Abstract
Age-related macular degeneration (AMD) is one of the leading cause of blindness among the elderly; however, current therapy options are limited. Epidemiological studies have shown that a diet that is high in ω-3 polyunsaturated (n-3) fatty acids can slow disease progression in patients with advanced AMD. In this study, we evaluated the effect of such a diet on the retinas of Ccl2−/−/Cx3cr1−/− mice, a model that develops AMD-like retinal lesions that include focal deep retinal lesions, abnormal retinal pigment epithelium, photoreceptor degeneration, and A2E accumulation. Ccl2−/−/Cx3cr1−/− mice that ingested a high n-3 fatty acid diet showed a slower progression of retinal lesions compared with the low n-3 fatty acids group. Some mice that were given high levels of n-3 fatty acids had lesion reversion. We found a shunted arachidonic acid metabolism that resulted in decreased pro-inflammatory derivatives (prostaglandin E2 and leukotriene B4) and an increased anti-inflammatory derivative (prostaglandin D2). We also measured lower ocular TNF-α and IL-6 transcript levels in the mice fed a diet of high n-3 fatty acids. Our findings in these mice are in line with human studies of AMD risk reduction by long-chain n-3 fatty acids. This murine model provides a useful tool to evaluate therapies that might delay the development of AMD.
Age-related macular degeneration (AMD) is the most common cause of legal blindness of elderly people in the world.1 The pathological features of AMD involve the destruction and deterioration of the photoreceptor and retinal pigment epithelium (RPE) specific to the macula. There are two types of AMD, the exudative or ‘wet’ form with choroidal neovascularization, and the atrophic or ‘dry’ form with atrophy of the photoreceptors and RPE. To date, except for the suppression of choroidal neovascularization of the late stage wet form, there is no definitive treatment for AMD. Care for earlier stage AMD and dry AMD is limited to risk factor management. Cessation of smoking, reduction in body mass, and taking specific vitamins and other nutrient supplements may help to slow disease progression.2
Omega-3 and ω-6 polyunsaturated fatty acids (n-3 PUFAs, or n-3 fatty acids; and n-6 PUFAs, or n-6 fatty acids) are two classes of PUFAs, which are metabolically and functionally distinct and often have opposing physiological effects. Mammals depend on dietary intake of n-3 fatty acids through sources such as fish oil, because mammalian cells lack enzymes necessary to synthesize the 18-C precursor of n-3 fatty acids and to convert n-6 to n-3 fatty acids.3 n-3 fatty acids are highly concentrated in the brain and retina and are believed to be important in neuronal development and damage repair.4 Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA, the precursor to DHA) are two major n-3 fatty acids and are concentrated in retina and retinal vascular endothelium.5 Photoreceptors are abundantly enriched in DHA with amounts up to approximately 50% of photoreceptor rod outer segment lipids.6 Vital retinal functions depend on the existence of an adequate proportion of DHA in retinal lipids.7 DHA prevents the apoptosis of photoreceptor cells that otherwise inevitably occurs during their early development in vitro.8
The n-3 fatty acids have been shown to exert many preventive and therapeutic actions for an array of diseases such as atherosclerosis and are recommended as a risk management option for AMD.2 Epidemiological retrospective studies of n-3 fatty acids or fish intake on the prevalence of advanced AMD suggest a protective relationship.5,9,10,11,12 However, no human clinical trials or intervention studies using an AMD animal model have evaluated the effect of n-3 fatty acids on AMD or AMD-like pathology in the literature.
We reported recently that Ccl2−/−/Cx3cr1−/− mice developed a broad spectrum of AMD-like pathologies with early onset and high penetrance.13,14 In this study, we hypothesized that dietary intake of n-3 fatty acids would alleviate the retinal lesions which develop spontaneously in Ccl2−/−/Cx3cr1−/− mice. To test this hypothesis, we raised the mice on diets that were low or high in n-3 fatty acids and measured a clinical endpoint (funduscopy), histopathology, as well as the level of 2-[2,6-dimethyl-8-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1E,3E,5E,7E-octatetra-enyl]−1-(2-hydroxyethyl)−4-[4-methyl-6(2,6,6-trimethyl-1-cyclohexen-1-yl) 1E,3E,5E,7E-hexatrienyl]-pyridinium (A2E) to evaluate the effects of those diets.
Materials and Methods
Animals
Ccl2−/−/Cx3cr1−/− mice and wild-type control (C57BL/6) were bred in-house. The study was conducted in compliance with the Association for Research in Vision and Ophthalmology statement for the use of animals, and all animal experiments were performed under protocols approved by the National Eye Institute’s Institutional Animal Care and Use Committee.
Experimental Protocol
Ccl2−/−/Cx3cr1−/− breeding pairs were divided into two groups and fed diets through pregnancy and lactation that were either high or low in long chain n-3 fatty acid content. The pups were weaned onto the same diets as their dams. The two pelleted diets used (provided by Dyets Inc., Bethlehem PA) were based on AIN-93G formulations with several modifications to obtain the low and high n-3 fatty acid levels required in this study.15 The two diets contained 10% fat by weight and had a similar content of linoleic acid and α-linolenic acid with EPA (C20:5n-3) and DHA (C22:6n-3), along with docosapentaenoic acid (C22:5n-3), as the nutritional variables. To confirm their fatty acid content, lipid profiles were performed for both diets before administration to the mice. The lipid composition for the major fatty acids present in each diet is indicated in Table 1. The efficiency of altering the n-3 fatty acids content in mice by those diets has been tested by measuring the n-3:n-6 ratio in the milk of the mother and the retina of pups in a similar study using identical diets as in this study.16 In addition, the lipid composition of the retina from mice on the two diets was measured with transmethylation and gas chromatography at 12 weeks of age as described previously.17
Table 1.
Nutrient and Fatty Acid Composition of Pelleted Diets Given to High n-3 Fatty Acid and Low n-3 Fatty Acid Groups
| Ingredient | Amount (g/kg) |
|---|---|
| Casein, ALACID, Vitamin free* | 200 |
| Carbohydrate: | 600 |
| Corn starch | 150 |
| Sucrose | 100 |
| Dextrose | 199 |
| Maltose-dextrin | 150 |
| Cellulose | 50 |
| Mineral and salt mix† | 35 |
| Vitamin mix‡ | 10 |
| l-Cystine | 3 |
| Choline bitatrate | 2.5 |
| Fatty acids | Low n-3 fatty acids (g/kg) | High n-3 fatty acids (g/kg) |
|---|---|---|
| Menhaden oil§ | — | 18 |
| Olive oil | 56 | 43.2 |
| Flaxseed oil | 0.5 | — |
| Safflower oil | 11.8 | 12.8 |
| Hydrogenated coconut oil | 31.7 | 26 |
| TBHQ¶ | 0.00374 | — |
| Mixed Tocopherol∥ | 0.0187 | — |
| Fatty acid composition | wt % of fatty acids | wt % of fatty acids |
|---|---|---|
| ∑ Saturated | 38.5 | 37.2 |
| ∑ monounsaturated | 45.5 | 39.3 |
| 18:2n-6 fatty acids (LA) | 14.8 | 14.6 |
| 18:3n-3 fatty acids (α-LNA) | 0.68 | 0.66 |
| 20:5n-3 fatty acids (EPA) | 0.09 | 1.9 |
| 22:5n-3 fatty acids (DPA) | — | 0.4 |
| 22:6n-3 fatty acids (DHA) | — | 1.9 |
| n-6/n-3 fatty acids | 19.2 | 2.9 |
ALACID casein (NZMP North America Inc, Santa Rosa, CA).
Dyets Inc., Bethlehem, PA. catalogue #210025.
Dyets Inc., Bethlehem, PA. catalogue #310025.
Omega Protein, Inc. (ingredient declaration: Menhaden oil, Tocopherol, TBHQ; fatty acid composition: wt % of Menhaden oil: 1.8% 18:3n3; 11% 20:5n-3; 2.2% 22:5n-3; 11.4% 22:6n-3).
TBHQ is tert-butylhydroquinone (Eastman Chemical Company, Kingsport, Tenneessee).
Cognis Nutrition, Cincinnati, OH.
Mice were fed until 8 months of age with either a high n-3 fatty acid or low n-3 fatty acid diet until being harvested for histology as well as other testing. Some were fed continuously until up to 12 months of age. Since we did not observe any retinal lesions in the wild-type (C57BL/6) control mice in our previous studies,13,14 the wild-type mouse was not included in our feeding experiment, but age-matched tissue samples of wild-type mice fed with a regular diet were used as the reference for certain measurements.
Fundus Photography
After pupil dilation, sequential funduscopic examinations were performed every 5 weeks from 3 to 5 weeks of age until 8 months of age, using a Kowa fundus camera (Kowa Optimed, Torrance, CA) and Volk 90D lens (Volk Optical, Mentor, OH) following intraperitoneal injection of ketamine (1.4 mg/mouse) and xylazine (0.12 mg/mouse) for systemic anesthesia and topical administration of 1% tropicamide ophthalmic solution (Alcon Inc, Fort Worth, Texas) for pupil dilation. All mice were identified with ear tags, each photograph was labeled, and the record was kept. We evaluated the lesion change by comparing the sequential photos taken in the same fundus area. Progression was defined as >10% increase of deep retinal and subretinal spot (lesion) number, >50% increase in spot diameter in at least 1/3 of the spots, >5 fused spots, or appearance of >2 chorioretinal scars in comparison with the previous observation. Regression was defined as >10% decrease of deep retinal lesions’ number, >50% decrease in spot diameter in at least 1/3 of the spots. To avoid a subjective bias, evaluation of the pictures was conducted without knowledge of the treatment by a masked observer. Each individual lesion was identified and viewed in sequential fundus photographs of that eye.
Histopathology
Eyes were harvested following euthanasia of the mice after 8 months on the diet. The tissues were fixed in 10% formalin for at least 24 hours. All tissues were then embedded in methacrylate. The eyes were serially sectioned in the vertical pupillary-optic nerve plane. Each eye was cut into 6 sections. All sections were stained with hematoxylin and eosin. If an ocular lesion was observed, another 6 to 12 sections would be cut through the lesion. These slides were also stained with Periodic Acid Schiff to highlight Bruch’s membrane and the basement membrane of small neovascular vessels.
Transmission Electron Microscopy
Electron microscopy was performed on 4% glutaraldehyde-formalin fixed whole eyes. The fixed neuroretina-RPE-choroid tissue was embedded in Ladd LX-112 epoxy resin. Six 1 μm-thick sections stained with toluidine blue were examined under light microscopy. Based on the lesions shown on the thick sections, ultrathin sections of these lesions were taken and were stained with uranyl acetate and lead citrate for examination under a JEOL/JEM-100B microscope. Two eyes in each diet group were used for transmission electron microscopic study.
A2E Extraction and Quantification
A2E is the major component of lipofuscin fluorophores generated from visual cycle flux of all-trans-retinal. The molecule is particularly relevant to aging and AMD pathogenesis.18 The mice were kept in the dark for >12 hours before being sacrificed. Whole eyes were removed in a dark room under dim red light and homogenized. At least three eyes were pooled for each measurement. A2E was extracted with chloroform/methanol as previously described.19 Detection and quantification was performed by liquid-chromatography mass spectrometry (LC/MS/MS) using a QTRAP 2000 linear ion trap tandem mass spectrometer (Applied Biosystems/MDS SCIEX, Concord, Ontario, Canada) with an Agilent 1100 LC system (Agilent, Wilmington, DE). A gradient of 80% to 98% methanol was used to separate A2E on a C18 column (Zorbax; Agilent) at a flow-rate of 0.3 ml/min. A2E was quantified using external A2E standards.
Enzyme-Linked Immunosorbent Assay
Because dietary n-3 can influence the metabolism of arachidonic acids, we measured several biological active metabolites of arachidonic acids. Serum was collected for different diet groups. Levels of serum prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and prostaglandin D2 (PGD2) were determined by monoclonal antibody-based enzyme-linked immunosorbent assay. Assays were performed by following the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI).
Quantification of Gene Expression by Reverse Transcription PCR
Approximately 100 retinal cells (RPE and neuronal cells) were microdissected from a frozen section of an ocular slide. The total RNA from the cells and universal mouse RNA (BD Bioscience, Palo Alto, CA) for assay normalization were subject to cDNA synthesis (Superscript II RNase H− Reverse Transcriptase, Invitrogen, Grand Island, NY). Real-time PCR was performed using a Stratagene Mx3000 Real-Time PCR System and Brilliant SYBR Green QPCR Master Mix (Stratagene, CA). The primers for TNF-α, IL-6, VEGFA, and VEGFR were synthesized by SuperArray and supplied as the RT2 Real-Time Gene Expression Assay kit. For the internal control, β-actin was amplified using primers 5′-CCCAGCACAATGAAGATCAA-3′ and 5′-ACATCTGCTGGAAGGTGGAC-3′. Following PCR, a thermal melt profile was performed for amplicon identification. To determine the Ct, the threshold level of fluorescence was set manually in the early phase of PCR amplification. ABI SDS 1.3.1 software and the 2−ΔΔCt analysis method were used to determine relative amounts of product using β-actin as an endogenous control. The fold change was normalized first by the level of β-actin from same cDNA sample. The average fold change due to gene manipulation was again normalized to the transcript level of the universal mouse RNA and presented graphically. Each sample was analyzed at least twice.
Statistical Analysis
The rates of progression and regression between groups were compared by χ2 test. Multiple means were compared by one-way analysis of variance (analysis of variance), followed by Duncan’s multiple range test for post hoc comparison of means. Differences were considered significant when P < 0.05.
Results
Two independent experiments were performed on Ccl2−/−/ Cx3cr1−/− mice fed diets either high or low in long chain n-3 fatty acids (Table 1) and yielded similar and reproducible results. The experimental end-point data (fundoscopy, histopathology, and A2E) were pooled and presented as below. The levels of the primary n-3 (EPA, DHA) or n-6 in the retina after 12 weeks on the low or high n-3 diet are shown in Table 2. The n-3 fatty acids accounted for 10.9% ± 1.02% and 20.6% ± 6.9% of the total lipid in the low and high n-3 fatty acid groups, respectively.
Table 2.
The Constituents of n-3 Fatty Acid in Eye Tissue after 12 Weeks of Low or High n-3 Diet Treatment
| Low n-3 diet
|
High n-3 diet
|
|
|---|---|---|
| Mean ± SD (wt % of total fatty acids) | Mean ± SD (wt % of total fatty acids) | |
| 12:0 | 0.47 ± 0.15 | 0.55 ± 0.44 |
| 14:0 | 1.49 ± 0.08 | 2.18 ± 1.24 |
| 16:0 | 18.00 ± 1.13 | 20.29 ± 2.00 |
| 18:0 | 13.21 ± 0.58 | 9.55 ± 4.45 |
| 20:0 | 0.44 ± 0.06 | 0.31 ± 0.15 |
| 22:0 | 0.56 ± 0.11 | 0.40 ± 0.21 |
| 14:1 | 0.15 ± 0.04 | 0.31 ± 0.21 |
| 16:1 | 4.61 ± 0.52 | 9.26 ± 5.94 |
| 18:1n9 | 25.31 ± 1.69 | 25.83 ± 6.92 |
| 18:1n7 | 2.88 ± 0.06 | 2.40 ± 0.25 |
| 20:1n9 | 0.78 ± 0.13 | 0.58 ± 0.21 |
| 18:2n6 | 3.50 ± 0.81 | 7.27 ± 3.76 |
| 18:3n6 | 0.03 ± 0.01 | 0.08 ± 0.03 |
| 20:2n6 | 0.32 ± 0.04 | 0.20 ± 0.23 |
| 20:3n6 | 0.38 ± 0.02 | 0.32 ± 0.09 |
| 20:4n6 | 6.74 ± 0.30 | 3.10 ± 1.84 |
| 22:4n6 | 1.35 ± 0.18 | 0.49 ± 0.39 |
| 22:5n6 | 1.45 ± 0.52 | 0.25 ± 0.12 |
| 18:3n3 | 0.08 ± 0.01 | 0.23 ± 0.20 |
| 20:5n3 (EPA) | 0.07 ± 0.00 | 0.21 ± 0.07 |
| 22:5n3 | 0.29 ± 0.01 | 0.62 ± 0.05 |
| 22:6n3 (DHA) | 10.31 ± 1.35 | 18.63 ± 3.32 |
| Total n-3 | 10.94 ± 1.02 | 20.60 ± 6.85 |
Clinical Ocular Features
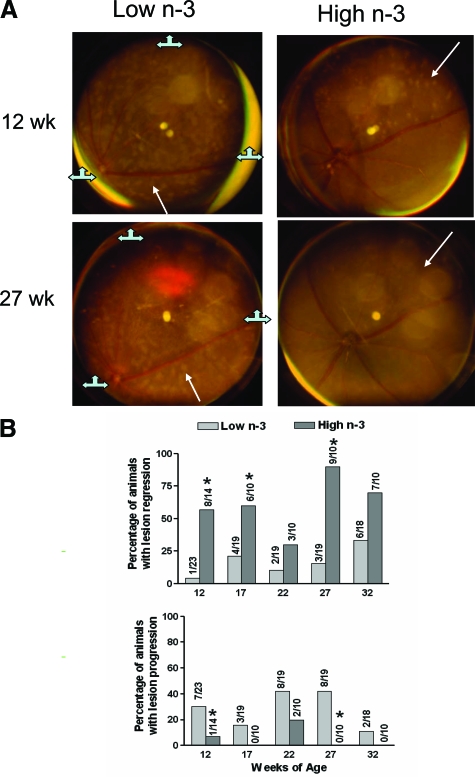
Mouse fundus photographs were taken to record the lesion location, number, size and character. At 9 weeks of age, we took baseline pictures of the fundus of all mice and found no obvious difference in lesions between the low-n-3 fatty acids and high n-3 fatty acids group of Ccl2−/−/Cx3cr1−/− mice. The sequential photographs taken at 12 weeks of age and 5-week intervals were compared as the lesions progressed or regressed in each follow-up examination. Less progression and greater regression of the retinal lesions were observed in the high n-3 fatty acids fed mice compared with the low n-3 fatty acids fed mice (Figure 1). Figure 1A demonstrates representative funduscopic images of a mouse on the low n-3 fatty acid diet with progression of the deep retinal lesions, and a mouse on the high n-3 fatty acid diet with regressed retinal lesions 15 weeks later after baseline measurements.
Figure 1.
Periodic monitoring of Ccl2−/−/Cx3cr1−/− mice fundus lesions. A: Representative funduscopic photographs from a low n-3-treated mouse show progressing deep retinal lesions characterized by increases in number and size, as well as scar formation within 15 weeks, and from a high n-3-treated mouse showing improving deep retinal lesions characterized by decreases in number and size within 15 weeks. Arrows indicate the same deep retinal local lesion in the sequential photographs: the size of the retinal lesion is enlarged in the mouse fed with low n-3 diet and the retinal lesion disappears in the mouse fed with high n-3 diet. The three markers in the low n-3 photo sets show the two images are slightly tiled. B: Summary of the observations. The regression and progression evaluation at 12th week was based on the comparison with the images taken at ninth week. *χ2 test P < 0.05 in comparison with the low n-3 fatty acid diet group.
More mice demonstrated a regression of retinal lesions in the high n-3 fatty acids group in comparison with the 9-week baseline and with the preceding observation (Figure 1B). At 12 weeks of age, 57% of the mice fed the high n-3 fatty acids exhibited lesion regression, in contrast, only 4% of the low n-3 fatty acids fed mice showed regressed lesions (P < 0.05). At 17 weeks, 60% of the high n-3 fatty acids fed but 21% of the low n-3 fatty acids fed mice had lesion regression (P < 0.05). At 22 weeks, 30% of the high n-3 fatty acids treated and 10% of the low n-3 fatty acids treated mice showed lesion regression. At 27 weeks, 90% of the high n-3 fatty acids treated and 16% of the low n-3 fatty acids treated mice showed lesion regression (P < 0.05). At 32 weeks, 70% of the high n-3 fatty acids treated and 33% of the low n-3 fatty acids treated mice showed lesion regression.
A higher percentage of mice demonstrated lesion progression in the low n-3 fatty acids group, in most examinations (Figure 1B). At 12 weeks, only 7% of high n-3 fatty acids fed but 30% of low n-3 fatty acids fed mice showed lesion progression (P < 0.05). At 17 weeks, high n-3 fatty acids treated mice were stable, but 16% of low n-3 fatty acids treated mice continued lesion progression. At 22 weeks, 20% of high n-3 fatty acids treated and 42% of low n-3 fatty acids treated mice showed lesion progression. At 27 weeks, no high n-3 fatty acids treated and 42% of low n-3 fatty acids treated mice showed lesion progression (P < 0.05). At 32 weeks, no high n-3 fatty acids treated and 11% of low n-3 fatty acids treated mice showed lesion progression.
Overall, a significantly smaller percentage of mice showed progression and a higher percentage showed regression in the high n-3 fatty acid diet group in comparison with low n-3 fatty acid diet group (P < 0.05).
Histological and Ultrastructural Features
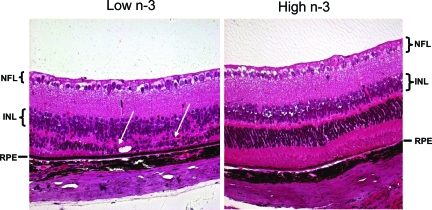
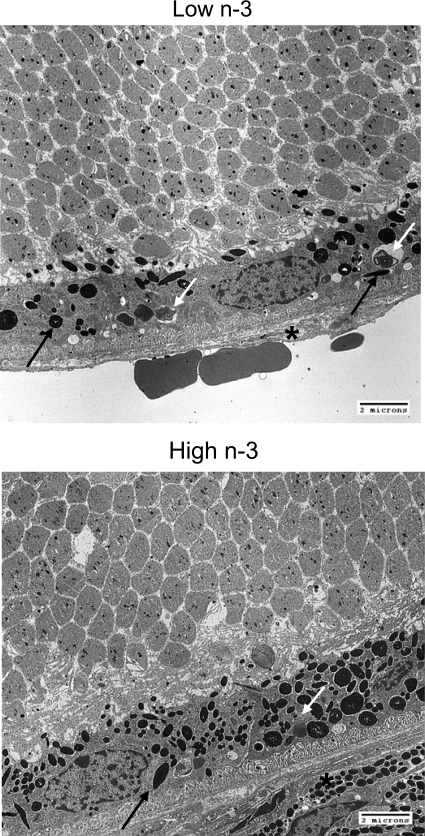
Histopathological examination was conducted on 14 eyes from 14 mice fed with the high n-3 fatty acids diet and 18 eyes from 18 mice fed with the low n-3 fatty acids diet for 8 months. Loss of photoreceptors and mild focal hypopigmentation of the RPE cells were found in 4 out of 14 eyes of the high n-3 fatty acids group. In contrast, moderate to extensive retinal photoreceptor dystrophy, atrophy and RPE alterations were identified in 12 out of 18 eyes of the low n-3 fatty acids group (χ2 test P < 0.05; representative images: Figure 2). These findings were confirmed by transmission electron microscopy (Figure 3). Loss of melanosomes and accumulation of lipofuscins were found to be more severe in the RPE cells of the low n-3 fatty acids mice, as compared with the high n-3 fatty acids group. No retinal lesions were seen in the normal control wild-type mice fed with regular diet.
Figure 2.
Representative ocular photomicrographs of Ccl2−/−/Cx3cr1−/− mice. Photomicrographs show focal loss of photoreceptors and retinal degeneration (between two arrows) in a mouse on a low n-3 fatty acid diet for 27 weeks (left). In contrast, the retina is relatively normal with minimal RPE hypotrophy (arrow) in a mouse on a high n-3 fatty acids diet for 27 weeks (right). Staining is H&E (original magnification, ×200) NFL, nerve fiber layer; INL, inner nuclear layer; RPE, retinal pigment epithelium.
Figure 3.
Representative retinal transmission electron micrographs of Ccl2−/−/ Cx3cr1−/− mice. Upper panel: Transmission electron micrograph of a retina from a mouse on a low n-3 fatty acids diet for 27 weeks shows a decrease of melanosomes (black arrows) and an increase of lipofuscin-containing lysosomes (white arrows) in the RPE cells. Bruch’s membrane (asterisk) is thickened. Lower panel: Transmission electron micrograph of a mouse on a high n-3 fatty acids diet for 27 weeks shows many melanosomes (black arrow) and few lipofuscin-containing lysosomes (white arrow) in the RPE cells. Bruch’s membrane (asterisk) appears normal. Scale bar = 2 μm.
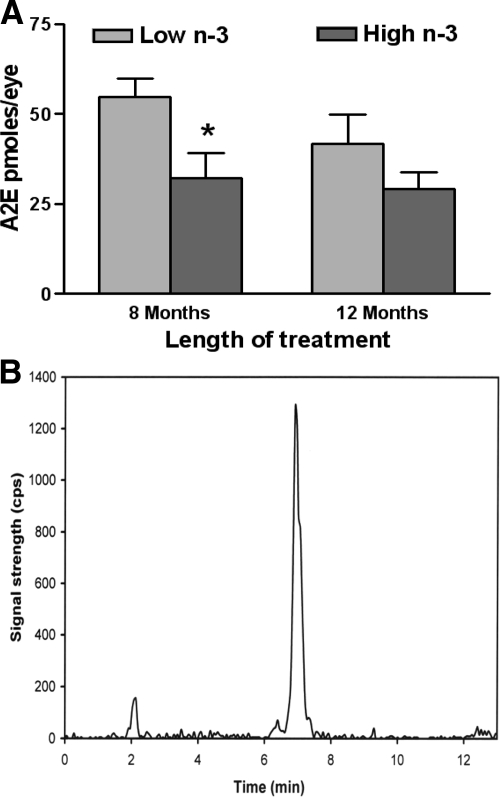
Accumulation of A2E
Two-fold higher A2E was detected in the eyes of low n-3 fatty acids fed mice relative to the high n-3 fatty acids fed mice after 8 months of feeding (P < 0.05) (Figure 4A). However, the differences were no longer significant after 12 months of feeding (P > 0.05) (Figure 4A), which might result from loss of RPE cells and photoreceptors, the source of A2E, in the late stage of the disease. Figure 4B demonstrates the high signal-to-noise ratio of the A2E detection by the LC/MS/MS system.
Figure 4.
Quantification of A2E in eyes of Ccl2−/−/Cx3cr1−/− mice. A: A2E, a major lipofuscin fluorophore that accumulates during AMD progression, was lower in the Ccl2−/−/Cx3cr1−/− retina in the high n-3 fatty acids (n = 4) compared with the low n-3 fatty acids group (n = 4) at 8 months of age (*P < 0.05), and 12 months of age (n = 4 in the high n-3 fatty acid group and n = 3 in the low n-3 fatty acid, P > 0.05). B: LC/MS/MS chromatography represents the A2E peak eluting at 6.9 minutes.
Profile of Arachidonic Acid Derivatives
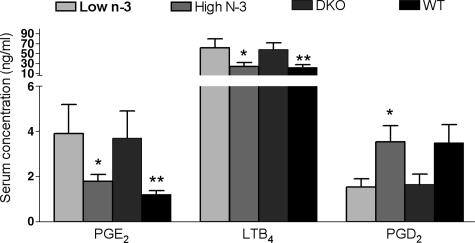
The concentrations of PGE2 and LTB4, two pro-inflammatory metabolites of arachidonic acid, were significantly lower in the serum of the high n-3 fatty acids fed mice than in the low n-3 fatty acids fed mice (Figure 5). In contrast, the serum concentration of PGD2, an anti-inflammatory metabolite, was significantly higher in the mice fed with high n-3 fatty acids as compared with the low n-3 fatty acids group (Figure 5). The levels of these metabolites from the high n-3 fatty acids group were similar to those of the wild-type age-matched control mice that ingested regular chow. The levels of those metabolites from the low n-3 fatty acids group were similar to those of the age-matched Ccl2−/−/Cx3cr1−/− mice that ingested regular chow. No wild-type mice showed any retinal lesions.
Figure 5.
The serum concentration of arachidonic acid metabolites in Ccl2−/−/ Cx3cr1−/− mice treated with low n-3 or high n-3 chow; wild-type mice or Ccl2−/−/Cx3cr1−/− mice with regular chow. The graph is plotted as mean ± SD. The concentrations of PGE2 and LTB4, pro-inflammatory metabolites of arachidonic acid, were lower, whereas PGD2, an anti-inflammatory metabolite of arachidonic acid was higher in the serum of the high n-3 fatty acids Ccl2−/−/Cx3cr1−/− group (n = 5) compared with that of low n-3 fatty acids diet group (n = 5). *P < 0.05 in comparison with the low n-3 fatty acids diet group. **P < 0.05 in comparison with the Ccl2/Cx3cr1 deficient mice with regular chow.
Transcripts Profile in the Retina
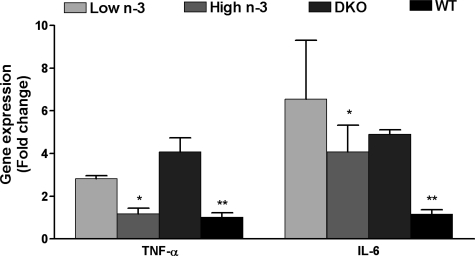
RT-PCR data demonstrated a significant reduction of ocular TNF-α and IL-6 mRNA detected by quantitative RT-PCR in the microdissected retinal cells of Ccl2−/−/Cx3cr1−/− mice fed with the high n-3 fatty acids diet, as compared with those fed with the low n-3 fatty acids diet (Figure 6). The levels of TNF-α transcript expression in the high n-3 fatty acids group were close to that of the wild-type control mice fed with regular chow, which depicted normal retina. In the retinal tissue for the mice that ingested regular chow, TNF-α and IL-6 transcripts were higher in Ccl2−/−/Cx3cr1−/− mice than the wild-type mice (Figure 6). We did not observe significant changes of VEGF and VEGFR expression between low or high n-3 diets (data not shown).
Figure 6.
Gene transcripts in the retina of Ccl2−/−/Cx3cr1−/− mice treated with low n-3 or high n-3 chow; wild-type mice or Ccl2−/−/Cx3cr1−/− mice with regular chow. The graph is plotted as mean ± SD. Lower TNF-α and IL-6 mRNA were detected by quantitative RT-PCR in the ocular tissue of Ccl2−/−/ Cx3cr1−/− mice fed with a high n-3 fatty acid diet (n = 6) as compared with those fed with a low n-3 fatty acid diet (n = 6). *P < 0.05 in comparison with Ccl2−/−/Cx3cr1−/− low n-3 fatty acids group. **P < 0.05 in comparison with the Ccl2/Cx3cr1 deficient mice with regular chow.
Discussion
In this study, the Ccl2/Cx3cr1-deficient mice were given a defined isocaloric diet either high or low in n-3 fatty acids (DHA and EPA). The results demonstrated an obvious benefit of long chain n-3 fatty acid intake in a mouse strain generated by knocking out a chemokine (Ccl2) and a chemokine receptor (Cx3cr1), which exhibits certain pathological features of human AMD-like lesions.13 Since aged mouse Bruch’s membrane structures are not considered similar to human, the characteristic age changes of drusen formation, often seen in humans, are rarely seen in aged mouse Bruch’s membrane.20 However, an increase in size and number of lipofuscin granules are reported in the RPE.21 Therefore, A2E measurement rather than identification of drusen should be the biomarker of mouse “AMD.” The diet started from conception and the clinical outcome can be observed as early as 9 weeks of age. Although there is a significant variation among individual animals, the overall outcome of lesion reversion or progression is striking between the two groups fed with high or low n-3 fatty acid diets.
The diet used in this study is designed to generate a differential ratio of n-3 fatty acids to n-6 fatty acids. The beneficial effects of n-3 fatty acids in various physiological and pathological situations are well-documented.22 The n-3 fatty acids act as an energy source as well as an important unique cell component, especially in cellular membranes. DHA is particularly rich in retinal photoreceptor outer segments.22 The highest concentrations in the body for DHA per unit area are found in the photoreceptor disk membranes and the overall percentage of DHA can reach 50% of total retinal fatty acids.23,24 The unique fatty acid composition in retinal photoreceptor outer segment disk membranes is essential for maintaining a healthy retina. DHA affects membrane function by altering permeability, membrane order, thickness, lipid phase properties, and the activation of membrane-bound proteins.5 The special structure of DHA, the position of the first unsaturated bond at the n-3 fatty acids (between Δ-20 and Δ-19) carbon, provides an advantage in efficiency of membrane dynamics over that observed in an otherwise structurally identical fatty acid with the first double bond at the n-6 carbon.24 The n-3 fatty acids may have a neuroprotective effect in our model, which results in decelerated retinal lesion development in Ccl2−/−/Cx3cr1−/− mice ingesting a high n-3 fatty acid diet.
The n-3 fatty acids and their derivatives play an extensive role in numerous biological processes, such as inflammatory cascades, apoptosis, and neuroprotection.5 These are related to direct actions on plasma membranes, altered inflammatory response and control of gene expression. We focus on the role of n-3 fatty acids in inflammation because the role of inflammation in AMD pathogenesis is evident.25,26 During AMD development, cellular debris derived from the aging RPE cells becomes sequestered below the RPE basal lamina into Bruch’s membrane, which could constitute a chronic inflammatory stimulus. The entrapped cellular debris then becomes the target of encapsulation by a variety of inflammatory mediators and elicits a local chronic inflammatory response that exacerbates the effects of primary pathogenic stimuli.26,27 During the late stage of the disease, more evidence can be found for inflammatory involvement.25,28 The immunological protein expression profile in Ccl2−/−/Cx3cr1−/− mice, the model used in this study, reveals increased complement C3 in Bruch’s membrane, RPE, choroidal capillaries, and particularly in the deep retinal lesions, relative to the wild-type controls.29 Complement component C3 and chemokine Ccl5 are elevated in the retina of these mice. Moreover, infiltration of mononuclear phagocytic cells, both macrophages and microglia are also found in the lesion site.29
We focused on the arachidonic acid metabolism pathway because it interacts with n-3 fatty acids metabolism in various ways such as plasma membranes.5 Neuronal membranes are composed of high levels of PUFA, especially DHA and arachidonic acids.22 Arachidonic acid (C20:4n-6) is the substrate for the synthesis of a range of biologically active compounds (eicosanoids) including prostaglandins, thromboxanes, and leukotrienes. These compounds can act as mediators in leukocyte chemotaxis and inflammatory cytokine production. When fish oil (high n-3 fatty acids) is provided, EPA is incorporated into cell membrane phospholipids at the expense of arachidonic acid, leading to less substrate available for eicosanoid synthesis.30 In our study, high n-3 fatty acids decreased the production of inflammatory eicosanoids (PGE2 and LTB4) as compared with the mice ingesting low n-3 fatty acids, which was correlated with amelioration of retinal lesions. An similar result was reported by Ira mete al.31 The reduced PGE2 and LTB4 could also be attributed to differential gene expression regulated by reactive mediators of n-3 fatty acids.32,33
We also observed an increased PGD2 in serum of the high n-3 fatty acids group. PGD2 is considered to be an anti-inflammatory mediator.34,35 The dehydration end-product of PGD2 is PGJ2, which represses several pro-inflammatory genes including tumor necrosis factor (TNF)-α, interleukin-1β, and inducible nitric oxide synthase.36,37 Even though the replacement of arachidonic acids with EPA reduces the generation of arachidonic acid metabolites in general; an increased PGI2 after fish oil supplementation has been reported.38 The newly identified EPA/DHA-derived inflammation-resolving mediator classes and their role on pathological retinal angiogenesis in mice provided the evidence to explain this pleiotropic effect of n-3 fatty acids.16,39 This phenomenon might be attributed to an altered profile of lipid mediators using arachidonic acid and/or EPA as the substrates during inflammation,39 or to modified expression of genes responsible for arachidonic acid metabolism.32,33
The protective effect of high n-3 fatty acids was correlated with the suppression of TNF-α and IL-6 in the eye in this study. TNF-α is an inflammatory cytokine and has been found in a subset of microglia that is closely associated with retinal vessels.16,40 Anti-TNF-α treatment has been reported to reduce both the size of, and leakage of, laser-induced choroidal neovasculature in mice.41 Interestingly, TNF-α increases PGE2 but decreases PGD2 synthesis by zymosan-stimulated murine macrophages.42 The role of n-3 on the interleukin (IL)-6 has been reported previously.43 Aqueous IL-6 levels are reported to significantly correlated with the sizes of AMD associated choriodal neovascular membranes.44 Even though vascular endothelial growth factor (VEGF) has been reported to be related to AMD,44 particularly neovascular AMD,45 we did not find an altered expression due to different diets. This may be because most Ccl2/Cx3cr1 deficient mice lack choroidal neovascularization.
In summary, a diet enriched in EPA and DHA can ameliorate the progression of retinal lesions in the Ccl2/Cx3cr1 deficient mice. We suggest that this mouse strain is useful for the screening of therapeutic agents for AMD. One of the mechanisms underlying lower disease progression by long chain n-3 fatty acids may be via a shunted arachidonic acid pathway, leading to an increase of anti-inflammatory derivatives such as PGD2 and decreases of pro-inflammatory derivatives such as PGE2, LTB4, TNF-α, and IL-6. The results in these mice are in line with the epidemiological studies of AMD risk reduction by long chain n-3 fatty acids.
Footnotes
Address reprint requests to Chi-Chao Chan, 10/10N103, NIH/NEI, 10 Center Dr., Bethesda, MD 20892-1857. E-mail: chanc@nei.nih.gov.
Supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health and the American Health Assistance Foundation.
This work was prepared as part of our official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TC, Lee L. Age related macular degeneration-new developments in treatment. Aust Fam Physician. 2007;36:359–361. [PubMed] [Google Scholar]
- Kang ZB, Ge Y, Chen Z, Cluette-Brown J, Laposata M, Leaf A, Kang JX. Adenoviral gene transfer of Caenorhabditis elegans n–3 fatty acid desaturase optimizes fatty acid composition in mammalian cells. Proc Natl Acad Sci USA. 2001;98:4050–4054. doi: 10.1073/pnas.061040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Sangiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Rotstein NP, Politi LE, German OL, Girotti R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest Ophthalmol Vis Sci. 2003;44:2252–2259. doi: 10.1167/iovs.02-0901. [DOI] [PubMed] [Google Scholar]
- Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33:3242–3253. [PubMed] [Google Scholar]
- Rotstein NP, Aveldano MI, Barrantes FJ, Politi LE. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J Neurochem. 1996;66:1851–1859. doi: 10.1046/j.1471-4159.1996.66051851.x. [DOI] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001;73:209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- Hodge WG, Barnes D, Schachter HM, Pan YI, Lowcock EC, Zhang L, Sampson M, Morrison A, Tran K, Miguelez M, Lewin G. Evidence for the effect of omega-3 fatty acids on progression of age-related macular degeneration: a systematic review. Retina. 2007;27:216–221. doi: 10.1097/01.iae.0000233322.83713.2d. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, Zhou M, Salem N, Jr, Bonner R, Tuo J. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–128. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Connor KM, Sangiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- Ben-Shabat S, Parish CA, Hashimoto M, Liu J, Nakanishi K, Sparrow JR. Fluorescent pigments of the retinal pigment epithelium and age-related macular degeneration. Bioorg Med Chem Lett. 2001;11:1533–1540. doi: 10.1016/s0960-894x(01)00314-6. [DOI] [PubMed] [Google Scholar]
- Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, Williams DS, Zhang K. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci USA. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima H, Kondo K. Extrusion of lysosomal bodies from apical mouse retinal pigment epithelium. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;216:209–217. doi: 10.1007/BF00408162. [DOI] [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Dyall SC, Michael-Titus AT. Neurological benefits of omega-3 fatty acids. Neuromolecular Med. 2008;10:219–235. doi: 10.1007/s12017-008-8036-z. [DOI] [PubMed] [Google Scholar]
- Gawrisch K, Eldho NV, Holte LL. The structure of DHA in phospholipid membranes. Lipids. 2003;38:445–452. doi: 10.1007/s11745-003-1082-0. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. DHA-rich phospholipids optimize G-Protein-coupled signaling. J Pediatr. 2003;143:S80–S86. doi: 10.1067/s0022-3476(03)00405-0. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Survey of Ophthalmology. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Barouch FC, Miller JW. The role of inflammation and infection in age-related macular degeneration. Int Ophthalmol Clin. 2007;47:185–197. doi: 10.1097/IIO.0b013e3180377936. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, Tuo J, Chan CC. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp Eye Res. 2008;86:675–683. doi: 10.1016/j.exer.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz J Med Biol Res. 2003;36:433–446. doi: 10.1590/s0100-879x2003000400004. [DOI] [PubMed] [Google Scholar]
- Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- Hertzel AV, Bernlohr DA. Regulation of adipocyte gene expression by polyunsaturated fatty acids. Mol Cell Biochem. 1998;188:33–39. [PubMed] [Google Scholar]
- Li CC, Lii CK, Liu KL, Yang JJ, Chen HW. n-6 and n-3 polyunsaturated fatty acids down-regulate cytochrome P-450 2B1 gene expression induced by phenobarbital in primary rat hepatocytes. J Nutr Biochem. 2006;17:707–715. doi: 10.1016/j.jnutbio.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Kojima F, Yang L, Crofford LJ. Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study. Prostaglandins Leukot Essent Fatty Acids. 2007;76:103–112. doi: 10.1016/j.plefa.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SM, Schumacher HR, Kim H, Kim M, Lee SH, Pessler F. Reduction of urate crystal-induced inflammation by root extracts from traditional oriental medicinal plants: elevation of prostaglandin D2 levels. Arthritis Res Ther. 2007;9:R64. doi: 10.1186/ar2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HY, Jeon WK, Kim BC. Up-regulation of heme oxygenase-1 expression through the Rac1/NADPH oxidase/ROS/p38 signaling cascade mediates the anti-inflammatory effect of 15-deoxy-delta 12,14-prostaglandin J2 in murine macrophages. FEBS Lett. 2008;582:861–868. doi: 10.1016/j.febslet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Reyes-Martin P, Ramirez-Rubio S, Parra-Cid T, Bienes-Martinez R, Lucio-Cazana J. 15-Deoxy-delta12,14-prostaglandin-J(2) up-regulates cyclooxygenase-2 but inhibits prostaglandin-E(2) production through a thiol antioxidant-sensitive mechanism. Pharmacol Res. 2008;57:344–350. doi: 10.1016/j.phrs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Knapp HR, Salem N., Jr Formation of PGI3 in the rat during dietary fish oil supplementation. Prostaglandins. 1989;38:509–521. doi: 10.1016/0090-6980(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- Carter DA, Dick AD. Lipopolysaccharide/interferon-gamma and not transforming growth factor beta inhibits retinal microglial migration from retinal explant. Br J Ophthalmol. 2003;87:481–487. doi: 10.1136/bjo.87.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Semkova I, Muther PS, Dell S, Kociok N, Joussen AM. Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res. 2006;83:1325–1334. doi: 10.1016/j.exer.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Fournier T, Fadok V, Henson PM. Tumor necrosis factor-alpha inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Biol Chem. 1997;272:31065–31072. doi: 10.1074/jbc.272.49.31065. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhou HR, Shi Y, Pestka JJ. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J Nutr. 2006;136:366–372. doi: 10.1093/jn/136.2.366. [DOI] [PubMed] [Google Scholar]
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Thill M, Strunnikova NV, Berna MJ, Gordiyenko N, Schmid K, Cousins SW, Thompson DJ, Csaky KG. Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:2696–2708. doi: 10.1167/iovs.07-0955. [DOI] [PubMed] [Google Scholar]