Abstract
Arginase has been reported to reduce nitric oxide bioavailability in cardiovascular disease. However, its specific role in retinopathy has not been studied. In this study, we assessed the role of arginase in a mouse model of endotoxin-induced uveitis induced by lipopolysaccharide (LPS) treatment. Measurement of arginase expression and activity in the retina revealed a significant increase in arginase activity that was associated with increases in both mRNA and protein levels of arginase (Arg)1 but not Arg2. Immunofluorescence and flow cytometry confirmed this increase in Arg1, which was localized to glia and microglia. Arg1 expression and activity were also increased in cultured Muller cells and microglia treated with LPS. To test whether arginase has a role in the development of retinal inflammation, experiments were performed in mice deficient in one copy of the Arg1 gene and both copies of the Arg2 gene or in mice treated with a selective arginase inhibitor. These studies showed that LPS-induced increases in inflammatory protein production, leukostasis, retinal damage, signs of anterior uveitis, and uncoupling of nitric oxide synthase were blocked by either knockdown or inhibition of arginase. Furthermore, the LPS-induced increase in Arg1 expression was abrogated by blocking NADPH oxidase. In conclusion, these studies suggest that LPS-induced retinal inflammation in endotoxin-induced uveitis is mediated by NADPH oxidase-dependent increases in arginase activity.
Uveitis is a damaging ocular condition that can lead to severe vision loss and blindness.1 Endotoxin-induced uveitis (EIU) is an experimental model that closely mimics human disease and is induced by administration of a single sublethal dose of lipopolysaccharide (LPS).2,3 EIU is characterized as an acute ocular inflammation that is comprised of the breakdown of the blood–ocular barrier and the infiltration of leukocytes in the anterior chamber and retina.4,5 Although precise mechanisms remain to be elucidated, cytokines and chemokines, such as vascular endothelial growth factor (VEGF), monocyte chemoattractant protein (MCP)-1, and tumor necrosis factor (TNF)-α, as well as nitric oxide (NO), released from inflammatory cells and ocular resident cells, including Muller cells and microglia in response to LPS, all contribute to the pathogenesis of EIU.6,7,8,9,10,11,12 In addition, increased oxidative stress induced by LPS or inflammatory cytokines seems to play a critical role in the regulation of the inflammatory cascade.13,14,15,16 Blockade of NAD(P)H oxidase, a major source of reactive oxygen species (ROS) formation, abolishes both leukocyte adhesion to the vasculature and production of MCP-1 in EIU.17,18
Arginase is a binuclear manganese metalloenzyme that catalyzes the hydrolysis of l-arginine to form urea and ornithine, which is a critical step for the urea cycle in the liver.19 There are two arginase isoforms which are encoded by separate genes with different intracellular localization. Arginase 1 (Arg1) is a cytosolic enzyme, while arginase 2 (Arg2) is mainly expressed in mitochondria. Arginase has a wide distribution in the body although Arg1 is strongly expressed in the liver and Arg2 is more evident in the kidney and prostate.20 Expression of arginase has been found in many cell types, including vascular endothelial cells, smooth muscle cells, and macrophages.21,22,23 Given that l-arginine is also an indispensible substrate for nitric oxide synthase (NOS) in NO formation, arginase is recently recognized as a critical regulator for NO production by competing with NOS for l-arginine.19 Associated with this mechanism, increased arginase activity has been linked to several diseases, such as atherosclerosis, hypertension, asthma, and endothelial dysfunction in diabetes.22,24,25,26 Increased expression of arginase has been described in a rat model for EIU.27 However, the specific role of arginase in EIU and mechanisms underlying arginase expression in this disease are unknown.
Materials and Methods
Treatment of Animals
All procedures with animals were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the institutional animal care and use committee (Animal Welfare Assurance no. A3307-01). Experiments were performed with C57BL/6J wild-type mice, mice deficient in arginase 2 (Arg2−/−), mice deficient in both arginase 1 and 2 (Arg1+/−Arg2−/−), and mice deficient in NOX2 (NOX2−/−). The NOX2−/− mouse was produced by Dinauer and colleagues.28 The C57BL/6J Arg1+/− and Arg2−/− mice developed by Cederbaum et al29 and O’Brien et al30 were provided by Dr. Steven Cederbaum with the permission of Dr. O’Brien. The knockout mice have been backcrossed for at least 10 generations on C57BL/6 mice.
EIU was induced by injection of lipopolysaccharide from Salmonella typhimurium (LPS, 4 mg/kg in PBS, i.p.; Sigma-Aldrich, St. Louis, MO). Control mice received vehicle alone.
Another group of wild-type mice was treated with the arginase inhibitor [S]-[2-boronoethyl]-l-Cysteine-HCl (BEC, EMD Chemicals, Gibbstown, NJ). For this study mice were injected with BEC (20 mg/kg in 0.9% saline, i.v.) 1 hour before the injection of LPS.
Tissue Culture
Rat Muller cells31 were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. The cells were used from passages 2 to 6 and starved in serum-free medium overnight before treatment. Primary rat microglia were isolated and seeded at a density of 1 × 105 cells/well in 24-well plates in Dulbecco’s modified Eagle’s medium/F12 (Mediatech, Manassas, VA) that was supplied with 10% fetal bovine serum and 1% Penicillin-Streptomycin solution (Invitrogen) as described.7 One day after seeding, cells were washed with Cellgro Complete (Mediatech) and incubated in the Cellgro Complete with various treatments.
Quantitative Reverse Transcription-PCR
Total RNA was isolated using an RNAqueous – 4PCR kit (Applied Biosystems, Austin, TX) according to the manufacture’s suggestion (for retina tissue) or using TRIzol reagent (Invitrogen) (for cells). Total RNA was reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Gene expression was determined by quantitative PCR with TaqMan Gene Expression Assays specific for 18S, Arg1, and Arg2 (Applied Biosystems), which was performed on a StepOne Plus thermocycler (Applied Biosystems). The cycle threshold, determined as the initial increase in fluorescence above background, was ascertained for each sample. 18S was used as internal control in the PCR reaction for normalization.
Western Blot Analysis
Retinas were homogenized in a radioimmunoprecipitation assay lysis buffer (Millipore, Billerica, MA) supplemented with 10 mmol/L NaF, 10 mmol/L Na4P2O7, 1 mmol/L phenyl methyl sulfonyl fluoride and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Twenty-microgram protein samples were subjected to 10% SDS polyacrylamide gel electrophoresis. Proteins were transferred onto a nitrocellulose membrane and the membrane was incubated overnight at 4°C with primary antibodies against Arg 1 (1:1000, BD Biosciences, San Jose, CA), actin (1:3000), and tubulin (1:5000, Sigma-Aldrich), followed by horseradish peroxidase-conjugated secondary antibody. Immunoreactive proteins were detected using the enhanced chemiluminescence system (GE Health care, Piscataway, NJ).
Arginase Activity Assay
Retinas or Muller cells were homogenized in ice-cold lysis buffer (50 mmol/L Tris-HCl, 0.1 mmol/L EDTA and EGTA, pH 7.5) containing protease inhibitors. The homogenate was centrifuged at 14,000 × g for 20 minutes and the supernatant was collected for the enzyme assay. Arginase activity was assayed as previously described.32 Briefly, the enzyme was activated by heating the lysate at 56°C in 25 mmol/L Tris buffer (pH 7.4) containing 5 mmol/L MnCl2. l-Arginine hydrolysis was then performed by incubating 50 μl of the activated lysate with 50 μl of 0.5 M/L l-arginine (pH 9.7) at 37°C for 60 minutes. The reaction was stopped in acid medium. The concentration of urea, which is the end product of l-arginine hydrolysis by arginase, was determined after adding 25 μl of 9% α-isonitrosopropiophenone. Protein concentration in the lysates was determined by bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). Arginase activity was calculated as mM Urea/mg protein.
Immunolocalization of Arginase 1
Mice were sacrificed and eye balls were embedded in optimal cutting temperature (OCT) compounds after treatment with vehicle or LPS (4 mg/kg, i.p.) for 16 hours. The OCT-frozen sections (10 μm) were fixed using 4% paraformaldehyde solution in PBS for 5 minutes at room temperature. Sections were washed several times with PBS, treated with proteinase K for 3 minutes, then permeabilized with 0.5% Triton X-100 for 30 minutes and blocked with 20% donkey serum for 1 hour at room temperature. Then sections were reacted with mouse monoclonal anti-arginase 1 antibody (5 μg/ml, BD Biosciences) or mouse IgG1 isotype control antibody (5 μg/ml) overnight at 4°C, followed by Cy3.0-conjugated AffiniPure Donkey Anti-Mouse antibody (1:500, Jackson Immuno Research, West Grove, PA) at room temperature for 1 hour. Images were taken by fluorescence microscopy at × 200. Fluorescence was very low in sections stained with isotype IgG1 and exposure time was increased to show the retinal tissue layers.
Flow Cytometry
Retinas were dissected and pooled retina samples (3 to 4 retinas) were incubated in Dispase (Sigma Aldrich, 37°C, 10 minutes) and filtered through a 70 μm nylon mesh to obtain a single cell suspension. Cells were centrifuged (1500 rpm, 10 minutes) and washed twice with PBS. Then cells were fixed and permeabilized using Becton Dickinson CytofixCytoperm ready-to-use buffer (500 μl for 15 minutes on ice). Cells were then washed twice with PBS and blocked with 20% donkey serum before being double-stained for cell markers and arginase 1 or for cell markers and cytokines or for cell markers and isotype control IgGs. The antibodies used and corresponding isotype control are: mouse anti-arginase 1 and mouse IgG1 (2.5 μg/ml, BD Biosciences), rabbit anti-VEGF and normal rabbit IgG (2 μg/ml, EMD Chemicals), phycoerythrin (PE)-conjugated rat anti-TNF-α and PE Rat IgG1 isotype control (0.4 μg/ml, eBioscience, San Diego and, CA), rabbit anti-MCP-1 (1 μg/ml, PeproTech, Rocky Hill, NJ), and normal rabbit IgG (1 μg/ml, EMD Chemicals). To show arginase or cytokines in neuroglia, arginase 1 and TNF-α were double-stained with rabbit anti-glial fibrillary acidic protein (GFAP) (1:50, neuroglia marker, from Sigma Aldrich). VEGF and MCP-1 were double-stained with mouse anti-GFAP (ready-to-use, Abcam, Cambridge, MA). To show arginase or cytokines in macrophage/microglia, arginase 1 and cytokines were double-stained with biotinylated rat anti-F4/80 (1:800, marker for activated macrophage/microglia, a gift from Dr. Sally S. Atherton). Cells were incubated with primary antibodies for 1 hour at 4°C and washed. Then cells were incubated with second antibodies for 40 minutes at 4°C. The following second antibodies were used at 1:500 dilution: PE-donkey anti-mouse (for arginase 1), PE-donkey anti-rabbit (for MCP-1 and VEGF), fluorescein isothiocyanate (FITC)-donkey anti-rabbit (for GFAP), FITC-donkey anti-mouse (for GFAP), and FITC-streptavidin (for F4/80). After staining, samples were washed twice and analyzed using four-color flow cytometry (FACS Calibur, BD Biosciences) and CellQuestTM software. Samples double-stained with control IgG and cell marker were used to set up the compensation to subtract the spillover signal of FITC from PE detector (PE-%FITC). Proper compensation was set up to make sure the median PE fluorescence intensities of FITC negative cells and positive cells were identical and were both gated as PE-negative population. Due to the limited number of cells from mouse retinas, reciprocal compensation to subtract the spillover signal of PE from FITC detector (FITC-%PE) was not set up since this effect was minimal. Then the percentage of cells that express cytokines or arginase in each cell type was identified after gating to exclude dead cells and debris using forward and side scatter plots.
Analysis of Leukocyte Adhesion
Adhesion of leukocytes to the wall of the retinal vessels was evaluated as described previously,17 with some modification. After the induction of deep anesthesia, the chest cavity was carefully opened, and a perfusion cannula with 0.2 mm internal diameter was introduced into the aorta. Drainage was achieved by opening the right atrium. The animals were then perfused with 6 ml of PBS to wash out nonadherent blood cells. Next, the animals were perfused with 6 ml FITC-labeled concanavalin A lectin (40 μg/ml in PBS, Vector Laboratories, Burlingame, CA) to label the adherent leukocytes and vascular endothelial cells. Residual unbound concanavalin A was removed by perfusion with PBS. The eyeballs were removed and fixed with 4% paraformaldehyde. The retinas were then dissected, mounted flat on glass slides and observed by fluorescence microscopy at × 200 magnification. The total number of adherent leukocytes per retina was determined.
Analysis of Retinal Morphology
Mice were sacrificed after treatment with vehicle or LPS (4 mg/kg, i.p, 16 hours) and eye balls were embedded in OCT compound. OCT-frozen sections (10 μm) were fixed and stained with H&E. Images were taken by using a Zeiss microscope at × 200.
Analysis of Leukocyte Infiltration and Protein Content in the Aqueous Humor
After the induction of deep anesthesia, aqueous humor was collected by anterior chamber puncture using a 30-gauge needle under a surgical microscope. The samples were used for either cell counting or protein concentration measurements. To quantify the number of infiltrated leukocytes, 1 μl of aqueous humor was mixed with 8 μl of 0.4% trypan blue stain solution and subjected to cell counting using a hemocytometer. Cells in five fields were counted, averaged and calculated as number of cells in 1 μl of humor. The protein concentration in the aqueous humor was determined by bicinchoninic acid assay and dilutions of bovine serum albumin as standards.
Nitrite and Nitrate Measurement
Retinas were homogenized in PBS with a pestle. Then the lysates were centrifuged at 14000 rpm for 10 minutes at 4°C and supernatants were collected. The level of nitrite in the supernatants was analyzed using NO-specific chemiluminescence. In brief, samples containing nitrite were refluxed in glacial acetic acid containing sodium iodide. Nitrite is quantitatively reduced to NO under these conditions, which can be quantified by a chemiluminescence detector after reaction with ozone in a NO analyzer (Sievers). To measure the total level of nitrite plus nitrate, supernatants were incubated with PBS containing nitrate reductase (0.25 unit/ml), NADPH (13 μg/ml), and FAD-Na2 (4 μg/ml) at 30°C for 1 hour to reduce nitrate to nitrite. Then the level of nitrite was analyzed using NO-specific chemiluminescence. Protein concentration in the supernatant was determined by bicinchoninic acid assay. The level of nitrite or nitrite plus nitrate was normalized to the protein concentration in the supernatant and calculated as percentage of control.
Dihydroethidium Assay for Superoxide Formation
To evaluate production of superoxide in situ, the oxidative fluorescent dye dihydroethidium (DHE) was used. DHE is freely permeable to cells and in the presence of superoxide is oxidized to ethidium bromide, which binds to DNA and fluoresces red. Frozen sections were pre-incubated in NADPH (100 μmol/L) for 20 minutes followed by DHE (2 μmol/L, 20 minutes, 37°C). DHE images from serial sections were obtained using an Axiovision fluorescence microscope, DHE is excited at 488 nm with an emission spectrum of 610 nm. The images were analyzed for reaction intensity by using the Metamorph Image System (Molecular Devices).
Statistical Analysis
The results are expressed as mean ± SEM. Group differences were evaluated by using one way analysis of variance followed by posthoc Student’s t-test. Results were considered significant at P < 0.05.
Results
Arg1 Expression and Activity Are Increased in EIU
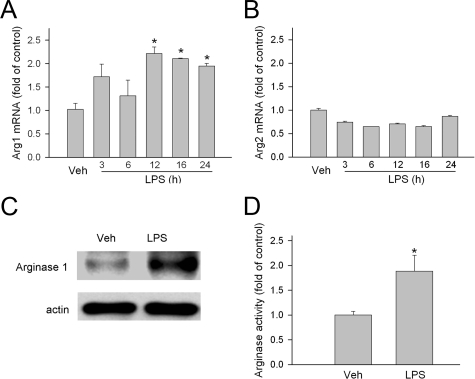
To evaluate whether arginase plays a role in acute retinal inflammation, the expression and activity of arginase were evaluated in a mouse model for EIU. Quantitative PCR analysis of mRNA revealed that both Arg1 and Arg2 were expressed in the retina. The mRNA level of Arg1 was significantly increased following LPS injection. It reached a peak at 12 hours (2.21 ± 0.14-fold of control) and was slightly decreased at 24 hours (1.94 ± 0.06-fold of control) (Figure 1A). The mRNA level of Arg2 was not changed (or even slightly decreased) (Figure 1B). Consistent with the increase in Arg1 mRNA, Arg1 protein was also increased 16 hours after the LPS injection as revealed by immunoblotting with a specific antibody for Arg1 (Figure 1C). The level of arginase activity in the retina was determined by measuring the amount of urea produced from l-arginine hydrolysis. LPS treatment significantly increased arginase activity by 1.9 ± 0.3-fold (Figure 1D). To assess the generality of this effect, studies were also performed in rats treated with LPS (1 mg/kg, i.p.). These experiments showed that arginase activity was increased by ∼1.8-fold within 12 or 24 hours following the LPS treatment (data not shown).
Figure 1.
Arginase expression and activity. A and B: Mice were injected with LPS (4 mg/kg, i.p.) and sacrificed at the time indicated. Arg1 and Arg2 mRNA in the retina were then determined by quantitative PCR and normalized to vehicle (Veh) control (n = 3 mice). *P < 0.05 compared with vehicle (saline). C: Mice were sacrificed 16 hours after injection with LPS (4 mg/kg, i.p.). Arg1 protein in the retina extract was detected by immunoblot. A representative blot is shown (n = 3 mice). D: Mice were sacrificed 16 hours after injection with LPS (4 mg/kg, i.p.). Then retinas were homogenized and arginase activity was determined as described in the Materials and Methods section. Mice that received vehicle (saline) injection were used as reference (n = 6 mice). *P < 0.05 compared with vehicle.
Arg1 Is Localized in Neuroglia and Macrophage/Microglia in EIU
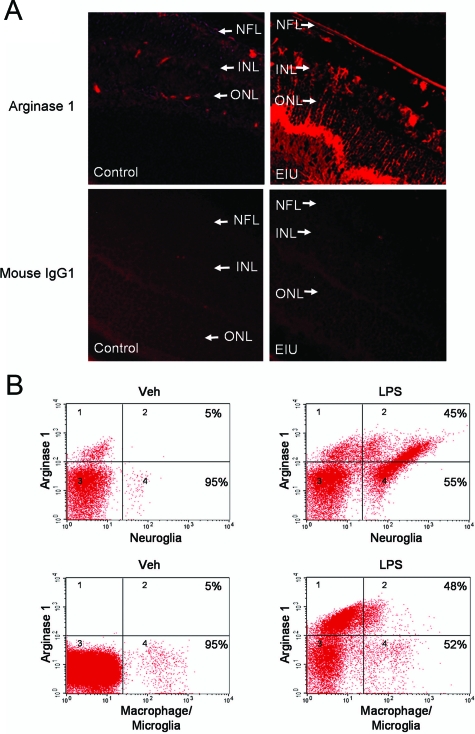
Prompted by the observation that Arg1 expression was up-regulated in EIU, we next determined the localization of Arg1 in retina sections. Immunolocalization analysis showed that immunoreactivity for Arg1 was localized to the nerve fiber layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, and outer limiting membrane in a pattern that corresponded to the processes of Muller cells, astrocytes, and microglia cells (Figure 2A). Immunofluorescence with an isotype control IgG was very weak and did not show any specific structure (Figure 2A), indicating specificity of the arginase antibody.
Figure 2.
Localization of Arg1. A: Immunohistochemistry of Arg1 expression (red) in retinal sections from mice injected with vehicle (veh, control) or LPS (4 mg/kg, i.p., EIU). Purified mouse IgG1 is used as control. LPS treatment (EIU) resulted in a prominent increase in Arg1 protein, which is localized to the nerve fiber layer (NFL), inner plexiform layer, inner nuclear layer (INL), outer plexiform layer, and outer limiting membrane. ONL is outer nuclear layer. Original magnification ×200. B: Flow cytometry analysis of Arg1 expression in retinal cell types. Retina samples were separated into single cell suspension and stained with Arg1 antibody in combination with one of the following antibodies against specific cell makers: GFAP for neuroglia and F4/80 for activated macrophage/microglia. Percentage indicates the proportion of cells that are dual-positive (right-upper quadrant). Representative data are shown (n = 3 experiments with pooled retinas).
To further investigate the expression of Arg1 in specific cell types, flow cytometry analysis was performed after retinal cells were separated into a single cell suspension by Dispase digestion and costained with both Arg1 antibody and cell type markers. GFAP was used as neuroglial marker. Retinal astrocytes express GFAP under both resting and activated conditions and Muller cells express GFAP after treatment with LPS. F4/80 was used as a marker for activated macrophage/microglia. As shown in Figure 2B, in the retina from vehicle-treated mice, a small fraction of GFAP-positive cells were located in quadrants 2 and 4 and a small fraction of Arg1-positive cells were located in quadrants 1 and 2. In the GFAP-positive population (quadrants 2 and 4), only 5% of cells were as also positive for Arg1 (quadrant 2). Parallel experiments using a Muller cell-specific antibody, CRALBP, showed that Muller cells are also largely negative for Arg 1 (data not shown). In contrast with the vehicle-treated controls, LPS treatment resulted in a prominent increase in the GFAP-positive cell population, which is consistent with previous findings that LPS treatment increases GFAP expression in both astrocytes and Muller cells.33,34 Interestingly, the percentage of Arg1-positive cells in the GFAP-positive population (neuroglia) also was robustly increased to 45% after LPS treatment, suggesting an increase of Arg1 expression in astrocytes and Muller cells during EIU. Similarly, analysis of Arg1 expression in the F4/80-positive population (activated microglia and macrophages) also revealed that Arg1-positive cells were increased from 5% to 48% following LPS administration (Figure 2B). Analysis of Arg1 expression in combination with CD11b, which is a marker for the entire macrophage/microglia population, also revealed that Arg1 positive cells were increased from 3% to 40% following LPS administration (data not shown). This observation is consistent with the report that there is an 80% overlap between CD11b-positive cells and F4/80-positive cells in the retina.35
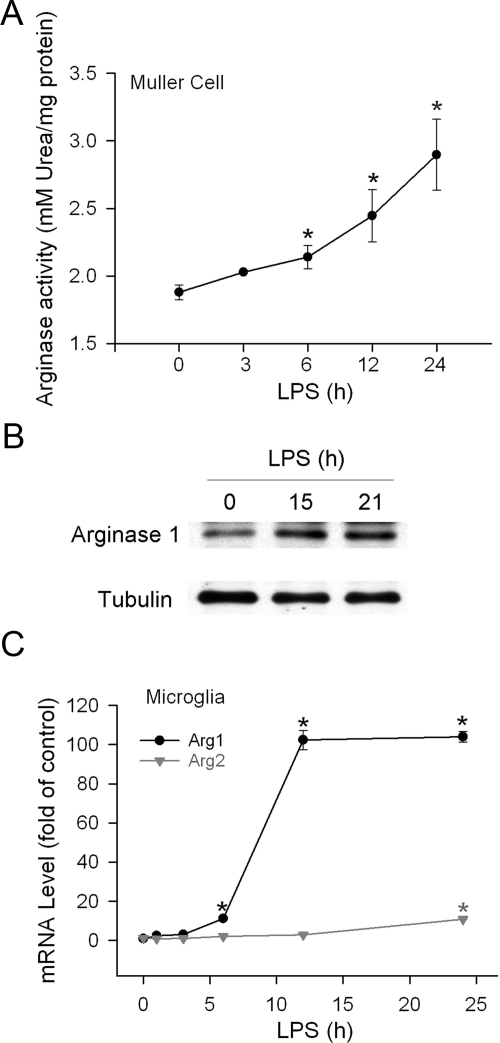
Arg1 Activity and Expression Are Increased in LPS-Treated Cells
The direct effect of LPS on Arg1 expression was assessed in cultured Muller cells and retinal microglia. Incubation of Muller cells with LPS resulted in significant increases in arginase activity in a time-dependent manner, which was 55% above basal level after cells were treated for 24 hours (Figure 3A). Consistent with the increase in arginase activity, analysis of Arg1 protein by immunoblot also demonstrated an increase in Arg1 protein levels in the LPS-treated cells (Figure 3B). In primary retinal microglial cells, LPS robustly increased mRNA level of Arg1, which peaked at 12 hours and was maintained at the same level after 24 hours. In contrast, the increase of Arg2 mRNA was marginal (Figure 3C).
Figure 3.
Arginase activity and expression in cultured retinal cells. A: Retinal Muller cells were treated with LPS (1 μg/ml) for the time indicated. Then cells were lysed and arginase activity was assayed by detecting urea production and normalized to the total amount of protein in the lysates (n = 3 experiments). *P < 0.05 compared with 0 hours treatment. B: Retinal Muller cells were treated with LPS (1 μg/ml) for the time indicated. Cells were then lysed and Arg1 and tubulin protein were determined by immunoblot. Representative blot is shown (n = 3 experiments). C: Retinal microglial cells were treated with LPS (30 ng/ml) for the time indicated. Total RNA was extracted and mRNA of Arg1 and Arg2 was measured by quantitative PCR. Cells without treatment were used as control (n = 4 experiments). *P < 0.05 compared with controls.
Inflammatory Cytokine Expression Is Abrogated by Blocking Arginase in EIU
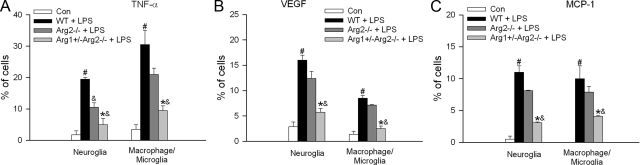
To address the role of arginase in the inflammatory response in EIU, a genetic approach was taken to evaluate LPS-induced inflammatory cytokine expression in mice that were completely lacking in Arg2 gene or deficient in one copy of the Arg1 gene and both copies of Arg2 gene. Most mice that are completely lacking the Arg1 gene die by 14 to 15 days postnatal due to ammonia poisoning. This study showed that LPS treatment induced a prominent increase in the percentage of TNF-α-positive neuroglial cells and macrophage/microglia in retinas from wild-type mice (Figure 4A). This increase was significantly reduced in neuroglia from the Arg2 deficient retinas (by 50%) but was only slightly reduced in macrophage/microglia from the same samples (by 35%, no statistically significant difference). However, additional loss of one copy of the Arg1 gene resulted in a marked decrease in TNF-α production in both neuroglia (reduced by 82%) and macrophage/microglia (reduced by 78%) (Figure 4A). LPS also significantly increased the production of VEGF and MCP-1 in both neuroglia and macrophage/microglia, as shown by an increase in the percentage of cells that were double-positive for GFAP or F4/80 and VEGF (Figure 4B) or MCP-1 (Figure 4C). This increase was dramatically blocked in mice deficient in one copy of the Arg1 gene and both copies of Arg2. However, absence of Arg2 alone did not significantly reduce the production of VEGF and MCP-1 in either cell type (Figure 4, B and C).
Figure 4.
Inflammatory cytokine expression in arginase deficient mice. Wild-type mice (WT), or mice completely lacking arginase 2 (Arg2−/−), or mice partially deficient in arginase 1 in addition to lack of arginase 2 (Arg1+/−Arg2−/−) were treated with LPS (4 mg/kg, i.p.) for 16 hours. Then retina samples were separated into single cell suspension and stained with A: TNF-α; B: VEGF; C: MCP-1, in combination with one of the following cell markers: GFAP for neuroglia, F4/80 for macrophage/microglia. The portion of positively stained cells in each cell population was determined by flow cytometry (n = 3 experiments with pooled retinas). Wild-type mice without LPS treatment were used as control (Con). #P < 0.05 compared with control. &P < 0.05 compared with WT+LPS. *P < 0.05 compared with Arg2−/− + LPS.
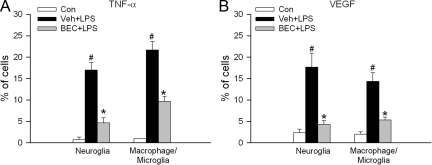
To further confirm these observations and also to examine the potential therapeutic benefit of arginase inhibition, mice were injected with a specific arginase inhibitor (BEC, 20 mg/kg, i.v.) before LPS administration. As shown in Figure 5A, the LPS-induced increase of TNF-α positive cells was significantly reduced by 76% in neuroglial cells and by 58% in macrophage/microglia in mice treated with BEC. Similarly, inhibition of arginase by BEC robustly reduced VEGF expression in neuroglia (72% reduction) and macrophage/microglia (87% reduction) (Figure 5B).
Figure 5.
Inflammatory cytokine expression in mice treated with arginase inhibitor. Mice were pretreated with vehicle (Veh) or arginase inhibitor (BEC, 20 mg/kg, i.v.) 1 hour before LPS injection. 16 hours after LPS treatment, mice were sacrificed and retina samples were separated into single cell suspension and stained with A: TNF-α; B: VEGF; in combination with one of the following cell markers: GFAP for neuroglia, F4/80 for macrophage/microglia. The portion of positively stained cells in each cell type population was determined by flow cytometry (n = 3 experiments with pooled retinas). Mice without LPS treatment were served as control (Con). #P < 0.05 compared with control. *P < 0.05 compared with Veh+LPS.
Leukostasis in EIU Is Abrogated by Blocking Arginase
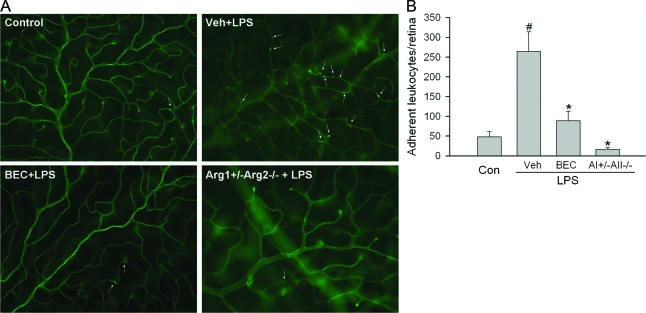
Leukocyte adhesion to the vessel wall (leukostasis) is a common feature of retinal vascular inflammation and is an important step in leukocyte infiltration. To further test whether increased arginase activity is involved in the vascular inflammatory reactions associated with EIU, experiments were performed to determine the effects of inhibiting arginase on LPS-induced leukostasis. Leukocytes and vessels were visualized in retina flat mount preparations by perfusion labeling with FITC-coupled concanavalin A lectin. As shown in Figure 6, A–B, the adhesion of leukocytes to the vessel walls was minimal in normal mice. LPS treatment prominently increased the number of adherent leukocytes by 5.5-fold. However, LPS-induced leukostasis was reduced by more than 80% by blockade of arginase activity with the specific inhibitor BEC or by deleting arginase alleles.
Figure 6.
Leukocyte adhesion in mice deficient in arginase or treated with arginase inhibitor. A: Wild-type mice were pretreated with vehicle (Veh) or arginase inhibitor (BEC, 20 mg/kg, i.v.) for1 hours. Then these mice or mice partially deficient in arginase (Arg1+/−Arg2−/−) were injected with LPS (4 mg/kg, i.p.) for 16 hours. Leukocyte adhesion in the retina vasculature was measured by fluorescence microscopy after cells were in vivo labeled with FITC-concanavalin A lectin. Representative images are shown. Arrows, adherent leukocytes. B: Quantitative data of adherent leukocytes per retina from each group (n = 3 to 6 mice). #P < 0.05 compared with control. *P < 0.05 compared with Veh+LPS.
Retina Morphology in EIU Is Preserved in Arg1+/−Arg2−/− Mice
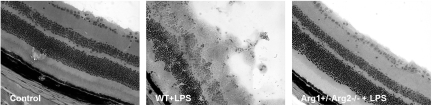
To evaluate whether arginase is involved in the retinal damage associated with inflammation, retina morphology was analyzed in frozen sections after H&E staining (Figure 7). In wild-type mice treated with LPS, the retinal lamination pattern was highly distorted and cellular membranes protruded from the retina into the vitreous cavity, suggesting extensive retinal damage. In contrast retinal morphology in Arg1+/−Arg2−/− mice treated with LPS was similar to that of the vehicle-treated controls.
Figure 7.
Retina morphology in arginase deficient mice. Wild-type mice (WT or mice partially deficient in arginase, Arg1+/−Arg2−/−) were treated with LPS (4 mg/kg, i.p.) for 16 hours. Retinal cryosections were stained with H&E. Wild-type mice without LPS treatment were used as control (Con). Original magnification ×200.
Anterior Uveitis Is Reduced in Arg1+/−Arg2−/− Mice
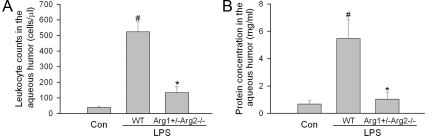
EIU is characterized as an acute ocular inflammation that affects both the anterior chamber and retina. To evaluate the role of arginase in anterior uveitis, leukocyte infiltration and protein leakage into the aqueous humor were examined in EIU animals. In wild-type mice, the average leukocyte count and protein concentration were increased by 13.6-fold (Figure 8A) and 8.0-fold (Figure 8B), respectively. In contrast, deleting arginase alleles significantly reduced LPS-induced leukocyte infiltration by 80% (Figure 8A) and protein leakage by 92% (Figure 8B).
Figure 8.
Anterior uveitis in arginase deficient mice. Wild-type mice (WT) or mice partially deficient in arginase (Arg1+/−Arg2−/−) were treated with LPS (4 mg/kg, i.p.) for 16 hours and aqueous humor was collected (n = 6 eyes). A: The cell number/μl was determined. B: Protein concentration/ml was determined. Wild-type mice without LPS treatment were used as control (Con). #P < 0.05 compared with control. *P < 0.05 compared with WT+LPS.
NOS Uncoupling Is Blocked by Deletion of Arginase Alleles
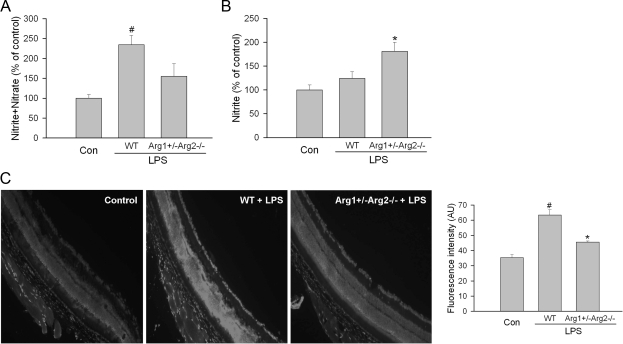
Because both arginase and NOS use l-arginine as substrate, experiments were performed to address the potential mechanism of the arginase-induced retinal injury by determining the effects of deleting arginase on total levels of nitrate plus nitrite versus nitrite alone. Nitrate formation is favored in the presence of excess superoxide.36 Analyses of total nitrate plus nitrite represent relative levels of total NO products rather than bioavailable NO. On the other hand, nitrite is the final product of NO autoxidation in water or hemoglobin-free media and is recognized as an indicator of the amount of bioavailable NO. Therefore, we compared the effects of LPS treatment on total nitrate plus nitrite versus nitrite only in wild-type and Arg1+/−Arg2−/− mice. This study showed that LPS treatment caused a significant increase in the total NO products (nitrite plus nitrate), in the wild-type mice as compared with the controls. This effect was significantly blunted by the arginase knockout (Figure 9A). Furthermore, measurement of nitrite alone showed that nitrite was significantly higher in the LPS treated Arg1+/−Arg2−/− mice than in either the control or LPS-treated wild-type mice (Figure 9B). Given that nitrate formation is favored in the presence of excess superoxide, these data suggest an effect of NOS uncoupling reducing bioavailable NO in wild-type mice. This hypothesis was supported by experiments using the DHE assay for superoxide formation. This study showed significant increases in superoxide formation in retinas from wild-type mice during EIU while arginase deficiency blocked this effect (Figure 9C). These results indicate an action of arginase in causing uncoupling of NOS to generate superoxide instead of NO and consequently decreasing bioavailable NO and increasing oxidative stress.
Figure 9.
Level of nitrite+nitrate, nitrite, and superoxide in arginase deficient mice. Wild-type mice (WT) or partially deficient in arginase (Arg1+/−Arg2−/−) were treated with LPS (4 mg/kg, i.p.) for 16 hours. A: Retina lysates were incubated with nitrate reductase to reduce nitrate to nitrite. Then the level of nitrite was determined by NO analyzer. B: Nitrite level in the retina lysates was determined by NO analyzer. C: Real-time DHE imaging of superoxide formation in retinas from LPS treated WT and Arg1+/−Arg2−/− mice or from control mice. Representative images and statistic of fluorescence intensity are shown. Wild-type mice without LPS treatment were used as control (Con). For (A) and (B) n = 3 mice for control and n = 9 mice for LPS treated mice. For (C), n = 3 mice for each group. #P < 0.05 compared with control. *P < 0.05 compared with WT+LPS.
Up-Regulation of Arginase Is Blocked by Inhibiting NOX2/NADPH Oxidase
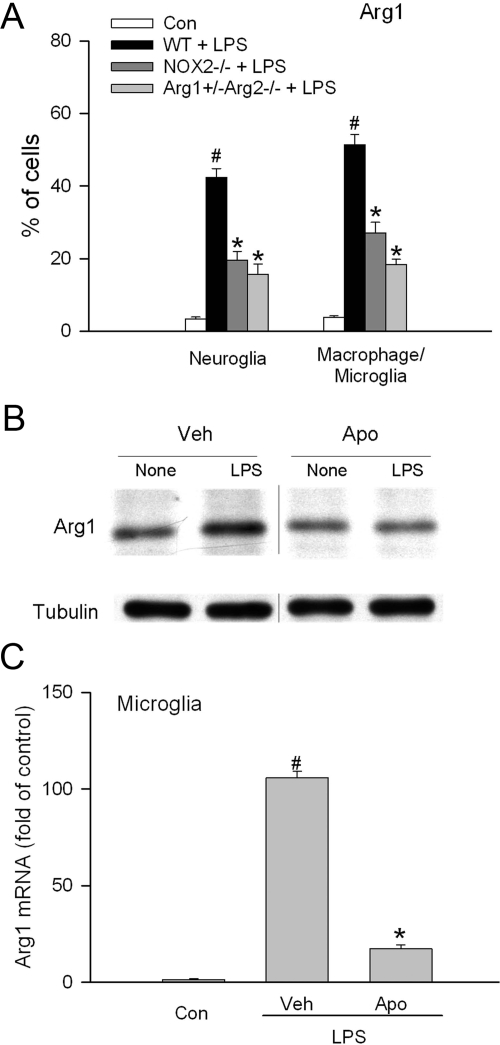
We have previously demonstrated that inhibition of NOX2/ NADPH oxidase blocks retinal vascular inflammation in EIU.17 To understand whether or not there is a causal link between arginase and NADPH oxidase, Arg1 expression in the retina and retinal cells were assessed following blockade of NOX2/NADPH oxidase. As shown in Figure 10A, LPS strongly induced the expression of Arg1 in both neuroglia and macrophage/microglia in the retina. However, in mice lacking NOX2 expression, this increase was significantly reduced by more than 50%, suggesting an involvement of NOX2/NADPH oxidase. As a positive control, Arg1 expression was reduced by almost 70% in mice deficient in one copy of the Arg1 gene and both copies of Arg2 gene. In cultured cells, apocynin, which is a specific inhibitor for NADPH oxidase, also abolished LPS induced Arg1 expression in both Muller cells and retinal microglial cells (Figure 10, B and C).
Figure 10.
Arg1 expression in mice deficient in NOX2 or cells treated with NADPH oxidase inhibitor. A: Wild-type mice (WT), mice deficient in NOX2 (NOX2−/−), or mice partially deficient in arginase (Arg1+/−Arg2−/−) were treated with LPS (4 mg/kg, i.p.) for 16 hours. Then retina samples were separated into single cell suspension and stained with arginase 1 antibody in combination with one of the following cell markers: GFAP for neuroglia, F4/80 for macrophage/microglia. The portion of positively stained cells in each cell type population was determined by flow cytometry (n = 3 experiments with pooled retinas). Wild-type mice without LPS treatment were used as control (Con). #P < 0.05 compared with control. *P < 0.05 compared with WT+LPS. B: Retinal Muller cells were pretreated with vehicle (Veh) or apocynin (Apo, 30 μmol/L, a specific inhibitor for NADPH oxidase) for 30 minutes and then stimulated with LPS (1 μg/ml) for 15 hours. Cells were then lysed and arginase 1 and tubulin protein were determined by immunoblot. Representative blot is shown (n = 3 experiments). The lane between “Veh+LPS” and “Apo+None” contained “Veh+LPS” for 21 hours. It was moved from this figure since this data has been shown in Figure 3B. C: Retinal microglial cells were pretreated with apocynin (500 μmol/L) or vehicle (Veh) for 30 minutes and then treated with LPS (30 ng/ml) for 24 hours. Total RNA was extracted and arginase 1 mRNA was determined by quantitative PCR. Cells without treatment were used as control (Con) (n = 4 experiments). #P < 0.05 compared with Con. *P < 0.05 compared with Veh.
Discussion
Nonhepatic arginase has been implicated in the pathogenesis of cardiovascular and lung diseases.19 We have shown that diabetes causes eNOS uncoupling and vascular endothelial cell dysfunction by activating arginase and thereby decreasing availability of l-arginine to eNOS, decreasing NO formation and increasing oxidative stress.22 In the present study, we provide the first evidence that arginase is critically involved in acute retinal inflammation. Using a model of EIU, we found that LPS induces increases in Arg1 expression and activity in the retina and retinal cells in vivo and in vitro. Based on the selective inhibition of arginase by gene deletion or pharmacological inhibitor, we clearly demonstrate that arginase is an indispensible component in retinal inflammation during EIU. Blockade of arginase not only reduces the production of inflammatory cytokines such as TNF-α, VEGF, and MCP-1 in retinal neuroglia and macrophage/microglia, but also abrogates leukocyte adhesion to the vessel wall, prevents retina damage, and reduces signs of anterior uveitis. Considering that uveitis is a leading cause of blindness with undefined mechanisms,1 reduction of arginase activity may provide a beneficial therapeutic approach for preventing the severe ocular inflammation and damage associated with this disease. In addition, since vascular inflammation is a common phenomenon in many eye diseases, including diabetic retinopathy and ischemic retinopathy,37,38,39 study of the role of arginase in these diseases is needed to determine whether or not arginase has a general role in retinal diseases.
Two isoforms of arginase, namely Arg1 and Arg2, have been identified and been shown to share similar profiles for the substrate but to have different intracellular distributions.19,20 Depending on specific disease conditions, Arg1 or Arg2 or both may be up-regulated and exert a dominant role. In cardiovascular diseases, Arg1 has been found to be up-regulated and associated with endothelial dysfunction in diabetes, aging and ischemia.22,40,41 In contrast, Arg2 activity is reported mainly responsible for the pathogenesis of atherosclerosis in that selective deletion of Arg2 reduces plaque burden.26 In our study, Arg1 seems to be dominant in EIU since the expression of Arg1 is up-regulated while Arg2 is unchanged or even slightly decreased. This in vivo observation is further supported by studies in vitro using cultured cells where LPS induces dramatic increase of Arg1 whereas the change in Arg2 is minimal. However, the basal activity of Arg2 seems to also contribute to the inflammatory response in EIU in that complete loss of Arg2 expression results in reduced cytokine expression, particularly the production of TNF-α in neuroglial cells. Nevertheless, additional loss of one allele of Arg1 almost completely blocks the up-regulation of inflammatory cytokines in both neuroglia and macrophage/microglia, which highlights the important role of Arg1. Given that complete loss of Arg1 is lethal, conditional knockout of Arg1 or Arg2 in retina is needed to address this question clearly in the future. Regardless of the arginase isoform involved, blockade of arginase activity with a pharmacological inhibitor appears to be beneficial for reducing acute retinal inflammation.
The precise mechanisms by which arginase regulates retinal inflammation remain to be elucidated. However, by determining the level of nitrite (indicator of bioavailable NO) or nitrite plus nitrate (indicator of nitrative stress) in the retina, we provide the first evidence that arginase negatively regulates the amount of bioavailable NO while increasing nitrative stress in EIU. This finding is consistent with the notion that arginase serves as a competitive consumer of l-arginine. A similar effect of arginase in inhibition of normal function of NOS has been shown in many other diseases.19 NO is important second messenger that regulates many physiological and pathological events, including vascular dilation and vascular inflammation. NO seems to be pro-inflammatory in EIU given that increased iNOS expression is associated with disease progression and pharmacological inhibition of iNOS activity has been shown to reduce the inflammatory response and limit the pathogenesis of EIU.9 However, conflicting results have been reported in that several studies have shown that NO can be anti-inflammatory via modifying lipid to form nitroalkenes, which exert anti-inflammatory functions or by blocking platelet aggregation and leukocyte adhesion or inhibiting nuclear factor (NF)-κB activity.42,43,44,45,46 NO causes nitrosylation of the p65 and p50 subunits of NF-κB and consequently reduces their DNA binding ability.42,43 Corresponding to these findings, inhibition of arginase in lung epithelial cells was found to increase the nitrosylation of p50 and reduce the transcriptional activity of NF-κB.47 Thus, the beneficial effect of blocking iNOS in EIU may not be due to reducing NO production. Studies have shown that iNOS produces superoxide as well as NO when its substrate l-arginine is deficient,48 in a process referred to as ‘NOS uncoupling.’ As a consequence, peroxynitrate, a highly reactive inflammatory and toxic molecule,49 is formed by the reaction between NO and superoxide. The mechanism of NOS uncoupling is demonstrated by our findings that arginase deficiency prevents superoxide formation and increases the amount of bioavailable NO. Based on these observations, arginase may be involved in inflammation through at least two mechanisms. First, increased arginase activity will reduce NO production and consequently reduce the formation of anti-inflammatory nitroalkenes, increase platelet aggregation and leukocyte adhesion or enhance NF-κB activity due to decreases in NF-κB nitrosylation. Second, by reducing the availability of l-arginine, arginase will cause uncoupling of NOS, hence increasing formation of superoxide and peroxynitrate, which are well-known inflammatory players. As a consequence, inhibition of arginase will provide a tool for reducing inflammatory responses. As we demonstrate here, inhibition of arginase reduces inflammatory cytokine expression, blocks leukostasis, preserves retinal structure and prevents anterior inflammation in EIU. In agreement with our finding, inhibition of arginase has been shown to reduce leukocyte infiltration in asthma and to inhibit inflammatory cytokine production from splenocytes derived from a mouse model of experimental autoimmune encephalomyelitis.25,50 In addition to its effects on NOS activity, arginase may regulate inflammatory responses via its products, particularly polyamines, which are generated from ornithine. Polyamines have multiple physiological and pathological functions including vascularization, tissue repair, and neural toxicity.51,52,53 However, it is still questionable whether their function is pro-inflammatory or anti-inflammatory.54,55
We have previously demonstrated that inhibiting NOX2/NADPH oxidase blocks inflammatory responses in mouse models of EIU and diabetic retinopathy.17,18 In the current study, we provide new evidence of a role for NADPH oxidase in the regulation of arginase in EIU. Deleting the NOX2 subunit of NADPH oxidase or inhibiting enzyme activity with apocynin blocks LPS-induced arginase up-regulation in vivo and in vitro. Taken together with the finding that arginase activity is required for inflammation during EIU, we propose a mechanism in which arginase acts as a downstream mediator for NADPH oxidase in inflammatory responses. NADPH oxidase generated ROS may initiate the up-regulation of arginase activity, which in turn results in more production of ROS and peroxynitrate due to NOS uncoupling. Given that LPS induces cytokine production in macrophages and glial cells, the increase of arginase expression in EIU and LPS-treated cells can be a direct effect of LPS or an indirect effect via LPS-induced cytokine production by autocrine and/or paracrine mechanisms. It is likely that this process involves the induction of cytokines by LPS treatment since the increase in arginase was not prominent until 12 hours after treatment. Further studies are required to fully address the mechanism of LPS-induced increases in arginase 1 expression and to elucidate the specific role of NADPH oxidase in this process.
LPS is well-known to induce the macrophage M1 phenotype that is characterized by increases in iNOS and decreases in arginase 1.56 Our findings that arginase 1 expression is increased in retinal microglia in EIU seem contradictory to this notion. However, several studies have shown that LPS induces arginase 1 expression in bone marrow-derived macrophages and in peritoneal macrophages,57,58,59,60 indicating that the response we have observed in retinal microglia is not unique to this cell type. In addition, the retina is composed of multiple cell types that regulate each other in both physiological and pathological conditions. LPS may initiate the inflammatory response and induce production of pro-inflammatory cytokines in different cell types, such as Muller cells, macrophage/microglia, and astrocytes. As a consequence, arginase 1 expression in microglia in EIU may arise from direct effects of LPS as well as from indirect impact of other pro-inflammatory cytokines.
In summary, our data provide the first evidence that arginase is involved in the pathogenesis of an ocular disease. Using genetic and pharmacological approaches, we demonstrate that arginase serves as an oxidative stress modulated gene and is an important mediator for inflammatory responses in EIU. The action of arginase may involve a mechanism of NOS uncoupling. Together with the roles of arginase in cardiovascular,19 lung,25 and autoimmune diseases,50 this study highlights the therapeutic potential of blocking arginase with pharmacological inhibitors. Insight into mechanisms by which arginase regulates inflammatory responses may open important avenues for developing therapeutic approaches for many acute and chronic inflammatory diseases.
Acknowledgments
We are grateful to Tahira Lemtalsi and Jennifer Iddings for their excellent technical assistance. We thank Dr. Steven Cederbaum at UCLA for providing us with C57BL/6J Arg1+/− and Arg2−/− founder mice. We thank Dr. Sylvia B. Smith for Rat Muller cells, Dr. Gregory I. Liou for retinal microglia, and Dr. Sally S. Atherton for biotinylated rat anti-F4/80 used in this research.
Footnotes
Address reprint requests to Dr. Ruth B. Caldwell, Vascular Biology Center, Medical College of Georgia, Augusta, GA, 30912-2500. E-mail: rcaldwel@mail.mcg.edu.
Supported by National Eye Institute Grants R01 EY04618 and R01 EY11766, and Veterans Administration MRA (R.B.C.); National Heart, Lung, and Blood Institute Grant R01 HL70215 (R.W.C.); and the Greater Southeast Affiliate’s Postdoctoral Fellowship AHA0725604B (W.Z.)
W.Z. and B.B. contributed equally to this work.
Present affiliation of Babak Baban, Immunotherapy Center and Cancer Center, Medical College of Georgia.
References
- Read RW. Uveitis: advances in understanding of pathogenesis and treatment. Curr Rheumatol Rep. 2006;8:260–266. doi: 10.1007/s11926-006-0006-6. [DOI] [PubMed] [Google Scholar]
- Kogiso M, Tanouchi Y, Mimura Y, Nagasawa H, Himeno K. Endotoxin-induced uveitis in mice. 1. Induction of uveitis and role of T lymphocytes. Jpn J Ophthalmol. 1992;36:281–290. [PubMed] [Google Scholar]
- Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Cousins SW, Guss RB, Howes EL, Jr, Rosenbaum JT. Endotoxin-induced uveitis in the rat: observations on altered vascular permeability, clinical findings, and histology. Exp Eye Res. 1984;39:665–676. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Thillaye B, de Kozak Y. Retino-choroidal changes in endotoxin-induced uveitis in the rat. Ophthalmic Res. 1992;24:162–168. doi: 10.1159/000267163. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, Kociok N, Kirchhof B, Joussen AM. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, Khan S, Khalifa Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49:5526–5531. doi: 10.1167/iovs.08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulaki V, Iliaki E, Mitsiades N, Mitsiades CS, Paulus YN, Bula DV, Gragoudas ES, Miller JW. Inhibition of Hsp90 attenuates inflammation in endotoxin-induced uveitis. FASEB J. 2007;21:2113–2123. doi: 10.1096/fj.06-7637com. [DOI] [PubMed] [Google Scholar]
- Goureau O, Bellot J, Thillaye B, Courtois Y, de Kozak Y. Increased nitric oxide production in endotoxin-induced uveitis. Reduction of uveitis by an inhibitor of nitric oxide synthase. J Immunol. 1995;154:6518–6523. [PubMed] [Google Scholar]
- Goureau O, Hicks D, Courtois Y, De Kozak Y. Induction and regulation of nitric oxide synthase in retinal Muller glial cells. J Neurochem. 1994;63:310–317. doi: 10.1046/j.1471-4159.1994.63010310.x. [DOI] [PubMed] [Google Scholar]
- Tuaillon N, Shen DF, Berger RB, Lu B, Rollins BJ, Chan CC. MCP-1 expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2002;43:1493–1498. [PubMed] [Google Scholar]
- Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Victor VM, Rocha M, De la Fuente M. N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic Res. 2003;37:919–929. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rojas M, Lilly B, Tsai NT, Lemtalsi T, Liou GI, Caldwell RW, Caldwell RB. NAD(P)H oxidase regulates CCL2 production during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:3033–3040. doi: 10.1167/iovs.08-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–132. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- Erdely A, Kepka-Lenhart D, Clark M, Zeidler-Erdely P, Poljakovic M, Calhoun WJ, Morris SM., Jr Inhibition of phosphodiesterase 4 amplifies cytokine-dependent induction of arginase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;290:L534–L539. doi: 10.1152/ajplung.00326.2005. [DOI] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci USA. 2001;98:9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- Maarsingh H, Zuidhof AB, Bos IS, van Duin M, Boucher JL, Zaagsma J, Meurs H. Arginase inhibition protects against allergic airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- Koga T, Koshiyama Y, Gotoh T, Yonemura N, Hirata A, Tanihara H, Negi A, Mori M. Coinduction of nitric oxide synthase and arginine metabolic enzymes in endotoxin-induced uveitis rats. Exp Eye Res. 2002;75:659–667. doi: 10.1006/exer.2002.2062. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Deignan JL, Livesay JC, Yoo PK, Goodman SI, O'Brien WE, Iyer RK, Cederbaum SD, Grody WW. Ornithine deficiency in the arginase double knockout mouse. Mol Genet Metab. 2006;89:87–96. doi: 10.1016/j.ymgme.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol. 2001;21:811–813. doi: 10.1128/MCB.21.3.811-813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Lee JH, Choi KR, Kim D, Yoo HS, Oh S. Cytochemical alterations in the rat retina by LPS administration. Neurochem Res. 2007;32:1–10. doi: 10.1007/s11064-006-9215-7. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest Ophthalmol Vis Sci. 2003;44:3083–3093. doi: 10.1167/iovs.02-1014. [DOI] [PubMed] [Google Scholar]
- Chen B, Keshive M, Deen WM. Diffusion and reaction of nitric oxide in suspension cell cultures. Biophys J. 1998;75:745–754. doi: 10.1016/S0006-3495(98)77564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- Spirin KS, Saghizadeh M, Lewin SL, Zardi L, Kenney MC, Ljubimov AV. Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr Eye Res. 1999;18:490–499. doi: 10.1076/ceyr.18.6.490.5267. [DOI] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- Baker PR, Schopfer FJ, O'Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free Radic Biol Med. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol. 2007;36:645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, Chen YH. Arginase and autoimmune inflammation in the central nervous system. Immunology. 2003;110:141–148. doi: 10.1046/j.1365-2567.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima e Silva R, Saishin Y, Saishin Y, Akiyama H, Kachi S, Aslam S, Rogers B, Deering T, Gong YY, Hackett SF, Lai H, Frydman BJ, Valasinas A, Marton LJ, Campochiaro PA. Suppression and regression of choroidal neovascularization by polyamine analogues. Invest Ophthalmol Vis Sci. 2005;46:3323–3330. doi: 10.1167/iovs.04-1210. [DOI] [PubMed] [Google Scholar]
- Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, Mantelli B, Bronte V, Zanovello P. Role of arginine metabolism in immunity and immunopathology. Immunobiology. 2007;212:795–812. doi: 10.1016/j.imbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Pernet V, Bourgeois P, Di Polo A. A role for polyamines in retinal ganglion cell excitotoxic death. J Neurochem. 2007;103:1481–1490. doi: 10.1111/j.1471-4159.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- Soulet D, Rivest S. Polyamines play a critical role in the control of the innate immune response in the mouse central nervous system. J Cell Biol. 2003;162:257–268. doi: 10.1083/jcb.200301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–673. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol. 1998;274:R775–R782. doi: 10.1152/ajpregu.1998.274.3.R775. [DOI] [PubMed] [Google Scholar]
- Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem. 1997;272:3689–3693. doi: 10.1074/jbc.272.6.3689. [DOI] [PubMed] [Google Scholar]