expression in Response to Stromal Cells
Following 7 days of co-culture of cancer cells on top of matrix containing fibroblasts or stellate cells, Ezrin staining was observed in the basal aspect of cells (as opposed to the apical aspect when gels were without stromal cells), in keeping with our previous observations.11 There was an increase in Ezrin-positive cellular processes invading the matrix and an increase in the number of invading cells in the presence of stromal cells as compared with gels without stromal cells (Figure 6, A and B, and Supplemental Movie 2, at http://ajp.amjpathol.org).
Figure 6.
Ezrin expression in invading processes and cells. (A) (Capan-1) and (B) (PaCa-3) organotypic gels with no stromal cells (a), MRC-5 (b), and PS-1 (c) cells respectively with Ezrin immunofluorescent stain rendered as a gray image and the invading cells and processes shown by arrows. Quantification of number of Ezrin-positive processes (median and interquartile ranges) of cells (d), as well as whole cells (e) shows a significant increase of these (Mann-Whitney U-test; *P < 0.005) in response to the presence of stromal cells, which did not stain for Ezrin. Supplemental Movie 2 (http://ajp.amjpathol.org) shows the animation of z-stacks across one-cell thickness, along with nuclear stain to demonstrate the depth of invasion. Scale bar = 20 μm.
We also investigated the possibility of changes in other markers for epithelial–mesenchymal transition such as cytokeratin,17 which was reduced on exposure to stromal cells, and vimentin,17 which was not expressed in cancer cells even in the presence of stromal cells (which uniformly expressed this marker) (see Supplemental Figure 2, http://ajp.amjpathol.org).
E-Cadherin and β-Catenin Expression in Pancreatic Cancer Tissues
E-cadherin and β-catenin staining in organotypic cultures seemed to mimic the situation observed in clinical specimens. Table 2 summarizes the clinical details of 51 patients with pancreatic ductal adenocarcinoma (PDAC); the median age was 66.5 (range, 45 to 81), male: female ratio was 31:20, median follow-up was 26 months, and median survival was 18.6 months after surgery and adjuvant therapy. With β-catenin there was minimal staining of the normal pancreatic small ducts. In contrast, there was a demonstrable increase in the cytoplasmic staining of cancer cells in 18 of the 51 patients studied, with a minority of specimens exhibiting nuclear localization (Table 3 and Figure 7A). Most normal pancreatic ducts demonstrated strong membranous E-cadherin expression, which was reduced in cancers.18,19 Additionally, E-cadherin expression was more cytoplasmic in carcinoma cells (Table 3 and Figure 7B). Changes in β-catenin and E-cadherin expression were not related to any significant survival difference (Table 3). These changes were demonstrable in all stages of pancreatic cancer progression: invasion into surrounding stroma, peri-neural invasion and lymph node metastasis (Figure 7).
Table 2.
Clinical Details Relating to Patient Samples
| Log-rank (Mantel-Cox)* | ||
|---|---|---|
| T stage | ||
| T1 | 8 | 0.259 |
| T2 | 15 | |
| T3 | 28 | |
| N stage | ||
| N0 | 21 | 0.676 |
| N1 | 30 | |
| Differentiation | ||
| Well | 6 | 0.062 |
| Moderate | 26 | |
| Poor | 19 | |
| Venous and Neural | ||
| Nil | 4 | 0.936 |
| Venous | 15 | |
| Neural | 13 | |
| Both | 19 | |
| Resection margin | ||
| Negative | 18 | 0.045 |
| Positive | 33 | |
| Chemoradiotherapy | ||
| None | 11 | 0.807 |
| Chemotherapy | 22 | |
| Radiotherapy | 8 | |
| Both | 10 |
Comparison of Kaplan-Meier survival curves across sub-groups for each variable known to affect survival of patients with pancreatic ductal adenocarcinoma.
Table 3.
β-Catenin and E-Cadherin Immuno-Staining of Pancreatic Ductal Tissues
| Normal (%) | PDAC (%) | Log-rank (Mantel-Cox) PDAC* | |
|---|---|---|---|
| β-catenin | |||
| Total score | |||
| Weak (0–4) | 15 (83.3) | 28 (44.9) | 0.425 |
| Strong (5–6) | 3 (16.7) | 23 (45.1) | |
| Membranous | |||
| Absent | 13 (72.2) | 34 (66.6) | 0.597 |
| Present | 5 (27.8) | 17 (33.3) | |
| Cytoplasmic | |||
| Absent | 18 (100) | 33 (74.8) | 0.514 |
| Present | 0 (0) | 18 (35.2) | |
| E-cadherin | |||
| Total score | |||
| Weak (0–4) | 8 (44.4) | 34 (66.6) | 0.344 |
| Strong (5–6) | 10 (55.6) | 17 (33.3) | |
| Membranous | |||
| Absent | 5 (27.8) | 28 (54.9) | 0.630 |
| Present | 13 (72.2) | 23 (45.1) | |
| Cytoplasmic | |||
| Absent | 15 (83.3) | 30 (58.8) | 0.919 |
| Present | 3 (16.7) | 21 (41.2) |
Comparison of Kaplan-Meier survival curves for different staining patterns in patients with pancreatic ductal adenocarcinoma. Normal, normal pancreatic tissue, score for small pancreatic ducts; PDAC, Pancreatic ductal adenocarcinoma.
Figure 7.
β-catenin and E-cadherin expression in patient samples. A: Shows β-catenin expression in normal (a) and pancreatic cancer ducts (b, c, d). A(a) demonstrates no expression in small ducts of normal pancreas (arrowheads), with robust cell–cell junction expression in acinar cells (arrows). A(b, c, d) show heterogeneous expression of β-catenin at the cell–cell junctions, cytoplasm (arrowheads), and nucleus (arrows) when cells are invading the surrounding stroma (b), the perineural region (c, N = nerve), and lymph nodes (d, LN = lymph node). Scale bar = 20 μm. (B) shows E-cadherin expression in normal (a) and pancreatic cancer ducts (b, c, d). B(a) demonstrates strong membranous expression in ducts of normal pancreas (arrowheads) and in acinar cells (arrow). B(b, c, d) show heterogeneous expression of E-cadherin with pancreatic ductal adenocarcinoma cells not expressing E-cadherin (arrowheads) or a more cytoplasmic expression (arrows) when invading the surrounding stroma (b), the perineural region (c, N = nerve) and lymph nodes (d, LN = lymph node). Scale bar = 20 μm.
Discussion
Pancreatic organotypic culture, described here, provides the first, robust in vitro model, which could be used to dissect out interactions of pancreatic cancer cells with the extracellular matrix and ‘normal’ stromal cells in a biomimetic system. Pancreatic ductal adenocarcinoma is characterized by an intense desmoplastic stroma, that can account for a large proportion of the tumor mass.20 This abundant stroma, composed of extracellular matrix (mainly collagen type I) and stromal cells (predominantly activated fibroblasts and stellate cells), has been thought to participate actively in tumor development and progression but has not been studied in a 3D, physiologically relevant, experimental system.21,22 We demonstrate, using this model, the ability of stromal cells to modulate E-cadherin, β-catenin and Ezrin expression in cancer cells. Our model has, therefore, the potential to investigate the impact and roles of stromal components on pancreatic cancer cells in an easily controllable system that could reduce the need for animal experimentation.
Organotypic Culture Models
Organotypic models have been used extensively in skin, esophageal, brain, and breast cancers, where they have helped in determining the mechanisms of tumor invasion,4,7,23 the effect of drugs,24 the impact of radiotherapy,25 as well as normal physiological functions.16,26 However, in pancreatic research, the use of 3D models has been limited. Pancreatic endocrine tissue has been cultured as organotypic slices from perinatal mouse embryos27 and pancreatic cancer cell lines have been cultured on type I collagen-glycosaminoglycan scaffolds28 and in Matrigel.29 Little attention hitherto has been paid to the likely contribution of stromal cells, such a characteristic of pancreatic cancer, to tumor cell behavior.
PSCs have been identified as the key cells responsible for the stromal reaction in pancreatic cancer and chronic pancreatitis, and a small proportion of these cells may have extra-pancreatic origin.30,31,32 Recently, two other groups have developed immortalized human PSC lines.22,33 However these lines (both were tumor-derived) did not express key stellate cell proteins including desmin and GFAP. Moreover large T-cell antigen (SV40), which can induce phenotypic and genetic changes,34 was used for immortalization. The possibility must exist therefore that these lines are not truly representative of characteristic normal (not tumor-associated) stellate cells. Our PSC line, termed PS-1, was sourced from normal human pancreas, immortalized with hTERT and exhibited all PSC characteristics such as lipid droplets and expression of key cytoskeletal proteins (Figure 1).12,13 Moreover, the use of a relatively ‘normal’ stellate cell line might be advantageous to dissect out the ‘early’ events in cancer–stroma cross talk.
Morphology of Pancreatic Cancer Cells in Organotypic Cultures
The well-differentiated pancreatic carcinoma cells Capan-1 and, to a lesser extent, PaCa-3 cells, developed into structures resembling ducts with a lumen when cultured on top of extracellular matrix gels (irrespective of the presence of stromal cells). The luminal space was occupied by loosely packed, apoptotic cells while the luminal wall consisted of a well-organized multicellular, proliferative layer. Staining for ERM proteins indicated a distinct apico-basal polarity of epithelial cells forming the lumen. ERM protein family members bind to the actin cytoskeleton and play a role in cell adhesion, orientation, and motility.35,36 Recent studies suggest that ERM proteins are essential for cavity formation during embryogenesis of the digestive tract.35 Here, p-ERM, the active form of ERM proteins, occurred mainly in cells lining the large luminal space (Figure 3). Interestingly p-ERM also was concentrated at the margins of newly forming microlumens when observed in three-dimensionally rendered images, which suggests that the role of p-ERM here is similar to its role in developmental processes.35
We additionally cultured Capan-1 and PaCa-3 spheroids (formed on poly-2-hydroxyethylmethacrylate) on top of organotypic gels containing MRC-5 fibroblasts or stellate cells (Supplemental Figure 1, http://ajp.amjpathol.org). Interestingly, Capan-1 spheroids flattened when cultured on top of extracellular matrix, irrespective of the presence or absence of stromal cells, possibly suggesting that anoikis-induced lumen formation (on poly-2-hydroxyethylmethacrylate) employs different biological mechanisms from physiologically relevant systems (on extracellular matrix). Pre-established spheroids’ shape is disrupted when in contact with extracellular matrix.15 Given this, and that single cell suspensions differentiated into spherical 3D structures resembling neoplastic pancreatic ductal epithelium, we discontinued using pre-established spheroids.
Changes Evoked by Stromal Cell Presence
For both cancer cell lines, fewer cells were observed when they were cultured as a monolayer in organotypic culture with either type of stromal cell. This result was surprising, since activated stellate cells supposedly stimulate tumor growth in vivo and in vitro.21,22,37 Though such findings may be due to several factors, we speculated that a reduction in cell–cell or cell–matrix adhesion, due to loss of membranous E-cadherin, led to cancer cell loss (see Supplemental Figure 3, http://ajp.amjpathol.org). We were unable to show much impact of stromal cell presence on proliferative indices but showed significant changes in levels and distribution of various cell adhesion-related molecules. Thus, E-cadherin at cell–cell junctions was down-regulated (Figure 4) with a concomitant increase in cytoplasmic β-catenin expression (Figure 5). Such a reduction in E-cadherin has been demonstrated at tumor edges of pancreatic cancer,18 as well as in pancreatic cancers with an undifferentiated phenotype.19 We have not yet used tumor-derived stellate cells/fibroblasts in our assay since this methodology has inherent limitations and advantages. Tumor-derived fibroblasts are altered phenotypically and may therefore not represent the ‘early’ cross talk between cancer and stromal cells. Further experiments are now underway to explore whether tumor-derived, as distinct from normal, stromal cells influence the cancer cells differently. Interestingly, in colorectal cancer, it has been suggested that on dissociation of β-catenin from E-cadherin, β-catenin binds to actinin-438 and that this change in molecular binding partners occurs when tumor cells infiltrate surrounding stroma. While we have not addressed in our system the mechanisms responsible for these changes directly, they could relate to the Wnt signaling pathway, known to be dysregulated in pancreatic cancer.3
E-Cadherin and β-Catenin
β-Catenin mutations typically are absent in the more common pancreatic ductal adenocarcinoma but frequently are seen in rarer solid pseudo-papillary tumors.39 Recent animal models suggest that activation of Wnt signaling in pancreatic ductal PDAC, possibly downstream of the Hedgehog pathway, may be important.10,39,40 Up to 13% of patients with PDAC demonstrate nuclear localization of β-catenin and 78% show cytoplasmic localization.10 In our patient samples, we found similar patterns with no β-catenin expression in the small ducts of normal pancreas. Tumors differ from normal tissue not only in epithelial cell morphology, but also in the amount of stromal response; however, the influence of the stromal compartment on the eventual outcome of PDAC development has not been studied. We show here that presence of stromal cells leads to stabilization of β-catenin. Recently, Wang et al have suggested that overexpression of ataxia-telangiectasia group D complementing gene, due to unknown mechanisms, leads to β-catenin stabilization in pancreatic cancer.41 The possibility therefore exists that stromal cells, such as stellate cells, may contribute to this phenomenon in cancer cells. Convergence of E-cadherin, β-catenin, and Wnt signaling may explain, in part, our observations in the organotypic cultures and we are investigating this possibility.42,43 Additionally, Brabletz and colleagues recently have suggested that, predominantly in colorectal cancers, epithelial–mesenchymal transition is dominant at the invasive tumor edge.44 This is stimulated by unknown microenvironmental factors resulting in an increase in β-catenin expression with concomitant decrease in E-cadherin expression.44 It is plausible that ‘epithelial–mesenchymal transition’-like changes in pancreatic cancer are, at least in part, induced by stromal cells, as demonstrated by us here, and that these need to be targeted to halt tumor progression. Perhaps arguing against this concept of epithelial–mesenchymal transition as being a driving force in our studies is the fact that we failed to detect positivity for vimentin expression in the transformed cells (see Supplemental Figure 2, http://ajp.amjpathol.org).
Ezrin
Translocation of Ezrin, from the apical to the basal compartment of cancer cells, was instigated by stromal cell presence in our model. We previously described similar findings, in patient samples, during transition from early pancreatic neoplastic lesions to cancer.11 Moreover, stromal cells are instrumental in the appearance of invasive processes, derived from cancer cells, which protrude into the underlying stroma in the organotypic culture (Figure 6). The reciprocal relationship of Ezrin and E-cadherin has been detailed in mouse, fly, and C. elegans development leading to proper alignment of actin fibers, cellular orientation, and apico-basal polarity.45,46,47 In Drosophila embryos, which have only Moesin (of the three ERM proteins), Ezrin presence disrupts E-cadherin distribution and actin assembly at adherens junctions, through recruitment of btsz (bitesize), a synaptotagmin-like protein.45 In early mouse embryos, phosphorylated Ezrin controls E-cadherin expression reciprocally in baso-lateral and apical membranes.46 Similarly, in C. elegans, control of intestinal tube formation and integrity is mediated by E-cadherin and ERM homologs; in this instance controlled by crumbs and Disk large.47 The many similarities between clinical specimens and organotypic cultures shown by us suggest that stromal cells may be contributing to the malignant behavior of cancer cells by modulating cell–cell adhesion, perhaps through epithelial–mesenchymal transition and Ezrin re-distribution, resulting in invasion.
In summary, we describe an organotypic model, which could prove invaluable for studying various aspects of pancreatic tumor progression without the need for experimental animals. This pancreatic cancer model demonstrates that, even in our overly simplified system containing only cancer cells, stromal cells and matrix proteins (three most prominent aspects), there is cellular cross talk between various components. The model provides a means for testing hypotheses and dissecting out mechanisms involved in cancer progression as well as the potential to incorporate other matrix components, such as immune and endothelial cells, to further investigate epithelial-stromal interactions and to test drugs targeting specific compartments of cancer and/or stroma.
Supplementary Material
Acknowledgments
We are grateful to Prof Hans Clevers (Director, Hubrecht Institute, Utrecht, The Netherlands) and Dr. John F. Marshall, Prof Gareth J. Thomas, and Mr. Sabari Vallath (Tumor Biology Lab, Charterhouse Square, London) for their helpful discussions and critical review of the manuscript. We thank Ms. Jennifer Sandle and Dr. Ningfeng F. Li for help with immortalization; Dr. Joanne Chin-Aloeng and Dr. James Norman for staining with Oil Red O; Mr. Andrew Clear for help in creating tissue micro arrays and Ms. Emma Nye for help with quantification of the immunofluorescent stain.
Footnotes
Address reprint requests to Hemant M. Kocher, M.S., M.D., F.R.C.S., Centre for Tumour Biology, Institute of Cancer, Barts and the London School of Medicine and Dentistry, Charterhouse Square, London EC1M 6BQ. E-mail: Hemant.Kocher@bartsandthelondon.nhs.uk. or h.kocher@qmul.ac.uk.
Supported by Research Advisory Board Ph.D. studentship (F.E.M.F.) and by the Department of Health (UK) Clinician Scientist fellowship (H.M.K.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- O'Sullivan A, Kocher HM: Pancreatic cancer. BMJ Clin Evid 2007, 11:409–436. Available online at http://clinicalevidence.bmj.com (accessed Oct 2008). Subscription only. [PubMed] [Google Scholar]
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Nystrom ML, Thomas GJ, Stone M, Mackenzie IC, Hart IR, Marshall JF. Development of a quantitative method to analyse tumour cell invasion in organotypic culture. J Pathol. 2005;205:468–475. doi: 10.1002/path.1716. [DOI] [PubMed] [Google Scholar]
- Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–4671. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, Segara D, Dawson AC, Kench JG, Henshall SM, Sutherland RL, Dlugosz A, Rustgi AK, Hebrok M. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher HM, Sandle J, Mirza TA, Li NF, Hart IR. Ezrin interacts with cortactin to form podosomal rosettes in pancreatic cancer cells. Gut. 2009;58:271–284. doi: 10.1136/gut.2008.159871. [DOI] [PubMed] [Google Scholar]
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NF, Kocher HM, Salako MA, Obermueller E, Sandle J, Balkwill F. A novel function of colony-stimulating factor 1 receptor in hTERT immortalization of human epithelial cells. Oncogene. 2009;28:773–780. doi: 10.1038/onc.2008.412. [DOI] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyasu H, Ellis LM, Evans DB, Abbruzzese JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E, Fidler IJ. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin Cancer Res. 1999;5:25–33. [PubMed] [Google Scholar]
- Winter JM, Ting AH, Vilardell F, Gallmeier E, Baylin SB, Hruban RH, Kern SE, Iacobuzio-Donahue CA. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, Johnstone CN, Klein-Szanto AJ, El-Deiry WS, Cukierman E, Herlyn M, Rustgi AK. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A, Frozza RL, Jager E, Figueiro F, Bavaresco L, Salbego C, Pohlmann AR, Guterres SS, Battastini AM. Selective cytotoxicity of indomethacin and indomethacin ethyl ester-loaded nanocapsules against glioma cell lines: an in vitro study. Eur J Pharmacol. 2008;586:24–34. doi: 10.1016/j.ejphar.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Savas A, Warnke PC, Ginap T, Feuerstein TJ, Ostertag CB. The effects of continuous and single-dose radiation on choline uptake in organotypic tissue slice cultures of rabbit hippocampus. Neurol Res. 2001;23:669–675. doi: 10.1179/016164101101199018. [DOI] [PubMed] [Google Scholar]
- Hebner C, Weaver VM, Debnath J. Modeling Morphogenesis and Oncogenesis in Three-Dimensional Breast Epithelial Cultures. Annu Rev Pathol. 2008;3:313–339. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- Meneghel-Rozzo T, Rozzo A, Poppi L, Rupnik M. In vivo and in vitro development of mouse pancreatic beta-cells in organotypic slices. Cell Tissue Res. 2004;316:295–303. doi: 10.1007/s00441-004-0886-6. [DOI] [PubMed] [Google Scholar]
- Grzesiak JJ, Bouvet M. Determination of the ligand-binding specificities of the alpha2beta1 and alpha1beta1 integrins in a novel 3-dimensional in vitro model of pancreatic cancer. Pancreas. 2007;34:220–228. doi: 10.1097/01.mpa.0000250129.64650.f6. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun. 2007;358:698–703. doi: 10.1016/j.bbrc.2007.04.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache F, Pendyala S, Bhagat G, Betz KS, Song Z, Wang TC. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57:1113–1120. doi: 10.1136/gut.2007.143271. [DOI] [PubMed] [Google Scholar]
- Pinzani M. Pancreatic stellate cells: new kids become mature. Gut. 2006;55:12–14. doi: 10.1136/gut.2005.074427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, Apte MV. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- Jesnowski R, Furst D, Ringel J, Chen Y, Schrodel A, Kleeff J, Kolb A, Schareck WD, Lohr M. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005;85:1276–1291. doi: 10.1038/labinvest.3700329. [DOI] [PubMed] [Google Scholar]
- Li NF, Broad S, Lu YJ, Yang JS, Watson R, Hagemann T, Wilbanks G, Jacobs I, Balkwill F, Dafou D, Gayther SA. Human ovarian surface epithelial cells immortalized with hTERT maintain functional pRb and p53 expression. Cell Prolif. 2007;40:780–794. doi: 10.1111/j.1365-2184.2007.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SC, Fehon RG. Understanding ERM proteins–the awesome power of genetics finally brought to bear. Curr Opin Cell Biol. 2007;19:51–56. doi: 10.1016/j.ceb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, Moller P, Gschwend JE, Seufferlein T, Gress T, Adler G, Bachem MG. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512–519. doi: 10.1242/jcs.03347. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Honda K, Idogawa M, Ino Y, Ono M, Tsuchida A, Aoki T, Hirohashi S, Yamada T. E-cadherin regulates the association between beta-catenin and actinin-4. Cancer Res. 2005;65:8836–8845. doi: 10.1158/0008-5472.CAN-05-0718. [DOI] [PubMed] [Google Scholar]
- Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133:2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, Fearon ER, Ljungman M, Simeone DM. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15:207–219. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature. 2006;442:580–584. doi: 10.1038/nature04935. [DOI] [PubMed] [Google Scholar]
- Dard N, Louvet-Vallee S, Santa-Maria A, Maro B. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol. 2004;271:87–97. doi: 10.1016/j.ydbio.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Segbert C, Johnson K, Theres C, van Furden D, Bossinger O. Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev Biol. 2004;266:17–26. doi: 10.1016/j.ydbio.2003.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.