Abstract
Using immunohistochemistry with antibodies against the phosphoserine residues in both S6rp and 4E binding protein 1, we identified the activation of the mammalian target of rapamycin (mTORC)1 pathway in 29 cases of AIDS-related lymphoma. These cases represented a diverse spectrum of histological types of non-Hodgkin lymphoma (24 cases) and classic Hodgkin lymphoma (five cases). mTORC1 was also activated in the hyperplastic but not involuted follicles of HIV-associated lymphadenopathy in eight cases, supporting the notion that mTORC1 activation is a common feature of transformed lymphocytes irrespective of either their reactive or malignant phenotype. We also found that in B-cell lines that represent diffuse large B-cell lymphoma, Burkitt lymphoma, Epstein-Barr virus-infected lymphocytes, and human herpesvirus 8-positive primary effusion lymphoma, inhibitors of Syk, MEK, and, seemingly, phosphoinositide 3 kinases suppressed mTORC1 activation, in particular when these inhibitors were used in combination. These findings indicate that AIDS-related lymphoma and other histologically similar types of lymphomas that are derived from transformed B lymphocytes may display clinical responses to inhibitors that directly target mTORC1 or, possibly, upstream activators of the mTORC1 pathway.
The incidence of lymphomas in HIV-positive patients is nearly 200 times higher than in those uninfected by the virus. Lymphomas accounts for an increasing percentage of AIDS-defining illness, particularly from the advent of highly active antiretroviral therapy therapy.1,2 These AIDS-related lymphomas (ARLs) typically represent the proliferation of enlarged, transformed B lymphocytes, which usually fall into the category of diffuse large cell lymphoma (DLBCL), with morphology ranging from centroblasts to immunoblasts. Other histological types such as Burkitt lymphoma (BL), Hodgkin lymphoma, and T/null cell anaplastic large cell lymphoma are also overrepresented among ARLs.1,2,3,4 The pathogenesis of ARL is poorly understood. It has been postulated that cell proliferation occurring in the setting of severe immunosuppression and driven by chronic antigenemia resulting at first in the polyclonal and ultimately in the monoclonal lymphoproliferation plays a key role in lymphomagenesis in HIV patients. In addition, cell infection by the Epstein-Barr virus (EBV) and human herpesvirus 8 (HHV8) most likely contributes to the malignant cell phenotype in some subtypes of ARL, with the association of primary effusion lymphoma with HHV8 being essentially universal.1,2,3,4 Regardless of the histological type of ARL, chemotherapy is typically ineffective and new treatment approaches are clearly needed to combat this group of lymphomas. In addition to ARL, HIV patients develop a benign reactive lymphadenopathy, particularly early after the infection as an overall ineffective response to the virus. This immune response is characterized by florid follicular hyperplasia that over time may lead to follicular involution and lymphocyte depletion.
Mammalian target of rapamycin (mTOR) is a ubiquitously expressed serine/threonine kinase involved in key cellular functions including protein synthesis and proliferation.5,6 mTOR associates with several proteins including either raptor or rictor to form the mTORC1 and mTORC2 complexes, respectively, with the signaling pathways activated by mTORC1 being much better characterized.4,5,6,7 Accordingly, it is well established that mTORC1 activates p70S6 kinase 1 and inhibits 4E binding protein 1 (4E-BP1). In turn, p70S6 kinase 1 phosphorylates an S6 protein of the 40S ribosomal subunit (S6rp) at several sites including serines 235 and 236. The exact mechanisms of mTORC1 activation are less understood but both phosphoinositide 3 kinases (PI3K)/Akt8,9,10 and extracellular regulated (ERK)/mitogen-activated kinase (MEK) kinases11,12 signaling pathways have been found to activate mTORC1 with members of the insulin growth factor family providing the primary signal, at least in some instances. The highly potent and specific inhibitors from the rapamycin family can functionally inactivate mTORC1. In addition to being used as immunosuppressants, mTORC1 inhibitors are evaluated as therapeutic agents in various types of cancer,5,6 with high efficacy already documented in renal cell carcinoma.13
Syk is a protein tyrosine kinase expressed in B lymphocytes,14 monocytes/macrophages,15 mast cells,16 and other cell types. Syk has been found to be involved in signal transduction through several types of receptors including the antigen B-cell receptor17 and at least three different receptors for the Fc component of immunoglobulins G and E.14,15,16,18,19,20 A recent report suggests that Syk may be involved in mTORC1 activation in a follicular and possibly other types of B-cell lymphoma.21 Although inhibitors of either PI3K/Akt or MEK/ERK signaling pathways did fully inhibit mTORC1 activation in transformed B lymphocytes, at least when applied alone,22 these pathways have been found to contribute to mTORC1 stimulation in two types of T-cell lymphoma.23,24
In this study, we identified the activation of the mTORC1 pathway in all ARL cases examined, regardless of their specific histological classification and immunophenotype. mTORC1 was also activated in the hyperplastic follicles of the HIV-associated lymphadenopathy. Furthermore, we found that in the different types of transformed B-lymphocytes cell lines, inhibition of Syk, MEK, and, seemingly, PI3K resulted in suppression of the mTORC1 activation, in particular when the combination of the inhibitors was used. These findings indicate that ARL and histologically similar types of lymphoma may benefit from targeted therapy with inhibitors of mTORC1 or, possibly, its upstream activators.
Materials and Methods
Patient Samples
We examined tissue samples from a total of 37 HIV patients. These samples comprised 24 cases of non-Hodgkin lymphoma, 5 cases of Hodgkin lymphoma, and 8 reactive HIV-associated lymphadenopathy specimens. The cases were identified in the files of the Department of Pathology and Laboratory Medicine at the hospitals affiliated with the University of Pennsylvania. The samples included mostly lymph node specimens, as well as small diagnostic and large excisional biopsies of different solid organs. All cases were classified as HIV-related lymphadenopathy or ARL lymphomas based on the clinical history including HIV seropositivity, tissue and cell morphology, immunohistochemistry, flow cytometry, and molecular assays. Eight of the 17 evaluated ARL expressed the EBV genome as determined by in situ hybridization for EBV small RNA. Four out the 7 EBV small RNA-positive cases were also positive for late membrane protein 1. One of the plasmablastic lymphoma cases was positive for the HHV8-associated antigen latency-associated nuclear antigen-1. The study was performed according to the Institutional Review Board-approved protocol.
Cell Lines
The cell lines used in this study have been described in detail before.22,23 The LY18 and Val lines were derived from the DLBCL of germinal center origin. The Raji, Daudi, and Ramos cell lines were obtained from BL. The MM and HH lines were established in our laboratory by the EBV-mediated immortalization of peripheral blood B lymphocytes. BCBL-1, BC-1, and JSC-1 were derived from primary effusion lymphoma and are HHV8-positive. Sez-4 and MyLa3675 were from cutaneous T-cell lymphoma. All cell lines were cultured in RPMI (Invitrogen) supplemented with 10% fetal bovine serum (Mediatech, Manassas, VA).
Inhibitors
Piceatannol, Syk inhibitors I and II, and wortmannin were purchased from Calbiochem and rapamycin and U0126 were purchased from Cell Signaling Technology (Beverley, MA). The inhibitors were reconstituted in dimethyl sulfoxide to the suitable stock concentration and diluted shortly before the experiment to the final concentrations depicted in the Figures.
Immunohistochemistry
Immunohistochemistry was performed using antibodies that recognize phospho (p)-4E-BP1 (Ser65) and p-S6rp (Ser 235/236) (both from Cell Signaling) and a streptavidin horseradish immunoperoxidase method, as described previously.23,24,25 In brief, 5-μm sections from formalin-fixed, paraffin-embedded tissue specimens were deparaffinized in xylene and rehydrated in graded alcohol. After the deparaffinization, heat induced antigen retrieval was performed by boiling the slides in 10 μmol/L citrate buffer for 20 minutes. The sections were then blocked for 10 minutes with a peroxidase blocking system from Dako. Sequential 20- to 30-minute incubations included the following: 2% normal goat serum, affinity purified rabbit polyclonal primary antibodies at optimized dilution; secondary biotinylated anti-rabbit IgG, the streptavidin-biotinylated horseradish peroxidase complex reagent (Dako). The sections were then exposed to the chromagen diaminobenzidine plus from Dako for 5 minutes and were counterstained in hematoxylin, dehydrated, cleared, and mounted. Two pathologists examined the stained slides independently with 100% concordance. The samples were designated as positive when at least 50% of the lesional cells were positive in the properly stained regions with >90% of the cells staining in the majority of cases. Small resting lymphocytes served as an internal negative and plasma cells as positive control for both p-S6rp and p-4E-BP1. Endothelial cells served as an additional positive internal control for p-S6rp. Plasma cells served as internal positive control whenever applicable for the three immunohistochemical antibodies. Interpretations were based on the cell morphology and correlation with standard diagnostic immunohistochemical stains such as CD3, CD5, CD4, CD8, CD79a, CD20, CD 15, CD30, and late membrane protein-1.
Western Blot
The assay was performed as previously described.22,23,24 In brief, the cells were washed briefly in PBS, centrifuged and lysed in an radioimmunoprecipitation assay buffer supplemented with 0.5 mmol/L phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktails I and II from Sigma and protease inhibitor cocktail from Roche (Nutley, NJ), according to the manufacturer’s specifications. For normalization of the gel loading, the protein extracts were assayed with the Lowry method (Bio-Rad Dc protein assay). Typically, 40 μg of the protein was loaded into each lane. To examine protein expression and phosphorylation, the membranes were incubated with antibodies from Cell Signaling specific for p-S6rp (Ser235/236), total S6rp, p-Syk (Tyr525/526), or phosphorylated-signal transducer and activator of transcription (STAT)3 (Tyr705), or ß-actin antibody (Santa Cruz Biotech, Santa Cruz, CA). Next, the membranes were incubated with the appropriate secondary, peroxidase-conjugated antibodies. The blots were developed using the Lumingen PS-3 detection reagent from Amersham Biosciences.
Bromodeoxyuridine Incorporation
The assay was performed as previously described,22,24 In brief, cell proliferation was evaluated in bromodeoxyuridine (BrdU) incorporation assay using the commercially available kit Cell Proliferation enzyme-linked immunosorbent assay (Roche) according to the manufacturer’s protocol. In brief, cells were seeded in 96-well plates (Corning) at concentration of 1 × 104 cells/well in RPMI medium supplemented with 10% fetal bovine serum, cultured for 48 hours in the presence of kinase inhibitors and labeled with BrdU (Roche) for 4 hours. The amount of incorporated BrdU was determined by incubation with a specific antibody conjugated with peroxidase followed by colorimetric conversion of the substrate and optical density evaluation in the enzyme-linked immunosorbent assay plate reader.
Results
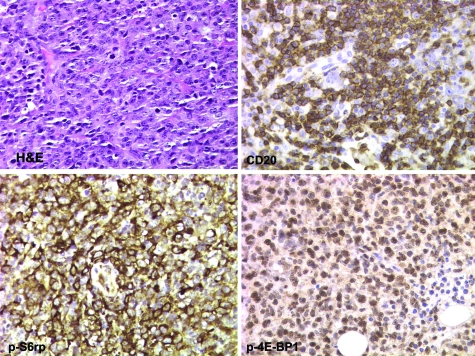
To determine the activation status of the mTORC1 signaling pathway in ARL tissues, we evaluated a total of 29 cases of ARL, 5 of which were classified as Hodgkin lymphoma (classical type), and 24 as non-Hodgkin lymphoma. The non-Hodgkin lymphoma cases comprised DLBCL (16 cases), anaplastic large cell lymphoma, anaplastic lymphoma kinase-negative (2), follicular lymphoma, grade 3/3 (1), BL (2), plasmablastic lymphoma (2), and peripheral T-cell lymphoma (1). We used in this analysis the phospho-specific antibodies against S6rp (Ser235/236) and p-4E-BP1 (Ser65), which we have validated extensively in our recent studies.23,24,25 Whereas these antibodies have proven highly suitable for immunohistochemistry, an antibody against phosphorylated p70S6K (Thr389) did not, due to its cross-reactivity with p-p90S6K, which is not phosphorylated by mTORC1.25 The representative results of the current evaluation of the mTORC1 signaling pathway activation in ARL are depicted in Figures 1 to 3. We began our analysis with the most frequent ARL type, DLBCL. The large lymphoma cells strongly expressed the p-S6rp and p-p4E-BP1 in all cases examined (Figure 1 depicts a representative case). In most cases, reactivity was identified in the vast majority, if not all, of the malignant-appearing cells. The mTORC1 activation was present in the ARL regardless of their EBV-genome expression status.
Figure 1.
Activation of the mTORC1 signaling pathway in ARL, DLBCL type. A section of a lymph node reveals high-grade histology with large atypical cells, atypical mitoses, and brisk apoptotic rate. Immunohistochemical stains for a B-cell marker CD20, mTORC1-dependent phosphorylation of S6rp (at Ser235/236) and 4E-BP1 (at Ser65). All images are at ×400 magnification.
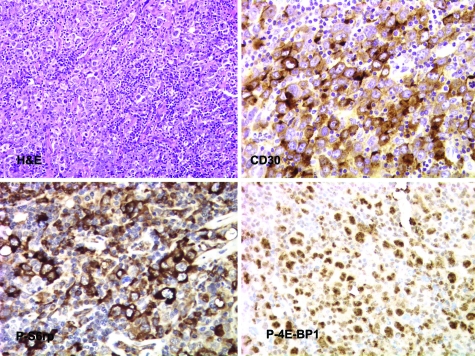
Figure 2.
Activation of the mTORC1 pathway in ARL with anaplastic large cell morphology. A section of a lymph node shows large atypical cells with irregular nuclei intermixed with smaller lymphocytes. Immunohistochemical stains for anaplastic large cell lymphoma-associated CD30 antigen, and phospho-(p-)S6rp (Ser235/236) and p-4E-BP1 (Ser65). All images are ×400 magnifications.
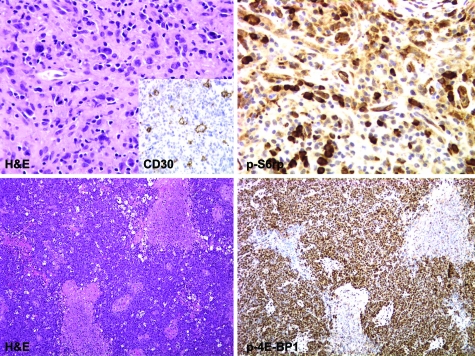
Figure 3.
mTORC1 signaling in ARL, Hodgkin lymphoma (upper panels), and Burkitt-type lymphoma (lower panels). Upper panels: Section of a lymph node shows scattered large atypical Reed-Sternberg cells in a background of fibrosis and small lymphoplasmacytic infiltrate staining for CD30 antigen (inset) and p-S6rp (Ser235/236) staining (×400 magnifications). Lower panels: Sheets of medium-sized lymphocytes in a background of necrosis and scattered macrophages stained for p-4E-BP1 (Ser65) (×100 magnifications).
The subsequent analyses of other ARL subtypes indicated that activation of the mTORC1 signaling pathway is ubiquitous in these lymphomas, irrespective of their histological classification. In addition, mTORC1 was activated in BL (Figure 2 top right panel) and was also seen in Reed-Sternberg cells of Hodgkin lymphoma (Figure 2D, lower right panel). The staining was particularly prominent in cases of anaplastic large cell lymphoma (Figure 2). Finally, activated mTORC1 was also seen in Reed-Sternberg cells of Hodgkin lymphoma (Figure 3, top panel) and in BL (Figure 3, bottom panel). In total, p-S6rp could be detected in 28 out of the 29 cases and p-p4E-BP1 in all 22 cases examined.
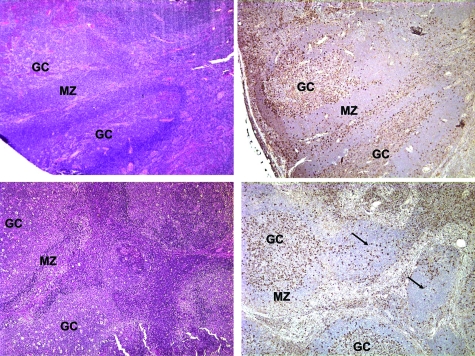
The prevalence of mTORC1 activation in various ARL histological types indirectly supported our previous notion25 that the mobilization of the mTORC1 signaling pathway may be a feature of lymphocyte activation in general rather than specifically of the malignant cell transformation. Indeed, when we next evaluated eight cases of the reactive, HIV-associated lymphadenopathy, marked mTORC1 activation was identified in the germinal centers of the floridly hyperplastic follicles and, to a lesser degree, in enlarged lymphocytes in the interfollicular areas (Figure 4). The remaining cells, including essentially all cells in the follicle mantle zone, were negative. Of note, in the areas of follicular involution where only a few to no enlarged cells are typically present, mTORC1 activation was markedly decreased or absent.
Figure 4.
mTORC1 activation pattern in reactive HIV-related lymphadenopathy. Florid follicular hyperplasia and follicular hyperplasia and involution (upper and lower left panels, respectively) stained for p-S6rp (right panels). The arrows point to the involuted follicles. All images are 400x magnifications. GC, germinal center; MZ, mantle zone.
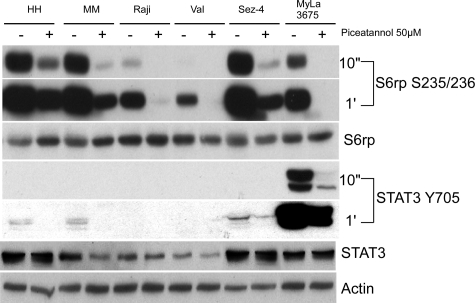
One of the key, yet still poorly understood issues is the exact mechanism of mTORC1 activation. A recent report21 suggested that Syk kinase is responsible for mTORC1 activation in at least some types of malignant B lymphocytes. This conclusion was made mainly by using two cell lines derived from follicular lymphoma, Karpas-422 and RL and the Syk inhibitor called piceatannol shown to be fully effective in regard to mTORC1 activation when applied at the high dose of 50 μmol/L. In our hands, piceatannol inhibited mTORC1 activation in the variety of transformed B-cell lines derived from DLBCL, BL, and EBV-infected lymphocytes (Figure 5). This observation suggests that Syk may indeed play a role in mTORC1 mobilization in the large spectrum of B-cell lymphomas. However, mTORC1 inhibition occurred not only in the transformed B cells but also in cutaneous T-cell lymphoma cell lines in which PI3K/Akt signaling pathway and, to the lesser degree, MEK/ERK pathway activate mTORC1.23 Furthermore, phosphorylation of the control protein STAT3 was also suppressed in all cell lines. These observations raise serious concerns regarding the specificity of piceatannol as the selective Syk inhibitor.
Figure 5.
Effect of piceatannol on mTORC1 activation. Transformed B-cell lines (EBV-immortalized MM and HH, BL-derived Raji, and DLBCL-derived Val) and cutaneous T-cell lymphoma cell lines (Sez-4 and MyLa 3676) were incubated for 30 minutes with 50 μmol/L of piceatannol and evaluated for expression of p-S6rp and p-STAT3. The phosphorylation of both proteins was examined after film exposure for 10 seconds and 1 minute.
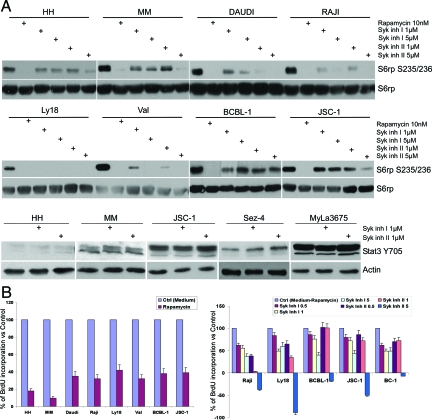
To address the question of the putative role of Syk more precisely, we used two potent Syk inhibitors that are structurally very different from piceatannol and from each other using rapamycin, a direct inhibitor of mTORC1, as control. As shown in Figure 6A, both Syk inhibitors (labeled Syk inhibitor I and II) displayed significant suppressive effect on the mTORC1 activation in the spectrum of B-cell lines representing DLBCL, BL, EBV + lymphoblastoid cell lines (LCL) and, in addition, HHV8-positive primary effusion lymphoma. However, the degree of inhibition varied among the cell lines and overall was less pronounced when compared with rapamycin. As anticipated, the inhibitors had no detectable effect on phosphorylation of STAT3 in either B- or T-cell lines.
Figure 6.
Role of Syk in mTORC1 activation and cell proliferation. A: The listed transformed B-cell lines were incubated for 30 minutes with the depicted doses of the mTORC1 inhibitor rapamycin or Syk inhibitors designated inhibitor I and II. Subsequently, they were evaluated for S6rp S235/236 phosphorylation and S6rp expression by Western blotting. As a specificity control, the selected B- and T-cell lines exposed to the Syk inhibitors were also examined for STAT3 Y705 phosphorylation. B: B-cell lines cultured for 48 hours in the presence of medium or 100 nmol/L of rapamycin (left panel) or medium, rapamycin, or Syk inhibitors at the depicted μM concentrations (right panel) were evaluated for BrdU incorporation in the colorimetric conversion assay. The results are presented as a percentage of the optical density (O.D.) value in relationship to cell cultures with medium (left panel) or medium versus rapamycin calculated as 100% and 0%, respectively.
We evaluated next the effect of Syk inhibitors on the cell line BrdU incorporation as compared with rapamycin (Figure 6B). As expected from the previous studies,22,26,27 the direct mTORC1 inhibition by rapamycin profoundly inhibited proliferation of the transformed B-cell lines used in this study (left panel). Similarly, both Syk inhibitors suppressed cell proliferation in the dose-dependent fashion. However, their effect was invariably much weaker than of rapamycin despite being used at the relatively high doses of up to 100 and 300 times the IC50 concentration required to inhibit Syk kinase in vitro in the cell-free conditions. The Syk inhibitor II applied at the relatively high dose of 5 μmol/L was very inhibitory likely due to an mTORC1 independent effect.
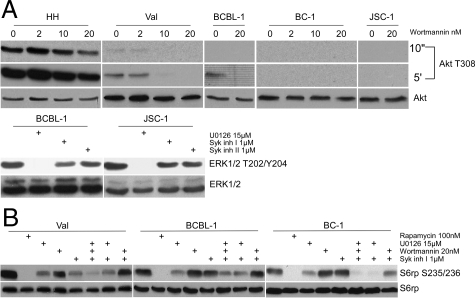
Because of the only partial impact of Syk inhibition on mTORC1 activation, we next compared this effect to the result of inhibition of PI3K/Akt and MEK/ERK, previously found by us to contribute to mTROC1 activation in T-cell lymphomas.23,24 As reported previously22 and shown in Figure 7A (upper panel), with the notable exception of the EBV-carrying cells, the other types of transformed B-cells including three HHV8-positive cell lines, expressed small to nondetectable amounts of phosphorylated Akt suggesting a low degree to possibly lack of activation of the PI3K/Akt pathway. Where visible, the Akt phosphorylation was inhibited by a potent PI3K inhibitor wortmannin in a dose-dependent fashion. Activation of the MEK/ERK pathway as determined by ERK phosphorylation was also diverse (Figure 7A, lower panel and ref. 22), but seemingly more frequent including its presence in the HHV8-positive cell lines. The ERK phosphorylation could be inhibited by the well-characterized MEK inhibitor U0126,22,23,24 while the control Syk inhibitors displayed no effect. As shown in Figure 7B, U0126 partially inhibited mTORC1 activation as determined by S6rp phosphorylation. The effect of wortmannin was less clear. Of note, combination of the kinase inhibitors was overall more effective than any of the inhibitors alone, in particular when U0126 was used together with the Syk inhibitor.
Figure 7.
Mechanisms of mTORC1 activation. A: The listed transformed B-cell lines were incubated for 30 minutes with medium or inhibitors of the PI3K/Akt (wortmannin) and MEK/ERK (U0126) signaling pathways and examined by Western blotting using antibodies against phosphorylated Akt and ERK1/2, respectively. B: The selected B-cell lines were incubated for 30 minutes with the depicted inhibitors and examined by Western blotting for S6rp S235/236 phosphorylation and, as a control, S6rp expression.
Discussion
By using immunohistochemical staining with antibodies specific for the phosphorylated forms of S6rp (Ser235/236) and 4E-BP1 (Ser65), we have identified the activation of the mTORC1 signaling pathway in all of the examined cases of ARL, regardless of their histological type or phenotype. The high frequency of the mTORC1 activation in ARL can most likely be attributed to the activated cell characteristics of the lymphoma cells rather than the malignant cell transformation per se. Accordingly, we also identified the mTORC1 activation in the enlarged, activated cells in germinal centers and interfollicular areas, but not in small, resting lymphocytes including the entire mantle zones of the reactive, benign HIV-associated lymphadenopathy. The important difference between ARL and the benign lymphadenopathy is that whereas in the reactive lymphocytes the mTORC1 activation should be antigen-dependent and therefore transient, while in malignant cells it is driven by oncoproteins and therefore is persistent.
Despite the many studies devoted to mTORC1 and its inhibitors, the exact mechanisms leading to mTORC1 activation are still only partially understood in regard to both the primary stimuli that trigger the activation, as well as the signal transduction pathways upstream of mTORC1. This study as well as the one by Leseux et al21 suggest that Syk kinase plays an important role in mTORC1 activation in the transformed B cells. Our observation that mTORC1 suppression by Syk inhibitors was overall less effective than by rapamycin indicated that other kinases contribute to the full mTORC1 activation in at least some types of transformed B lymphocytes. Indeed, inhibition of MEK/ERK and, less clearly, PI3K/Akt resulted in partial inhibition of the mTORC1 activation. Of note, combination of the inhibitors, in particular the ones targeting MEK and Syk, was overall more effective than any of the inhibitors applied alone. This finding suggests that mTORC1 is simultaneously activated by different kinases, which to various degrees contribute to its activation, possibly in the cell type-specific manner. In general, these studies suggest that kinases upstream of mTORC1 represent therapeutic targets in ARL and similar B-cell lymphomas, as well as other malignancies, with the potential caveat that more than one kinase may need to be targeted to achieve an effective inhibition of mTORC1.
Whereas the early studies focused on insulin and insulin-like growth factor as the growth factors capable of activating mTORC1, subsequent analysis revealed that various growth factors, cytokines, other ligands, and their receptors can activate mTORC1. They include the CD40 ligand,28 thyroid-stimulating hormone,29 fibroblast growth factor-9,30 polycystein-1,31 and prostaglandin F2a.32 In regard to malignant lymphocytes, we found that in anaplastic T/null-cell lymphomas that expresses a chimeric, constitutively activated form of the anaplastic lymphoma kinase, the kinase induces mTORC1 activation.24 Furthermore, interleukins 2 and 15, but not 21, were able to activate mTORC1 in the cutaneous T-cell lymphoma cells.23 Others have found that notch is responsible for mTORC1 activation in T-cell acute lymphoblastic leukemia.33 Of note, proteins encoded by EBV- and HHV8, two oncogenic viruses from the herpesvirus family expressed in a subset of ARLs and other lymphoid and non-lymphoid malignancies, were reported to activate mTORC1. In regard to EBV, late membrane protein-2 (a cell membrane protein encoded by the virus mimicking signaling by an antigen B-cell receptor) has been reported to activate mTORC1.34 In turn, the KSHV (Kaposi sacroma herpes virus)/HHV8-encoded vGPCR, an analog of the human receptor for interleukin-8, activates mTORC1 on transfection into endothelial cells.35 Importantly, mTORC1 activation was required for the vGPCR (viral G protein coupled receptor)-induced cell transformation, supporting the notion that mTORC1 pathway plays a key role in oncogenesis.
Our findings of the seemingly ubiquitous mTORC1 activation in ARL may have therapeutic implications for these lymphomas and possibly for other histologically similar lymphomas derived from enlarged, transformed lymphocytes in patients who are not HIV-positive. While various rapamycin analogs differ in the pharmacokinetics and bioavailability, they all inhibit mTORC1 in the highly potent and specific manner. A number of clinical trials are currently underway to evaluate the efficacy of mTORC1 inhibitors in the large spectrum of malignancies with some yielding promising results.5,6 Based on the results of one such study,13 rapamycin’s analog RAD (rapamycin derivative) has emerged as the first line therapeutic agent for renal cell carcinoma. In pre-clinical studies, we have found that post-transplant lymphoproliferative disorders (PTLD)-type cell lines are highly sensitive to mTORC1 inhibition in vitro and in vivo as xenotransplants.26,36 Our follow-up study demonstrated universal mTORC1 activation in the PTLD tissues, further supporting the notion of mTORC1-directed therapy in PTLD.25 We,26 and in a much greater depth other investigators,27 have recently shown that cell lines derived primary effusion lymphoma, which invariably express genes of HHV8 and often also of EBV but in a restricted fashion, are also highly sensitive to mTORC1 inhibition both in vitro and in vivo, suggesting that the inhibition may also be effective in patients with primary effusion lymphoma. According to several independent studies on organ transplant patients who developed PTLD, substitution of the calcineurine inhibitor-based immunosuppressive therapy with an mTORC1 inhibitor resulted in a potent anti-PTLD effect leading to the full resolution of the PTLD lesions in some cases.37,38,39,40,41,42,43 Perhaps even more strikingly, a similar therapeutic switch in transplant patients who developed the Kaposi sarcoma resulted in a sustained complete remission in all patients.42,43,44,45
The potential usage of mTORC1 inhibitors in AIDS patients may raise the concern of further increasing their immunodeficiency. However, transplant patients are also immunocompromised and yet mTORC1 inhibitors have proven efficacious in such patients,37,38,39,40,41,42,43,44,45 as discussed above. This observation indicates that the direct effects of mTORC1 inhibitors on malignant cells by impairing their proliferation, survival, and angiogenesis, clearly outweigh immunosuppressive properties of the inhibitors. Furthermore, circumstantial evidence indicates that mTORC1 inhibition may have additional beneficial effects in AIDS patients by limiting the replication of HIV and the detrimental effects of the virus on the target CD4+ T lymphocytes by preventing cell syncytium formation and p53-triggered cell apoptosis.46,47 Finally, the application of mTORC1 inhibitors to treat ARL would represent an alternative to the current regimens of multi-agent chemotherapy which not only display a plethora of severe toxicities, but are also rather ineffective in the AIDS patients,48,49 even when combined with an anti-CD20 antibody.48
Footnotes
Address reprint requests to Mariusz A. Wasik, M.D., University of Pennsylvania Medical Center, Department of Pathology and Laboratory Medicine, 3400 Spruce Street, 7.106 Founders Pavilion, Philadelphia, PA 19104. E-mail: wasik@mail.med.upenn.edu.
Supported by NIH grants R01-DE-017337 and R01-CA89194 and UPenn Cancer Center Pilot Project Grant.
References
- Wood C, Harrington W., Jr AIDS related lymphomas. Cell Res. 2005;15:947–952. doi: 10.1038/sj.cr.7290372. [DOI] [PubMed] [Google Scholar]
- Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immuno-deficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106:128–135. doi: 10.1002/cncr.21562. [DOI] [PubMed] [Google Scholar]
- Krause J. AIDS-related non-Hodgkin’s lymphomas. Microsc Res Tech. 2005;68:168–175. doi: 10.1002/jemt.20230. [DOI] [PubMed] [Google Scholar]
- Raphael M, Borisch B, Jaffe ES. Lymphomas associated with infection by the human immune deficiency virus (HIV). Jaffe E, Harris NL, Stein H, Vardiman J, editors. Lyon: IARC Press,; World Health Organization Classification of Tumours, Tumours of the Hematopoietic and Lymphoid Tissues. 2001:pp 260–263. [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradettia MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- Sekulić A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/ Akt-dependent and independent prosphorylation of tuberin. J Biol Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- Hutchcroft JE, Harrison ML, Geahlen RL. B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem. 1991;266:14846–1484. [PubMed] [Google Scholar]
- Darby C, Geahlen RL, Schreiber AD. Stimulation of macrophage Fc gamma RIIIA activates the receptor-associated protein tyrosine kinase Syk and induces phosphorylation of multiple proteins including p95Vav and p62/GAP-associated protein. J Immunol. 1994;152:5429–5437. [PubMed] [Google Scholar]
- Hutchcroft JE, Geahlen RL, Deanin GG, Oliver JM. Fc epsilon RI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc Natl Acad Sci USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcroft JE, Harrison ML, Geahlen RL. Association of the 72-kDa proteintyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992;267:8613–8619. [PubMed] [Google Scholar]
- Agarwal A, Salem P, Robbins KC. Involvement of p72syk, a protein-tyrosine kinase, in Fc gamma receptor signaling. J Biol Chem. 1993;268:15900–15905. [PubMed] [Google Scholar]
- Jouvin MH, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet JP. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- Kiener PA, Rankin BM, Burkhardt AL, Schieven GL, Gilliland LK, Rowley RB, Bolen JB, Ledbetter JA. Cross-linking of Fc gamma eceptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268:24442–24448. [PubMed] [Google Scholar]
- Leseux L, HAmdi SM, Al Saati T, Capilla F, Recher C, Laurent G, Bezombes C. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- Wlodarski P, Kasprzycka M, Liu X, Marzec M, Robertson ES, Slupianek A, Wasik MA. Activation of mTOR in transformed B lymphocyte is nutrient-dependent but independent of Akt. MEK, IGF-I, and serum. Cancer Res. 2005;65:7800–7808. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- Marzec M, Liu X, Kasprzycka M, Witkiewicz A, Raghunath PN, El-Salem M, Robertson E, Odum N, Wasik MA. IL-2- and IL-15-induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4+ T lymphocytes. Blood. 2008;111:2181–2189. doi: 10.1182/blood-2007-06-095182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Kasprzycka M, Liu X, El-Salem M, Halasa K, Raghunath PN, Bucki R, Wlodarski P, Wasik MA. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26:5606. doi: 10.1038/sj.onc.1210346. [DOI] [PubMed] [Google Scholar]
- El-Salem M, Raghunath PN, Marzec M, Wlodarski P, Tsai D, Hsi E, Wasik MA. Constitutive activation of mTOR signaling pathway in post-transplant lymphoproliferative disorders (PTLDs). Lab Invest. 2007;87:29–39. doi: 10.1038/labinvest.3700494. [DOI] [PubMed] [Google Scholar]
- Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, Silberstein LE, Nowell PC, Schuler W, Shaw LM, Wasik MA. Immuno-suppressive macrolide SDZ RAD inhibits growth of human EBV-transformed B lymphocytes in vitro and in vivo; a potential novel approach to prevention and treatment of post-transplant lymphoproliferative disorders (PTLDs). Proc Natl Acad Sci USA. 2000;97:4285–4290. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Jr, Damania BA, Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109:2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata A, Kuwahara K, Ohmura T, Inui S, Sakaguchi N. Involvement of a rapamycin-sensitive pathway in CD40-mediated activation of murine B cells in vitro. Immunol Lett. 1999;68:301–309. doi: 10.1016/s0165-2478(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Suh JM, Song JH, Kim DW, Kim H, Chung HK, Hwang JH, Kim JM, Hwang ES, Chung J, Han JH, Cho BY, Ro HK, Shong M. Regulation of the phosphatidylinositol 3-kinase. Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem. 2003;278:21960–21971. doi: 10.1074/jbc.M300805200. [DOI] [PubMed] [Google Scholar]
- Wing LY, Chen HM, Chuang PC, Wu M-H, Tsai SJ. The mTOR-S6K1 but not PI3K-Akt signaling is responsible for fibroblast growth factor-9-induced cell proliferation. J Biol Chem. 2005;280:19937–19947. doi: 10.1074/jbc.M411865200. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Murchid NS, Larson CH. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvisais EM, Romanelli A, Hou X, Davis JS. AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2alpha. J Biol Chem. 2006;281:26904–26913. doi: 10.1074/jbc.M605371200. [DOI] [PubMed] [Google Scholar]
- Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Scott RS, Amirghahari N, Nathan CA, Young LS, Dawson CW, Sixbey JW. Modulation of the cell growth regulator mTOR by Epstein-Barr virus-encoded LMP2A. J Virol. 2005;79:5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10:133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W, Shaw L, Wasik MA. Immunosuppressive signal transduction inhibitor RAD suppresses growth of cells derived from post-transplant lymphoproliferative disorder at allograft protecting doses. Transplantation. 2003;75:1710–1717. doi: 10.1097/01.TP.0000063934.89714.19. [DOI] [PubMed] [Google Scholar]
- Garcia VD, Bonamigo Filho JL, Neumann J, Fogliatto L, Geiger AM, Garcia CD, Barros V, Keitel E, Bittar AE, Ferrera des Santos A, Roithmann S. Rituximab in association with rapamycin for post-transplant lymphoproliferative disease treatment. Transpl Int. 2003;16:202–206. doi: 10.1007/s00147-002-0487-9. [DOI] [PubMed] [Google Scholar]
- Jiménez-Rivera C, Avitzur Y, Fecteau AH, Jones N, Grant D, Ng VL. Sirolimus for pediatric liver transplant recipients with post-transplant lymphoproliferative disease and hepatoblastoma. Pediatr Transplant. 2004;8:243–248. doi: 10.1111/j.1399-3046.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Zaltzman JS, Prasad R, Chun K, Jothy S. Resolution of renal allograft-associated post-transplant lymphoproliferative disorder with the introduction of sirolimus. Nephrol Dial Transplant. 2005;20:1748–1751. doi: 10.1093/ndt/gfh884. [DOI] [PubMed] [Google Scholar]
- Cullis B, D'Souza R, McCullagh P, Harries S, Nicholls A, Lee R, Bingham C. Sirolimus-induced remission of post transplantation lymphoproliferative disorder. Am J Kidney Dis. 2006;47:e67–e72. doi: 10.1053/j.ajkd.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Mohsin N, Budruddin M, Kamble P, Khalil M, Pakkyarra A, Jha A, Mohammed E, Ahmed H, Ahmed J, Thomas S, Campistol JM, Daar A. Complete regression of cutaneous B-cell lymphoma in a renal transplant patient after conversion from cyclosporin to sirolimus. Transplant Proc. 2007;39:1267–1271. doi: 10.1016/j.transproceed.2007.03.092. [DOI] [PubMed] [Google Scholar]
- Iaria G, Anselmo A, De Luca L, Manuelli M, Lucchesi C, Tariciotti L, Monaco A, Sforza D, Nigro F, Abruzzese E, Tisone G. Conversion to rapamycin immunosuppression for malignancy after kidney transplantation: case reports. TransplantProc. 2007;39:2036–2037. doi: 10.1016/j.transproceed.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Boratynska M, Smolska D. Inhibition of mTOR by sirolimus induces remission of post-transplant lymphoproliferative disorders. Transpl Int. 2008;21:605–608. doi: 10.1111/j.1432-2277.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: a successful treatment for post transplantation Kaposi’s sarcoma. Transplantation. 2004;77:760–762. doi: 10.1097/01.tp.0000115344.18025.0b. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal- transplant recipients. N Engl J Med. 2005;352:317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, Leduc P, Ingber DE, Druillennec S, Roques B, Leibovitch SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M, Kroemer G. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 2002;21:4070–4080. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini JL, Hospital V, Stahl L, Jungas T, Verbeke P, Ojcius DM. Cell death and inflam-mation during infection with the obligate intracellular pathogen. Chlamydia Biochim. 2003;85:763–769. doi: 10.1016/j.biochi.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Mounier N, Spina M, Gabarre J, Raphael M, Rizzardini G, Golfier JB, Vaccher E, Carbone A, Coiffier B, Chichino G, Bosly A, Tirelli U, Gisselbrecht C. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–3840. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14–19. doi: 10.1097/01.qco.0000200295.30285.13. [DOI] [PubMed] [Google Scholar]