Abstract
Serological cell death biomarkers and circulating tumor cells (CTCs) have potential uses as tools for pharmacodynamic blood-based assays and their subsequent application to early clinical trials. In this study, we evaluated both the expression and clinical significance of CTCs and serological cell death biomarkers in patients with small cell lung cancer. Blood samples from 88 patients were assayed using enzyme-linked immunosorbent assays for various cytokeratin 18 products (eg, M65, cell death, M30, and apoptosis) as well as nucleosomal DNA. CTCs (per 7.5 ml of blood) were quantified using Veridex CellSearch technology. Before therapeutic treatment, cell death biomarkers were elevated in patients compared with controls. CTCs were detected in 86% of patients; additionally, CD56 was detectable in CTCs, confirming their neoplastic origin. M30 levels correlated with the percentage of apoptotic CTCs. M30, M65, lactate dehydrogenase, and CTC number were prognostic for patient survival as determined by univariate analysis. Using multivariate analysis, both lactate dehydrogenase and M65 levels remained significant. CTC number fell following chemotherapy, whereas levels of serological cell death biomarkers peaked at 48 hours and fell by day 22, mirroring the tumor response. A 48-hour rise in nucleosomal DNA and M30 levels was associated with early response and severe toxicity, respectively. Our results provide a rationale to include the use of serological biomarkers and CTCs in early clinical trials of new agents for small cell lung cancer.
Small cell lung cancer (SCLC) is initially chemosensitive but invariably relapses with a chemoresistant phenotype.1 A number of molecularly targeted therapies have been evaluated attempting to improve outcome, but none have succeeded to date.2 Ideally, early clinical trials should incorporate validated pharmacodynamic biomarkers, conducted to good clinical laboratory practice, that demonstrate both proof of mechanism (drug hits target) and proof of concept (tumor responds to drug).3 Although possible, serial biopsies are rare in SCLC, and the tissue obtained often insufficient for extensive molecular profiling. Thus, there is a pressing need for blood-based biomarkers that report therapeutic response.
Assays of drug-induced cell death are potential proof of concept biomarkers for multiple therapeutics.4 The M30 Apoptosense and M65 assays (Peviva, Bromma, Sweden) detect cytokeratin (CK) 18, expressed in epithelial but not hematopoietic cells, and released into the blood following cytoskeletal disassembly and degradation during apoptotic and/or necrotic cell death.5 The M30 antibody recognizes a caspase-cleaved neoepitope of CK18 that is only revealed during apoptosis, whereas the M65 assay detects full length and cleaved forms of CK18 reporting apoptosis and necrosis.6 Nucleosomal DNA (nDNA) results from cleavage of chromatin by apoptotic endonucleases into membrane bound DNA fragments that are phagocytosed by macrophages and subsequently released into the blood.7 nDNA release is also detected when levels of apoptosis overwhelm macrophage capacity for phagocytosis and a more necrotic cell fate ensues.7 We have previously validated these cell death biomarkers8,9 and optimized them for application to a busy, clinical setting.6 Here we report on the behavior and clinical utility of these assays in patients with SCLC.
Importantly, cell death assays may report host toxicity in addition to tumor response. However, circulating tumor cell (CTC) numbers can, in theory, be used to directly evaluate drug effect on malignant cells.10 The cytometric approach using CellSearch technology (Veridex Inc., Huntingdon Valley, PA) is now approved by the Food and Drug Administration for clinical decision-making in patients with metastatic breast, colorectal and prostate cancers.11,12,13 This is the first report on the use of this technology platform for CTC enumeration in patients with SCLC and the first direct comparison of serological biomarkers of cell death and cell death in CTCs.
This study was conducted to evaluate cell death assays (M30, M65, and nDNA) and CTC profiles in patients with SCLC undergoing standard chemotherapy, as a prelude to their incorporation as biomarkers in early clinical trials. The hypothesis tested was that increases in cell death biomarkers immediately following therapy would predict outcome and that given the metastatic potential of SCLC high numbers of CTCs would be detectable. The results from this study are most encouraging for the development of CTCs as pharmacodynamic biomarkers in SCLC, provide novel insights into the clinical significance of serological cell death assays, and demonstrate agreement between measures of cell death at the molecular and cellular level in this disease.
Materials and Methods
Patients
Blood samples were collected from SCLC patients undertaking chemotherapy at the Christie Hospital, Manchester, UK, and from healthy volunteers according to ethically approved protocols. Eligible patients had pathologically confirmed chemo-naive SCLC, staged and managed using standard protocols. Patients received platinum-based chemotherapy, given in combination with etoposide where age, performance status, and comorbidities allowed. One patient received non-platinum-based combination therapy due to disease related thrombocytopenia at baseline. Radical radiotherapy and prophylactic cranial irradiation were administered either concurrently with chemotherapy (starting with cycle 2) or sequentially following cycle 6 in patients with limited disease depending on clinical status and extent of disease.
Data were collected on World Health Organization performance status (PS), complete blood count, alkaline phosphatase, lactate dehydrogenase (LDH), sodium (Na), radiology, treatment received, toxicity, response, and survival. Early response was assessed by an independent radiologist on chest radiographs taken on days 1 and 15 of the first cycle of treatment.
Blood Sampling, Processing, and Serological Cell Death Enzyme-Linked Immunosorbent Assays
Blood samples (15 ml) were collected using the Vacutainer system (BD, Franklin Lakes, NJ) within 24 hours before the first treatment then 48 hours and 22 days after therapy. The first 5 ml of blood was discarded to minimize the risk of skin contamination. Additional samples were obtained from a subgroup of patients (n = 12) after 24 hours, on day 8, and on day 15 to explore the optimal time points for the assays. Plasma samples were collected in lithium-heparin tubes, refrigerated at 4°C for a maximum of 2 hours before centrifugation at 1000 × g for 10 minutes, and transferred immediately to −80°C. Serum samples were collected in silica tubes, clotted at room temperature for 30 minutes to 2 hours, before centrifugation at 2000 × g for 10 minutes and transferred immediately to −80°C.6 Plasma samples were analyzed for M30 and M65 (Peviva) using our previously described assays validated to good clinical laboratory practice.8,9 Serum samples were analyzed for nDNA [Cell Death Detection ELISA (Roche, Basel, Switzerland)] as previously described.14
CTC Enumeration
Blood samples (7.5 ml) were collected into CellSave tubes (Veridex), containing EDTA and a cellular preservative. Samples were maintained at room temperature and processed within 72 hours using the CellSearch platform (Veridex) as previously described.15 In brief, blood was diluted, centrifuged and incubated with ferrofluid particles coated with anti-EpCAM antibodies. After immunomagnetic enrichment, ferrofluid-labeled cells were permeabilized and fluorescently labeled using phycoerythrin-conjugated anti-CK antibodies (pan-keratin antibody C-11 that recognizes keratins 4, 5, 6, 8, 10, 13, and 18) to identify epithelial cells and allophycocyanin-conjugated anti-CD45 antibody to identify and discount white blood cells. 4′6-Diamidino-2-phenylindole (DAPI) was incorporated to identify cell nuclei and to reveal mitotic or apoptotic nuclear morphologies. Analysis of CD56 expression on CTCs was performed using Alexa Fluor 488-conjugated mouse anti-human CD56 antibody (BD PharMingen, San Diego, CA) analyzed in the fourth channel of the CellSearch system. The antibody concentration was 12 μg/ml and the integration time for CellSearch Analyzer II was configured to 0.4 second. The positivity of CD56 in SCLC CTCs was obtained using the research mode of CellSearch Analyzer II. After repeated magnetic separation, the fluorescently labeled cells were oriented to the cartridge surface for analysis using the CellTracks Analyzer II, a semiautomated fluorescent microscope. Captured images, processed by CellTracks software, were analyzed blinded without knowledge of patient data. CTCs were defined as nucleated cells staining positively for cytokeratin and negatively for CD45 and reported as CTC number per 7.5 ml of blood. Based on the nuclear and CK morphology, apoptotic and mitotic CTCs were enumerated in cases where CTC number was >100. The reproducibility, intra- and interassay variability, cell stability, and recovery precision of the CellSearch system have been validated by the manufacturer.
Statistical Analysis
Study design and statistical analysis was performed using SPSS for Windows (release 13.0.2004, SPSS Inc., Chicago, IL) where P values of ≤0.05 were considered significant. A minimum sample size of 36 cases was required for enzyme-linked immunosorbent assay and CTC analyses to detect with 80% power a difference in survival between good and poor prognosis groups of 100 days, with an accrual and median follow-up of 365 days.
In addition, data from Phase 1 trials have shown that progressive disease is associated with a 12.5% (SD 28%) increase in M65 at day 22.16 However, in patients responding to cytotoxic chemotherapy in other solid tumors, a decrease of 20% (SD 44%) has been seen.17 To detect this difference with 80% power (P = 0.05) assuming a 70% response rate 34 patients were required.
Variables were positively skewed and were log (base 2) transformed before analysis to stabilize the sample variance, and nonparametric tests were used to satisfy the assumptions of variance between sample groups. Cell death biomarker levels were analyzed as continuous variables. Differences between groups were tested using the Mann-Whitney U Test for continuous variables and the χ2 test for categorical variables. Relationships were examined using the non-parametric Spearman’s rho bivariate correlation with a two-tailed test for significance. Univariate survival analysis was performed using the Kaplan-Meier method, with data categorized into three groups based on upper (75th), middle (median), and lower (25th) quartiles, which were compared using the log rank (Mantel-Cox) test. Multivariate analysis, using a forward stepwise Cox proportional hazards regression model, tested for associations between the variables and survival (defined as time from consent to death). Separate multivariate analysis was performed for M30 and M65 due to the strong correlation between these variables. Subjects with missing data were removed before this analysis. This did not have an adverse effect on the power of the study. The REMARK guidelines for reporting results from prognostic biomarkers statistical analysis were followed.18
Results
Clinical Characteristics of Patients
A total of 88 patients were enrolled prospectively between October 2006 and December 2008; 78 patients were assessable for cell death biomarkers and 50 patients were assessed for CTCs. Insufficient blood volume prevented analysis of all biomarkers in all patients. Clinical characteristics are summarized in Table 1, and there were no significant differences between the total cohort, the cell death biomarker cohort, and the CTC cohort.
Table 1.
Clinical Characteristics of SCLC Patients
| Characteristic | Total cohort N = 88 | Cell death biomarker cohort N = 78 | CTC cohort N = 50 |
|---|---|---|---|
| Age, median years (range) | 67 (27–86) | 67 (27–86) | 67 (28–84) |
| Sex, n (%) | |||
| Male | 44 (50) | 39 (50) | 27 (54) |
| Female | 44 (50) | 39 (50) | 23 (46) |
| Stage, n (%) | |||
| Limited stage | 35 (40) | 33 (42) | 20 (40) |
| Extensive stage | 53 (60) | 45 (58) | 30 (60) |
| Liver metastases (% of extensive stage) | 32 (60) | 29 (64) | 19 (63) |
| Performance status, n (%) | |||
| 0 | 7 (9) | 6 (8) | 4 (8) |
| 1 | 48 (55) | 42 (54) | 27 (54) |
| 2 | 30 (34) | 28 (36) | 17 (34) |
| 3 | 2 (2) | 2 (3) | 2 (4) |
| Treatment, n (%) | |||
| Cisplatin + etoposide | 19 (22) | 17 (22) | 10 (20) |
| Carboplatin + etoposide | 55 (62) | 50 (64) | 29 (58) |
| Carboplatin | 13 (15) | 10 (13) | 10 (20) |
| Vincristine, adriamycin, cyclophosphamide | 1 (1) | 1 (1) | 1 (2) |
| Baseline laboratory values | |||
| Na, median (range) | 137 (113–149) | 137 (114–149) | 137 (114–145) |
| Na <132, n (%) | 15 (17) | 15 (19) | 7 (14) |
| LDH, median (range) | 548 (240–6183) | 562 (294–6183) | 583 (240–6010) |
| LDH >450, n (%) | 56 (64) | 50 (64) | 31 (62) |
Cell Death Biomarkers and CTCs before Chemotherapy
Before treatment all three cell death biomarkers were higher in 78 patients with SCLC compared with 85 healthy controls (median values for M30 were 268 versus 198 U/L P = 0.02, for M65 were 609 versus 245 U/L P < 0.0001, and for nDNA were 1.40 versus 0.30, respectively, P < 0.0001). CTCs were detected in 43 of 50 (86%) patients analyzed with median CTC number of 28 (range, 0–44,896, mean ± SD = 2915 ± 8115).
M65 and M30 correlated with each other, consistent with detection of all forms of CK18 by M65, and the apoptosis cleaved fragment by M30 (P < 0.001). In contrast, nDNA did not correlate with M65 or M30. Pretreatment CTC number correlated significantly with M30 and M65 (P < 0.001) but not with nDNA in 40 patients assessable for all biomarkers. Morphological assessment for apoptotic and mitotic CTCs was performed in 14 samples from 12 patients containing a minimum CTC number of 100. Typical examples of cellular morphology are shown in Figure 1A.
Figure 1.
SCLC CTC morphology and effect of chemotherapy on CTC number. Based on CK and DAPI staining profile, CTCs represent as a heterogeneous population among which there are mitotic, apoptotic, and aggregated CTCs (A). A majority of patients have decreased CTC number on day 22 after the first cycle of chemotherapy (B).
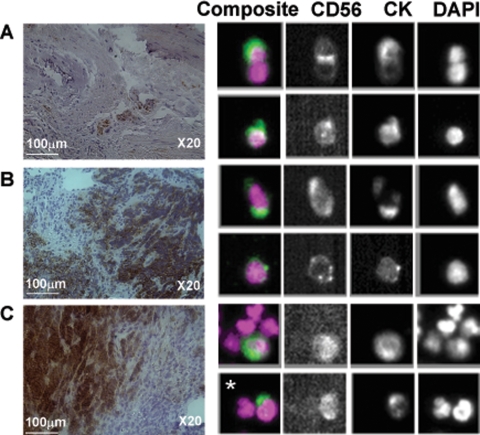
Apoptotic cells were observed in 13 of 14 evaluable patient samples (range, 0–5.9%) and mitotic cells were observed in 12 of 14 patient samples (range, 0–12.7%). A positive correlation was observed between apoptotic CTC number and serological levels of the apoptotic product M30 (P < 0.05). CD56 expression profiling was performed on 15 CTC samples in which the CTC number varied from 0 to 5884. In all samples with CTCs there were CD56 positive cells, in keeping with the IHC findings from matched tumor biopsies (Figure 2, A–C). This confirmed the dual epithelial-neuroendocrine nature and neoplastic origin of CTCs.
Figure 2.
CD56 in SCLC CTC and tumor biopsies. The CD56 staining profile is consistent in the matched tumor biopsies and CTC samples. A and B: Tumor biopsies from primary lung lesions and isolated CTCs from the same patients. C: Tumor biopsy from liver metastasis and paired CTCs. The asterisk shows a SCLC CTC with dual staining for CK and CD56 that can be seen next to a white blood cell (DAPI positive only).
Associations between Pretreatment Cell Death Biomarkers, CTCs, and Clinical Factors
M30, M65, and CTC number correlated with stage and PS. Patients with extensive stage disease had higher levels of M65 compared with patients with limited stage disease (P < 0.0001 by Mann-Whitney test). The median M30, M65, and CTC number for patients with extensive stage disease and limited stage disease were 379 U/L (range, 82–1331 U/L) versus 185 U/L (range, 68–466 U/L) for M30, 956 U/L (range, 185–4792 U/L) versus 390 U/L (range, 162–1370 U/L) for M65, and 237 (range, 1–44896) versus 2 (range, 0–91) for CTC number, respectively. Seven patients with no detectable CTCs all had limited stage disease SCLC.
M30, M65, and CTC number also correlated with the presence of liver metastases (P < 0.0001 by Mann-Whitney test) with median 424 U/L (range, 98–1331 U/L) versus 209 U/L (range, 68–725 U/L) for M30, 1363 U/L (range, 202–4792 U/L) versus 449 U/L (range, 162-2808 U/L) for M65, and 1197 (range, 2–44896) versus 4 (range, 0–487) for CTC number, respectively. M30, M65, and CTC number correlated with alkaline phosphatase, LDH (P < 0.001); and inversely with Na (P < 0.01). nDNA did not correlate with any clinical factors but correlated with LDH and alkaline phosphatase (P < 0.01).
Prognostic Significance Analysis
At the time of the analysis 60 patients in the total cohort had died and the median follow-up for surviving patients was 289 days (14–707 days). Of the CTC cohort of 50 patients, 32 patients had died with a median follow-up of 210 days. Of the 78 patient cohort with cell death biomarkers, 57 had died with a median follow-up of 336 days.
In univariate analysis, significant clinical and biochemical factors that adversely impacted on survival were stage, liver metastases, PS, Na, alkaline phosphatase, and LDH. Higher values of M30, M65, CTC number but not nDNA, were associated with shorter survival (Table 2). In the multivariable model, PS was the strongest factor for survival among standard clinical and biochemical factors. When cell death biomarkers were included in the model M65 (HR = 1.68 (95% confidence interval 1.13–2.51) P = 0.011) and LDH emerged as independent prognostic factors (HR 1.56 (95% confidence interval 1.06–2.29) P = 0.023), (Table 2). The median survival for patients with CTC number >300 (highest quartile) was 134 days compared with 443 days for patients with CTC number <2 (lowest quartile) (P < 0.005).The median survival for patients with M65 >1061 U/L (highest quartile) was 151 days compared with 388 days for patients with M65 <309 U/L (lowest quartile) (Figure 3) (P < 0.0001).
Table 2.
Prognostic Significance of Clinical Factors and Biomarkers
| Covariate | Univariate P value | Univariate HR | 95% Confidence interval | Risk group |
|---|---|---|---|---|
| Clinical factors | ||||
| Age | 0.091 | 1.02 | 1–1.05 | |
| PS | <0.001 | 2.63 | 1.8–4.1 | |
| Stage (limited versus extensive) | <0.001 | 0.38 | 0.22–0.66 | Extensive >limited |
| Liver metastases (Ab versus Pres) | <0.001 | 2.61 | 1.55–4.38 | Pres >Ab |
| Pre-therapy biomarkers | ||||
| LDH | <0.001 | 1.53 | 1.19–1.95 | High values |
| M30 | <0.001 | 1.74 | 1.3–2.33 | High values |
| M65 | <0.001 | 1.82 | 1.4–2.36 | High values |
| CTC no. | 0.015 | 1.1 | 1.02–1.18 | High values |
| Day 22 biomarkers | ||||
| LDH | 0.005 | 2.09 | 1.24–3.52 | High values |
| M30 | 0.011 | 1.54 | 1.1–2.14 | High values |
| M65 | 0.26 | 1.21 | 0.87–1.67 | |
| CTC no.
|
0.01
|
1.43
|
1.09–1.86
|
High values
|
| Covariate
|
Adjusted P value*
|
HR
|
95% Confidence interval
|
Risk group
|
| LDH | 0.023 | 1.56 | 1.06–2.29 | High values |
| M65 | 0.011 | 1.68 | 1.13–2.51 | High values |
Multivariable analysis.
Figure 3.
Kaplan-Meier curve demonstrating overall survival according to M65 determined before therapy.
Serial Assay of Cell Death Biomarkers and CTCs during Chemotherapy
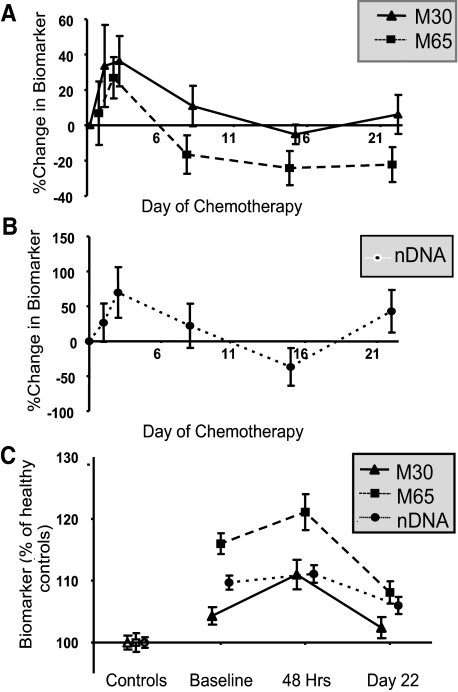
A pilot study to establish the optimal sampling times post therapy for the cell death assays in 12 patients demonstrated similar patterns with a rise in M30, M65 (Figure 4A), and nDNA (Figure 4B) 24 to 48 hours after chemotherapy, falling by day 22. Therefore the 48 hours and day 22 time points were selected for exploration in the larger cohort. Samples were evaluable for cell death biomarkers in 35 patients at 48 hours and 45 patients at day 22 (summarized in Figure 4C).
Figure 4.
Serial assays of serological cell death biomarkers during chemotherapy. A: Determination of optimal time points for sampling M30/M65 (n = 12). B: Determination of optimal time points for sampling nDNA (n = 12). C: Comparison of patient values (n = 39) with controls (n = 85).
M30 and M65 were higher at 48 hours compared with baseline [mean increase 50%, (95% confidence interval 19 to 81%) and 45%, (95% confidence interval 16 to 74%)], respectively, P < 0.001) (Figure 4C). By day 22 after treatment M30 values were similar to baseline, whereas M65 and nDNA were lower, but still higher than healthy controls (medians 363 U/L and 0.87 P < 0.001) (Figure 4C). Samples were evaluable for CTCs in 24 patients at day 22. The CTC number also decreased on day 22 (median CTC number = 1, range, 0–2960, mean ± SD = 210 ± 673 P < 0.05) (Figure 1B), and CTCs were detectable in only 60% of patients compared with 86% at baseline.
Relationship between Serial Assay of Cell Death Biomarkers with Survival, Chemotherapy Response, and Toxicity
Persistently elevated M30 (HR = 1.54, 95% confidence interval 1.10–2.14, P = 0.01) and CTC number (HR = 1.43, 95% confidence interval 1.09–1.86, P = 0.01) at day 22 were adverse prognostic factors in univariate analysis. There was a significant association between an increase in nDNA at 48 hours and response (n = 22) compared with stable disease (n = 10) (mean change +58% versus −41%, P < 0.05). Patients who developed toxicity requiring hospitalization had higher baseline levels of cell death biomarkers, potentially reflecting a higher disease burden and poorer performance status before therapy. The peak in M30 at 48 hours was significantly higher in patients requiring admission for toxicity (mean change 63% versus 15%, P < 0.05).
Discussion
Here we report for the first time the behavior of an integrated panel of cell death biomarkers, M30, M65, and nDNA, in patients undergoing standard chemotherapy treatment for SCLC together with enumeration and cell fate of CTCs. We demonstrate the large numbers of CTCs detectable in SCLC compared with other cancer types19 and their potential as a pharmacodynamic biomarker. While biomarker research is now an adjunct to most oncology drug trials, the data generated are usually exploratory, limited by the use of unvalidated assays and lacking sufficient robustness to inform on future clinical drug development. In our previous work these assays were validated and optimized to good clinical laboratory practice standard for clinical trials8,9 and the CellSearch technology for CTCs currently dominates the technology platforms for CTC enumeration with respect to reproducibility.15 Our current results will inform the application and interpretation of these bioassays when incorporated into upcoming trials of novel therapies for SCLC, not least those agents targeted to apoptosis regulatory components such as Bcl-2 family antagonists and inhibitors of the IAP family of proteins.20
Interest in serological biomarkers for SCLC has focused mainly on disease-related biomarkers rather than the mechanism-related biomarkers evaluated in the present study. Our data demonstrate the confounding influence of tumor burden and the prognostic importance of biomarkers related to the critical pathways of cell survival. Both M30 and M65 correlated with known clinical and biochemical prognostic factors in this study including stage and LDH. The latter, together with neuron specific enolase (NSE) and the precursor of gastrin-releasing peptide (proGRP) are among the best characterized prognostic markers of SCLC. Both serum NSE and proGRP are elevated in 60 to 90% of patients, correlate with stage and may predict for response to treatment21 and for survival.22 LDH is the most commonly used prognostic factor for SCLC in clinical trials although NSE may be superior.23 The results from this study suggest additional prognostic information from the measurement of M65 over LDH alone.
Although potentially counterintuitive, it is well recognized that increased tumor apoptosis and necrosis are associated with increased proliferation and higher grade and may be regarded as adverse biological factors.24 The disproportionately high pretherapeutic levels of M65 and nDNA with relatively normal M30 in this study are consistent with the large areas of necrosis commonly seen in SCLC at biopsy. There are conflicting reports for the clinical significance of pretherapeutic M30 in other types of cancer with one small series failing to confirm any associations25 but others noting correlation with stage.26 A recent study in lung cancer, biased to non-small cell lung cancer, but with 7 SCLC patients included, reported elevated baseline M30 as a poor prognostic factor consistent with our data.27 No data have yet been published on the prognostic significance of M65; however, tissue polypeptide antigen, which recognizes complexes of CKs 8, 18, and 19, has previously been reported as a prognostic factor in SCLC.28
The pattern of release of the cell death biomarkers into blood following chemotherapy treatment is consistent with pharmacodynamic relevance. Elevated levels of circulating free DNA are detectable in cancer patients including those with SCLC29 and several studies have supported a role for nDNA in monitoring and predicting clinical outcome.30 In a recent study of 128 patients with SCLC the utility of nDNA measured immediately before the first three cycles of therapy was compared with a panel of other putative SCLC biomarkers (CYFRA21-1, CEA, NSE, and proGRP). They found that falling levels of all biomarkers, including nDNA, at the start of cycle 2 could be used with reasonable sensitivity and specificity to predict early response to therapy. Interestingly in a multivariate model only PS and the value of nDNA immediately before the second cycle predicted response to therapy. In our study we also observed lower baseline nDNA levels in patients with early response to therapy, but did not see any prognostic significance of the level immediately before the second cycle.31 In our study we show for the first time a surge in nDNA at 48 hours following therapy in keeping with increased cell death, predicted for early response to chemotherapy treatment.
Several studies have reported increases in M30 after chemotherapy, although the ability of this peak to predict tumor response has varied between reports, disease type, and chemotherapy administered.27,32,33 We did not confirm a statistically significant relationship between radiological response and M30 or M65 dynamics in this study. This may be due to the high response and disease stabilization rate compared with the few non-responders in the cohort.
In this series, a rise in M30 at 48 hours after chemotherapy was found to correlate with severe toxicity requiring hospital treatment. Kramer et al previously attributed a rise in M65 to toxicity from estramustine among patients with prostate cancer on the basis of a lack of a corresponding rise in M30 and fall in prostate specific antigen but did not demonstrate any relationship to clinical toxicity.32 In this study both M30 and M65 rose in response to treatment; however, patients experiencing serious toxicity had a disproportionate rise in M30 at 48 hours, suggesting that M30 reports apoptosis of normal epithelium, in addition to tumor cell death. Indeed, recent reports have suggested utility of M30 in the assessment of hepatitis and sepsis.34,35 SCLC tumor markers proGRP and NSE may be complementary to M30 and M65 in future studies to determine the dominant process leading to CK18 release.
The choice of sample collection time points is vital in the application of these assays to early clinical trials. Consistent with our results, other studies have observed peak M30 and nDNA levels at 24 to 48 hours after chemotherapy.27,31,32 However, this may not hold true for novel agents36 where it would be prudent to perform pilot studies to select optimal time points.
Our findings illustrate several caveats to the interpretation of cell death biomarkers in early clinical trials due to confounding influences of tumor burden, prognostic significance and lack of specificity for cancer cells. In the absence of tumor biopsies, CTCs hold great promise as a surrogate tissue. Although SCLC is often referred to as a neuroendocrine tumor, it is an epithelial tumor that co-expresses neural, endocrine, and epithelial markers37 and so as we show, the latter can be exploited for detection of CTCs. This was first observed in a study by Kularatne et al who detected CTCs from 11 SCLC patients by flow cytometry using an anti-EpCAM antibody. However, the methodology proved frustrating for further clinical application due to a high false positive rate and considerable background noise.38 A second preliminary study of lung cancer patients conducted using unvalidated manual technology and where just 13 patients had SCLC, found CTCs in a similar proportion of patients to that reported here but with smaller numbers than we observed.39
To our knowledge this is the first report on CellSearch technology applied to patients with SCLC. We demonstrate detection of CTCs in the majority of patients, and in abundance compared with other types of cancer. In the study of Allard et al20 that used CellSearch to analyze samples from 964 cancer patients, including 90 patients with unspecified lung cancer subtype, the range of CTCs detected was comparable with that of the present study. The mean CTC number detected in SCLC patients in our study was substantially higher at 2915 ± 8115 than that of 61 ± 696 obtained for all 964 cancer patients, and 30 ± 178 for the 90 lung cancer patients. Also, the majority of SCLC patients (78%) had ≥2 CTCs compared with 36% of all samples and only 20% of the lung cancer patients’ samples in the Allard study, suggesting the latter to be predominantly non-small cell lung cancer.
The high prevalence and abundance of CTCs in this series of patients concurs with the known rapid doubling time, propensity for early metastasis, and aggressive clinical course in SCLC.1 Here, CTCs correlate with known prognostic factors, in addition to pretreatment M65. The agreement between levels of the circulating apoptotic-specific protein M30 and the proportion of morphologically apoptotic CTCs strengthens the evidence for M30 to report tumor apoptosis and CTCs to reflect underlying disease response.
The observation that only seven patients had no CTCs, all of whom had limited stage, raises the question of whether CTC number can identify patients with a higher chance of cure following radical chemoradiotherapy. CTCs decreased in all patients after one cycle of chemotherapy supporting CTC as a pharmacodynamic biomarker for SCLC. The abundance of CTCs in SCLC patients also suggests that this technology could be more readily exploited for molecular analysis of CTCs than may be possible for other tumor types. Further, larger studies are warranted to determine the predictive and prognostic value of CTCs in SCLC, to molecularly characterize CTCs and correlate their biology to that of the primary tumor.
In summary, the interpretation of biomarker assays can be complex due to disease and treatment related effects. The data presented here reinforce the importance of assessing fitness for purpose of biomarkers in relevant patient populations, before their incorporation into clinical trials. We conclude that both blood-borne cell death biomarkers and CTCs have the potential to enhance drug development as pharmacodynamic biomarkers and demonstrate for the first time a strong rationale to incorporate CTC analysis in early clinical trials for this disease.
Acknowledgments
We thank Linda Ashcroft for constructive criticism of the manuscript and Lynsey Priest and Nicky Griffiths for assistance with data management.
Footnotes
Address reprint requests to F. Blackhall or Caroline Dive, Clinical and Experimental Pharmacology, Paterson Institute for Cancer Research, Wilmslow Road, Manchester, M20 4BX, UK. E-mail: fiona.blackhall@christie or cdive@picr.man.ac.uk.
Supported by Cancer Research UK grant C147/A6058 and European Union CHEMORES FP6 contract LSHC-CT-2007-037665; Cancer Research UK China fellowship C480/A7421 (to J.-M.H.); and a clinical pharmacology fellowship from Cancer Research UK and AstraZeneca Ltd. (to A.G.).
J.-M.H. and A.G. contributed equally to this work.
References
- Murray N, Turrisi AT., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- Blackhall FH, Shepherd FA. Small cell lung cancer and targeted therapies. Curr Opin Oncol. 2007;19:103–108. doi: 10.1097/CCO.0b013e328011bec3. [DOI] [PubMed] [Google Scholar]
- Workman P, Aboagye EO, Chung YL, Griffiths JR, Hart R, Leach MO, Maxwell RJ, McSheehy PMJ, Price PM, Zweit J. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J Natl Cancer Inst. 2006;98:580–598. doi: 10.1093/jnci/djj162. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P. Apoptotic markers in cancer. Clin Biochem. 2004;37:605–617. doi: 10.1016/j.clinbiochem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Biven K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–1756. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- Greystoke A, Cummings J, Ward T, Simpson K, Renehan A, Butt F, Moore D, Gietema J, Blackhall F, Ranson M, Hughes A, Dive C. Optimisation of circulating biomarkers of cell death for routine clinical use. Ann Oncol. 2008;19:990–995. doi: 10.1093/annonc/mdn014. [DOI] [PubMed] [Google Scholar]
- Jiang N, Reich CF, Pisetsky DS. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood. 2003;102:2243–2250. doi: 10.1182/blood-2002-10-3312. [DOI] [PubMed] [Google Scholar]
- Cummings J, Ward TH, LaCasse E, Lefebvre C, St-Jean M, Durkin J, Ranson M, Dive C. Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2005;92:532–538. doi: 10.1038/sj.bjc.6602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, Durkin J, Dive C. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound ( AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42–48. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Keilholz U, Rossi CR, Donato N. Circulating tumor cells: the ‘leukemic phase’ of solid cancers. Trends Mol Med. 2006;12:130–139. doi: 10.1016/j.molmed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MG, Matera J, Allard WJ, Doyle GV, Terstappen L. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen L, Meropol NJ. Relationship of circulating tumor cells to tumor response: progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P, Bodenmuller H, Fertig G, Furst H, Schmeller N, Untch M, Seidel D. Nucleosomes in serum as a marker for cell death. Clin Chem Lab Med. 2001;39:596–605. doi: 10.1515/CCLM.2001.095. [DOI] [PubMed] [Google Scholar]
- Tibbe AG, de Grooth BG, Greve J, Liberti PA, Dolan GJ, Terstappen LW. Optical tracking and detection of immunomagnetically selected and aligned cells. Nature Biotechnol. 1999;17:1210–1213. doi: 10.1038/70761. [DOI] [PubMed] [Google Scholar]
- Greystoke A, Moore D, Butt F, Cummings J, Ranson M, Radford J, Blackhall F, Hughes A, Dive C: The utility of serological cell death biomarkers to guide development of new therapeutics. National Cancer Research Institute Cancer Conference, October 5–8, 2008, Birmingham UK, Abstract A92N, NCRI Nov 2008 available at http://www.ncri.org.uk/ncriconference/2008abstracts/abstracts/A92.htm [Google Scholar]
- de Haas EC, di Pietro A, Simpson KL, Meijer C, Suurmeijer AJ, Lancashire LJ, Cummings J, de Jong S, de Vries EG, Dive C, Gietema JA. Clinical evaluation of M30 and M65 ELISA cell death assays as circulating biomarkers in a drug-sensitive tumor, testicular cancer. Neoplasia. 2008;10:1041–1048. doi: 10.1593/neo.08620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Eur J Cancer. 2005;41:1690–1696. doi: 10.1016/j.ejca.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe A, Uhr JW, Terstappen L. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with non-malignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Taylor K, Micha D, Ranson M, Dive C. Recent advances in targeting regulators of apoptosis in cancer cells for therapeutic gain. Expert Opin Invest Drugs. 2006;15:669–690. doi: 10.1517/13543784.15.6.669. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Sloan JA, Rowland KM, Jr, Klee GG, Kugler JW, Mailliard JA, Wiesenfeld M, Krook JE, Maksymiuk AW, Shaw EG, Marks RS, Perez EA. Significance of neuron-specific enolase levels before and during therapy for small cell lung cancer. Clin Cancer Res. 2000;6:597–601. [PubMed] [Google Scholar]
- Bremnes RM, Sundstrom S, Aasebo U, Kaasa S, Hatlevoll R, Aamdal S. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39:303–313. doi: 10.1016/s0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- Jorgensen LG, Osterlind K, Genolla J, Gomms A, Hernadezj R, Johnson P, Lober J, Splinter T, Szturmowicz M. Serum neuron-specific enolase (S-NSE) and the prognosis in small-cell lung cancer (SCLC): a combined multivariable analysis on data from nine centres. Br J Cancer. 1996;74:463–467. doi: 10.1038/bjc.1996.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupa JD, de Bruine AP, Gerbers AJ, Leers MP, Nap M, Kessels AG, Schutte B, Arends JW. Simultaneous detection of apoptosis and proliferation in colorectal carcinoma by multiparameter flow cytometry allows separation of high and low-turnover tumors with distinct clinical outcome. Cancer. 2003;97:2404–2411. doi: 10.1002/cncr.11366. [DOI] [PubMed] [Google Scholar]
- Pichon MF, Labroquere M, Rezai K, Lokiec F. Variations of soluble fas and cytokeratin 18-asp 396 neo-epitope in different cancers during chemotherapy. Anticancer Res. 2006;26:2387–2392. [PubMed] [Google Scholar]
- Ueno T, Toi M, Biven K, Bando H, Ogawa T, Linder S. Measurement of an apoptotic product in the sera of breast cancer patients. Eur J Cancer. 2003;39:769–774. doi: 10.1016/s0959-8049(02)00865-1. [DOI] [PubMed] [Google Scholar]
- Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007;56:399–404. doi: 10.1016/j.lungcan.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Buccheri G, Ferrigno D. Serum biomarkers of non-neuron-endocrine origin in small-cell lung cancer: a 16-year study on carcinoembryonic antigen, tissue polypeptide antigen and lactate dehydrogenase. Lung Cancer. 2000;30:37–49. doi: 10.1016/s0169-5002(00)00123-9. [DOI] [PubMed] [Google Scholar]
- Board R, Williams S, Knight L, Shaw J, Greystoke A, Ranson M, Dive C, Hughes A. Detection of circulating tumor DNA in patients with small cell lung cancer. Ann NY Acad Sci. 2008;1137:98–107. doi: 10.1196/annals.1448.020. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J, Raith H, Feldmann K, Kremer AE, Muller S, Geiger S, Hamann GF, Seidel D, Stieber P. Clinical relevance of circulating nucleosomes in cancer. Ann NY Acad Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, von Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K, Hoffmann H, Raith H, Nagel D, Stieber P. Nucleosomes. ProGRP, NSE, CYFRA 21-1, and CEA in monitoring first-line chemotherapy of small cell lung cancer. Clin Cancer Res. 2008;14:7813–7821. doi: 10.1158/1078-0432.CCR-08-0678. [DOI] [PubMed] [Google Scholar]
- Kramer G, Schwarz S, Hagg M, Havelka AM, Linder S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer. 2006;94:1592–1598. doi: 10.1038/sj.bjc.6603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson MH, Ueno T, Pan Y, Xu R, Cai F, Heiko V, Thomas EM, Maike S, Walter EA, Stephan S, Elina A, Maria CS, Aleksandra MH, Masakazu T, Stig L. Cytokeratin-18 is a useful serum biomarker for early determination of response of breast carcinomas to chemotherapy. Clin Cancer Res. 2007;13:3198–3206. doi: 10.1158/1078-0432.CCR-07-0009. [DOI] [PubMed] [Google Scholar]
- Roth GA, Krenn C, Brunner M, Moser B, Ploder M, Spittler A, Pelinka L, Sautner T, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope m30 in critically ill patients. Shock. 2004;22:218–220. doi: 10.1097/01.shk.0000136098.49672.0e. [DOI] [PubMed] [Google Scholar]
- Kronenberger B, Wagner M, Herrmann E, Mihm U, Piiper A, Sarrazin C, Zeuzem S. Apoptotic cytokeratin 18 neoepitopes in serum of patients with chronic hepatitis C. J Viral Hepatol. 2005;12:307–314. doi: 10.1111/j.1365-2893.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Cummings J, Hodgkinson C, Odedra R, Sini P, Heaton SP, Mundt KE, Ward TH, Wilkinson RW, Growcott J, Hughes A, Dive C. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol Cancer Ther. 2008;7:455–463. doi: 10.1158/1535-7163.MCT-07-2136. [DOI] [PubMed] [Google Scholar]
- Brambilla E, Moro D, Gazzeri S, Brichon PY, Nagy-Mignotte HN, Morel F, Jacrot M, Brambilla C. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J Clin Oncol. 1991;9:50–61. doi: 10.1200/JCO.1991.9.1.50. [DOI] [PubMed] [Google Scholar]
- Kularatne BY, Lorigan P, Browne S, Suvarna SK, Smith MO, Lawry J. Monitoring tumor cells in the peripheral blood of small cell lung cancer patients. Cytometry. 2002;50:160–167. doi: 10.1002/cyto.10071. [DOI] [PubMed] [Google Scholar]
- Wu C, Hao H, Li L, Zhou X, Guo Z, Zhang L, Zhang X, Zhong W, Guo H, Bremner RM, Lin P. Preliminary investigation of the clinical significance of detecting circulating tumor cells enriched from lung cancer patients. J Thorac Oncol. 2009;4:30–36. doi: 10.1097/JTO.0b013e3181914125. [DOI] [PubMed] [Google Scholar]