Abstract
Regulation of both the expression and function of connexins in the vascular wall is important during atherosclerosis. Progression of the disease state is marked by vascular smooth muscle cell (VSMC) proliferation, which coincides with the reduced expression levels of connexin 43 (Cx43). However, nothing is currently known about the factors that regulate post-translational modifications of Cx43 in atherogenesis, which could be of particular importance, due to the association between site-specific Cx43 phosphorylation and cellular proliferation. We compared the effects of direct carotid applications of two oxidized phospholipid derivatives, 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC) and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC), on Cx43 expression and phosphorylation, and on cell proliferation. Since both POVPC and PGPC have been shown to act through different intracellular pathways, we hypothesized that each oxidized phospholipid species could induce differential Cx43 phosphorylation events in the cytoplasmically located carboxyl-terminal region of the protein, which could potentially enhance cell proliferation. Application of POVPC caused a reduction in VSMC Cx43 levels, enhanced its phosphorylation at serine (pS) 279/282, and increased VSMC proliferation both in vivo and in vitro. Treatment with PGPC enhanced VSMC pS368 levels with no associated change in proliferation. These oxidized phospholipid-induced Cx43 post-translational changes in VSMCs were consistent with those identified in ApoE−/− mice. Taken together, these results demonstrate that post-translational phosphorylation of Cx43 could be a key factor in the pathogenesis of atherosclerosis.
One of the markers of atherogenesis is a change in connexin 43 (Cx43) protein expression in the vascular smooth muscle cells (VSMCs) of the large vessels.1,2,3 Reductions in VSMC Cx43 protein expression and functionality has been associated with enhanced VSMC proliferation and differentiation, which is a central component of atherogenic progression.1,4 Connexin 43 expression and functionality is controlled in a large part through post-translational phosphorylation of sites within its cytoplasmically located carboxyl terminal region. Differential phosphorylation events of the Cx43 carboxyl terminus can influence Cx protein expression,5 targeting,6 function,3,7 and may act to control cellular proliferation.3,8,9 Post-translational modifications of Cx43 may therefore be integral to cell cycle progression in VSMCs. However, no evidence exists that Cx43 is phosphorylated in VSMCs during atherogenic progression.
During atherogenesis, minimally modified low density lipoproteins, the oxidative products of low-density lipoprotein, accumulate in vessel walls.10 Oxidized phospholipids (OxPLs), active components in minimally modified low density lipoproteins, have been identified as a major contributing factor in pro-atherogenic events, enhancing further lipid accumulation and oxidation, and are thought to promote key changes in VSMC phenotype, triggering the disease state.11 Several biologically active derivatives of minimally modified low density lipoprotein, eg, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC), as well as its component lipids, 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC) and 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC), have been identified as critical markers of atherosclerotic progression.12 In clinical studies, administration of lipid-lowering drugs reduces cell proliferation,13 and POVPC and PGPC identified in fatty streak lesions are associated with increased proliferation of the VSMC in vitro.14,15,16 It is unclear how POVPC and PGPC promote these alterations, but these lipids have been shown to have differing biological activities, promoting activation of mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) cascades respectively.14,17 The differential activation of signaling cascades by these OxPL species implicates a possible pathway in which they could individually mediate Cx43’s functionality through carboxyl terminus phosphorylation. Kinase mediated phosphorylation of Cx43 carboxyl terminus sites through MAPK (eg, phosphorylation at serine (pS) 279/282) and PKC (eg, pS368) have been shown to reduce Cx43 functionality, channel docking, and affect cell proliferation rates.18,19
In this study, we tested the hypothesis that POVPC and PGPC differentially mediate Cx43 phosphorylation and induce changes in the proliferative state of VSMC in vivo and in vitro. Following application directly to carotid arteries, POVPC and PGPC mediated site-specific Cx43 phosphorylation at pS279/282 and pS368 respectively. Applications of POVPC caused reductions in total Cx43 (Cx43-T) and enhanced pS279/282, which corresponded to an increase in VSMC proliferation in vivo and in vitro. While PGPC enhanced pS368, no changes in VSMC proliferation were observed. In keeping with these findings, Cx43 was phosphorylated at the pS279/282 and pS368 sites in ApoE−/− mice, but not in wild-type mice. These changes implicate key roles for both POVPC and PGPC in the specific post-translational modifications of Cx43 and as possible initiating factors and markers of early atherogenic changes in the vessel wall.
Materials and Methods
Mice
All C57/Bl6 mice (Taconic) used for pluronic applications were 6- to 8-week-old males. For comparisons of Cx43 expression and phosphorylation in atherosclerotic mice, 15-week-old ApoE−/− mice (Jackson labs) and age-matched C57/BL6 (Taconic) mice were used. All mice were kept on a standard 12-hour light/dark cycle and fed a normal chow diet. Food and water was available ad libitum. All animals were used according to the University of Virginia Animal Care and Use Committee guidelines.
Carotid Surgeries
Surgeries were performed as described20; briefly, mice were anesthetized with an i.p. injection of ketamine-xylazine, the left common carotid artery exposed, and PGPC or POVPC was topically applied (mixed in pluronic gel, F-127) at the stated dosages to the outside of the carotid wall. As shown in Figure 1A, application of the lipids in F-127 permits specific localization to the carotids. Twenty-four hours after the surgery, mice were euthanized with an i.p. injection of 60 to 90 mg/kg pentobarbital, and the carotids were placed in liquid N2-cooled 4-methyl butane and transferred to optimal cutting temperature compound for frozen sectioning or fixed in 4% paraformaldehyde for whole-mount analysis.
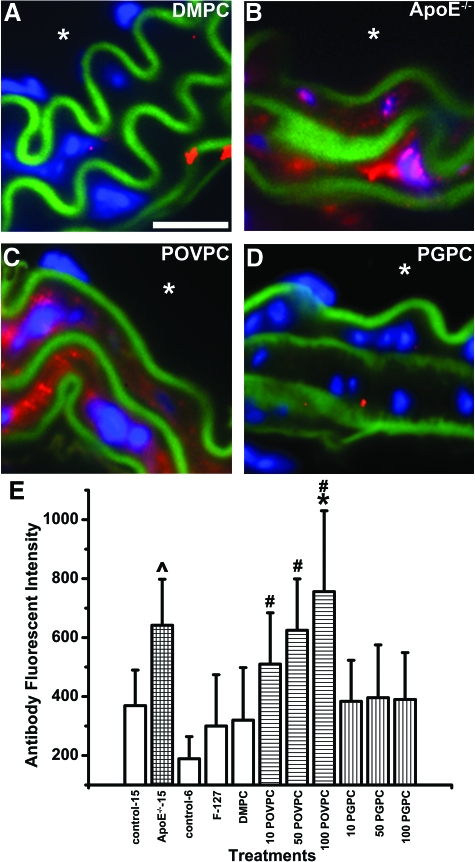
Figure 1.
Expression of Cx43-T in carotid VSMC in vivo. The image sequence in (A) is of an intact whole mouse on a LI-COR Odyssey imager. Pluronic gel mixed with Alexa 633 (700 nm, red) was topically applied to a mouse carotid for 24 hours before a tail-vein injection with a bolus of 800 nm conjugated IgG to mark the vasculature (green). In image sequence (B–E), representative carotid images show Cx43-T expression in the carotid VSMC of DMPC (B), ApoE−/− (C), POVPC (D), and PGPC (E) mice. In (B–E), blue indicates nuclei 4-6-Diamidino-2-phenylindole, green represents autofluorescence of the elastic lamina, red indicates Cx43-T, and the asterisk indicates the luminal side of the vessel. Scale bar = 20 μm (B), representative for (B–E). In (F), the fluorescent intensities from secondary antibodies used to detect Cx43-T were plotted according to different carotid experimental conditions: 15-week-old C57/Bl6 (control-15), 15-week-old ApoE−/− mice fed a standard chow diet (ApoE−/−−15), 6-week-old C57/Bl6 (control-6) mice with only pluronic gel applied to the carotid (F-127), mice with 50 μg DMPC applied to the carotid (DMPC), mice with 10/50/100 μg POVPC (10 POVPC/50 POVPC/100 POVPC respectively) applied to the carotid and mice with 10/50/100 μg PGPC (10 PGPC/50 PGPC/100 PGPC) applied to carotid. In (F), #P < 0.05 when compared with 6-week-old C57/Bl6, and *P < 0.05 when compared with 6-week-old C57/Bl6 with 50 μg DMPC applied to carotid.
Antibodies
Immunocytochemistry and Western blot analysis of samples were performed using antibodies for rabbit polyclonal Cx43, which recognizes Cx43 in its phosphorylated and non-phosphorylated states (Cx43-T, Sigma21), rabbit anti-Cx43 pS279/282 (pS279/28219), rabbit anti-Cx43 pS368 (pS368) obtained from Cell Signaling technologies (as verified19), mouse anti-Cx37 (α diagnostics22), mouse anti-Cx40 (α diagnostics22) rabbit anti-Cx45 (Kind gift T.H. Steinberg23), and monoclonal β-tubulin (Sigma). Antibodies were visualized with donkey anti-rabbit Alexa 594 for immunocytochemistry or using 680/800 nm conjugated secondary antibodies (LI-COR) for Western blotting.
Immunocytochemistry
Immunocytochemistry on mouse carotids treated with oxidized phospholipid species was performed as described.20 Images of single antibody stains to be used for quantification were obtained on an Olympus FV200 confocal microscope (objective = 60 × 0.9 NA) with intensity and exposure settings held constant for each antibody tested. For quantification of pixel intensities in carotids, 10 μm × 20 μm boxes were placed between layers of elastic lamina in the VSMC layers (as described24). In each image, at least three areas of VSMC were recorded; at least three images were used per mouse per treatment (n = 3).
Transmission Electron Microscopy
Carotids were placed in 4% paraformaldehyde and 2.5% glutaraldehyde overnight at 4°C. Transmission electron microscopy processing was performed by post-fixing with 1% osmium tetroxide, followed by dehydration in a gradient of alcohols and embedded in Epon. Thin sections (approximately 75 nm) were cut and carbon coated and imaged on a Joel 1230 transmission electron microscope.
Cell Culture
Primary cultures of rat aortic smooth muscle cells (ASMC, kind gift G. K. Owens, see supplemental Figure S1 at http://ajp.amjpathol.org, as described25) were grown in Dulbecco’s Modified Eagles Medium-F12 containing 10% fetal bovine serum, l-glutamine (1.6 mmol/L), penicillin (100 units/ml), and streptomycin (100 μg/ml). To induce a quiescent phenotype, cells were incubated for 48 hours in insulin-free, serum-free Dulbecco’s Modified Eagles Medium-F12 supplemented with l-ascorbic acid (35 μg/ml), apo-transferrin (5 μg/ml), sodium selenite (6.25 μg/ml), l-glutatmine (1.6 mmol/L), penicillin (100 units/ml), and streptomycin (100 μg/ml). Quiescence was verified by flow cytometry (as described26). For positive controls, quiescent cells were incubated with platelet-derived growth factor (PDGF-BB 20 ng/ml) in insulin-free, serum-free media for 24 hours.
In Vitro Cx Expression
Treated ASMC cells (75% to 80% confluent) were lysed in 20 mmol/L Tris-HCL plus Na3VO4 (500 μmol/L), NaF (10 mmol/L), 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (10 μmol/L), 1× complete protease inhibitors (Sigma), and phosphatase inhibitors (Sigma). Membrane fractions were separated via centrifugation (100,000 × g, 5 minutes, as described27). Membrane protein extracts (15 μg), were analyzed by Western blot (10% SDS), transferred to nitrocellulose, probed for protein expression with primary antibodies (as described), and secondary antibody fluorescence was detected using a LI-COR Odyssey infrared scanner. All fluorescence intensities were normalized against β-tubulin for loading.
VSMC Proliferation
Mice were pre-treated with an i.p. injection of the thymidine analogue 5-ethynyl-2′-deoxyuridine (EDU, 100 μg, Invitrogen) 48 hours before carotid application of the OxPLs.28 Carotid surgeries were performed as described above with control mice solely anesthetized with ketamine or with F-127 applications. Treated mice had POVPC or PGPC (100 μg) in F-127 applied to the left common carotid artery. Twenty-four hours after the surgery, mice were euthanized with an i.p. injection of 60 to 90 mg/kg pentobarbital and the carotids were removed. Whole carotids fixed in 4% paraformaldehyde were washed in PBS and permeabilized using 0.5% Triton X-100, and incorporated EDU was conjugated to an Alexa Fluor 594-azide using the Click-IT reagent kit (Invitrogen). Following staining, carotids were cut laterally up the vessel and washed overnight in PBS, and cell nuclei were stained using Sytox green (488 nm, Invitrogen). Carotids (n = 4 for each treatment) were pinned open and visualized by confocal (Olympus Fluoview) microscopy at three randomized sections along the length of each carotid. Z-stack images, approximately 30 μm, with approximately 300 to 500 cells in view, were compressed to a single file for counting. Total cells viewed were between 3500 to 4500 nuclei for each treatment. Positive EDU incorporation was determined as previously described for nuclear bromodeoxyuridine and EDU staining28,29 and confirmed in ligated carotids from control 6–8 week C57/Bl6 mice (see supplemental Figure S2 at http://ajp.amjpathol.org, as described30) and in small intestinal epithelial cells (see supplemental Figure S3 at http://ajp.amjpathol.org). We confirmed the specificity of localization of this treatment by analysis of proliferation in the aortic and mesenteric vessels from POVPC treated mice, which were negative for proliferation (see supplemental Figure S3 at http://ajp.amjpathol.org). The number of nuclei incorporating EDU was normalized against the total nuclei in each image.
For in vitro studies using ASMC, quiescence was induced and the cells (75% to 80% confluence) were treated with a combination of EDU (20 μg, as described31) plus POVPC or PGPC (5 μg/ml, as described15) for 24 hours. Cells were prepared for flow cytometry by fixation in 70% ethanol and permeabilized using 0.1% Triton X-100, and EDU was conjugated to Alexa Fluor 488-azide using the Click-IT reaction kit (Invitrogen). Negative control cells were incubated for a total of 72 hours in insulin-free, serum-free media and processed, as per the staining protocol, with and without EDU for 24 hours, or with PDGF plus EDU for 24 hours as positive controls. A total of 20,000 viable gated cells were collected for each treatment (n = 3, FACSCalibur BD Biosciences). Positive and negative gates in the 488/FL1-H channel (EDU positive) were based on the controls (above) and applied to each sample using FloJo software. Normalized percentages of positive cells were compared with total cell numbers.
Statistics
Significance was determined compared with the relevant controls (as described) using One-way analysis of variance (Bonferroni post hoc test) at significances of *P < 0.05 and ***P < 0.001.
Results
POVPC but not PGPC Reduces Total VSMC Cx43 Expression in Vivo
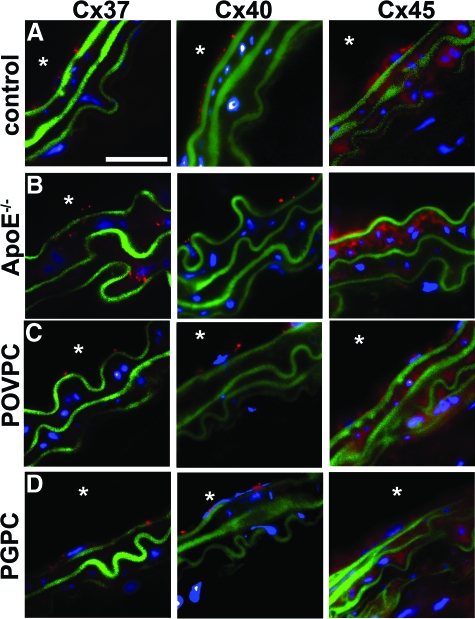
To investigate whether reductions in Cx43 during early atherogenesis1 are associated with the presence of specific OxPLs, we examined total Cx43 (Cx43-T) levels in mouse carotids under control and treated conditions. In 6- to 8-week C57/Bl6 mice, Cx43-T was shown to be expressed in VSMC layers, which was not significantly altered in anesthetic control mice, mice treated with vehicle pluronic F-127, or mice treated with the control lipid DMPC (Figure 1, B and F). In ApoE−/− mice, Cx43-T expression was reduced compared with age matched C57/Bl6 mice (Figure 1, C and F). Applications of POVPC to carotids produced significant, dose-dependent, reductions in VSMC Cx43-T (Figure 1, D and F). However, PGPC applied to carotids did not significantly alter the expression of Cx43-T (Figure 1, E and F). To investigate changes in other vascular connexins, we compared expression of Cx 37, 40, and 45 in control, OxPL-treated, and in ApoE−/− mice, and identified only elevated levels of Cx37 in ApoE−/− carotids (Figure 2, A–D). These data implicate POVPC, but not PGPC, as causative in reducing Cx43-T in the VSMC of carotids, similar to levels identified in carotids of ApoE−/− mice.
Figure 2.
Non-Cx43 vascular connexins expression in carotids. Images show representative carotids from 6-week-old mice treated for 24 hours with (A) DMPC (control), (C) POVPC (100 μg) or (D) PGPC (100 μg), and (B) 15-week ApoE−/− mice. Carotids from mice were analyzed for connexin expression by immunofluorescence using antibodies against mouse anti-Cx37, mouse anti-Cx40, and rabbit anti-Cx45. In each image, blue represents nuclei (DAPI), green represents autofluorescence of the elastic lamina, and red represents Cx staining (as labeled). Scale bar = 20 μm in (A), representative for all images; and the asterisk indicates the luminal side of the vessels.
POVPC and PGPC Differentially Phosphorylate VSMC Cx43 in Vivo
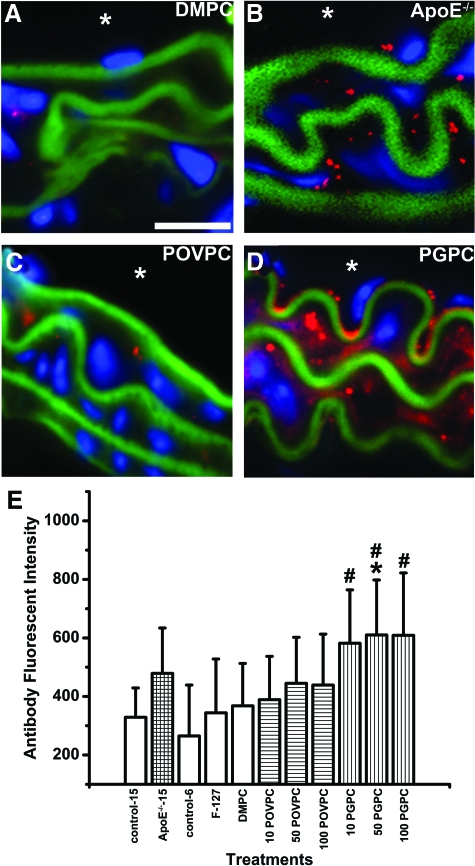
Targeting, functionality, and expression of Cx43 are highly regulated through phosphorylation at sites within its carboxyl terminus,3,4,5,6,7 yet no data currently exist describing Cx43 phosphorylation in atherogenic states. In all control mice, Cx43 was poorly phosphorylated at pS279/282 in carotid VSMC, but levels were significantly elevated in ApoE−/− mice, as compared with age-matched controls (Figure 3, A, B, E). Significant dose-dependent increases in pS279/282 were observed in the carotids of POVPC, but not PGPC-treated mice (Figure 3, C–E). Low amounts of Cx43 pS368 were detectable in all control mice, with elevated levels identified in ApoE−/− mice, as compared with age-matched controls (Figure 4, A, B, E). Applications of PGPC, but not POVPC enhanced levels of Cx43 pS368, as compared with controls (Figure 4, C–E). These findings demonstrate that POVPC and PGPC differentially phosphorylate Cx43 in vivo.
Figure 3.
Expression of Cx43-pS279/282 in carotid VSMC in vivo. In the image sequence (A–D), representative carotid images show Cx43-pS279/282 expression in the VSMCs of DMPCs (A), ApoE−/− (B), POVPC (C), and PGPC (D) mice. In (A–D), blue indicates nuclei (DAPI), green represents autofluorescence of the elastic lamina, red indicates pS279/282, and the asterisk indicates the luminal side of the vessel. Scale bar = 20 μm (A); representative for (A–D). In (E), the fluorescent intensities from secondary antibodies used to detect Cx43-pS279/282 were plotted according to different carotid experimental conditions: 15-week-old C57/Bl6 (control-15) mice, 15-week old-ApoE−/− mice fed a standard chow diet (ApoE−/−-15), 6-week-old C57/Bl6 (control-6) mice with only pluronics applied to the carotid (F-127), mice with 50 μg DMPC applied to the carotid (DMPC), mice with 10/50/100 μg POVPC (10 POVPC/50 POVPC/100 POVPC respectively) applied to the carotid, and mice with 10/50/100 μg PGPC (10 PGPC/50 PGPC/100 PGPC) applied to carotid. In (E), P̂ < 0.05, when compared with 15-week-old C57/Bl6 mice, #P < 0.05, when compared with 6-week-old C57/Bl6 mice, and *P < 0.05, when compared with 6-week-old C57/Bl6, with 50 μg DMPC applied to carotid.
Figure 4.
Expression of Cx43-pS368 in carotid VSMCs in vivo. In the image sequence (A–D), representative carotid images show Cx43-pS368 expression in the VSMC of DMPC (A), ApoE−/− (B), POVPC (C), and PGPC (D) mice. In (A–D), blue indicates nuclei (DAPI), green represents autofluorescence of the elastic lamina, red indicates pS368, and the asterisk indicates the luminal side of the vessel. Scale bar = 20 μm in (A); representative for (A–D). In (E), the fluorescent intensities from secondary antibodies used to detect Cx43-pS368 were plotted according to different carotid experimental conditions: 15-week-old C57/Bl6 (control-15) mice, 15-week-old ApoE−/− mice fed a standard chow diet (ApoE−/−-15), 6-week-old C57/Bl6 (control-6) mice with only pluronics applied to the carotid (F-127), mice with 50 μg DMPC applied to the carotid (DMPC), and mice with 10/50/100 μg POVPC (10 POVPC/50 POVPC/100 POVPC respectively) applied to the carotid and mice with 10/50/100 μg PGPC (10 PGPC/50 PGPC/100 PGPC) applied to the carotid. In (E), #P < 0.05, when compared with 6-week-old C57/Bl6, and *P < 0.05, when compared with 6-week-old C57/Bl6 with 50 μg DMPC applied to carotid.
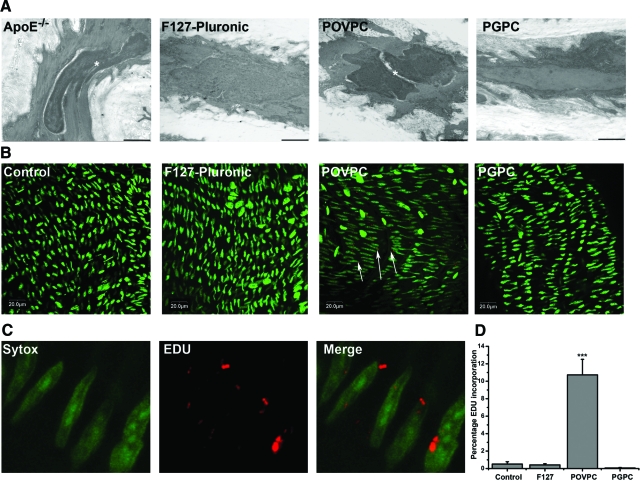
The presence of OxPLs has been linked with enhanced proliferation in vitro,14,15 similar reductions in Cx43 expression and changes in its phosphorylation status are thought to increase cell proliferation.3,18,32,33,34,35 Qualitative ultrastructural evaluations using transmission electron microscopy demonstrated, in control and PGPC-treated mice, compact layers of VSMCs with normal, regular-shaped nuclei, while in ApoE−/− and POVPC-treated mice, irregular-shaped, electron-dense nuclei indicative of heterochromatic DNA and proliferative VSMCs were identified (Figure 5A). These results demonstrate that POVPC elicits signs of VSMC proliferation in treated carotids.
Figure 5.
Cell proliferation in carotid VSMCs following POVPC and PGPC treatments. Qualitative ultrastructural analysis of representative carotids following application of F127 Pluronic, POVPC, PGPC, and in ApoE−/− mice demonstrates dividing VSMC nuclei in ApoE−/− mice and POVPC-treated mice (A). Bars represent 1 μm and the asterisk highlights dividing nuclei (A). Representative images in (B) show EDU incorporation for: anesthetic control, F-127 pluronic gel, POVPC, and PGPC treatments. Images in (C) are high magnifications of the EDU incorporation in VSMCs of carotids following POVPC treatment. The total nuclei were compared with the number of nuclei incorporating EDU as a normalized percentage compared with F-127 control (D). In each image green represents nuclei, red represents EDU, and arrows indicate areas of positive EDU incorporation. ***P < 0.001.
POVPC but not PGPC Promotes Cell Proliferation in Vivo
So as to quantitate our ultrastructural data, we next examined EDU uptake after lipid application. In anesthetic and F-127 control mice, only 0.51% ± 0.27% and 0.41% ± 0.24% (respectively) of all VSMC nuclei stained positive for EDU incorporation (Figure 5, B and D). The levels of VSMC incorporating EDU in mouse carotids treated with POVPC were significantly increased compared with controls (10.73% ± 3.10%, Figure 5, B, D), but not in PGPC treated carotids (0.06% ± 0.10%, Figure 5, B–D). Changes in proliferation following application of lipids were independent of macrophage recruitment to the site of treatment (see supplemental Figure S4 at http://ajp.amjpathol.org). These results show that POVPC, but not PGPC, acts to specifically enhance the proliferation of VSMC in vivo.
POVPC and PGPC Differentially Regulate Cx43 Expression, Phosphorylation, and Proliferation in Vitro
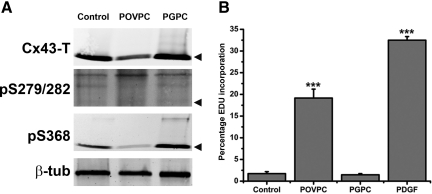
We correlated in vivo observations with cultured ASMC by measuring Cx43 expression and phosphorylation following OxPL treatments. Under control conditions, ASMC express detectable levels of Cx43-T, which were reduced by treatments with POVPC, but not PGPC (Figure 6A). Treatments with POVPC and PGPC enhanced the levels of pS279/282 and pS368 respectively (Figure 6A). When the ASMCs were treated with POVPC, there was a significant increase in cell proliferation (19.16% ± 2.05%) compared with non-treated controls (1.73% ± 0.45%) and PGPC treated cells (1.46% ± 0.27%, Figure 6B). These results are strikingly similar to our observations in vivo.
Figure 6.
Representative Western blot images from ASMCs treated with either POVPC or PGPC. Expression of Cx43-T, pS279/282 and pS368 were determined and normalized for loading with β-tubulin (A). Black arrows in (A) represent the Cx43 P0 band. Flow cytometric analysis is shown of EDU incorporation in ASMCs following treatments with POVPC, PGPC, or PDGF as a positive proliferation marker (B). The graph shows the percentage of cells incorporating EDU for each treatment, as compared with negative controls. ***P < 0.001.
A summary of the in vivo and in vitro data for Cx43 following lipid applications is shown in supplemental Table S1 at http://ajp.amjpathol.org.
Discussion
During atherogenesis, OxPL accumulation is thought to modulate changes in VSMC,36,37 which occur concurrently with alterations in the expression of Cx43.1,38,39 The mechanisms for altered Cx43 expression in atherogenesis have not been described, but may contribute to disease progression. In this paper, we have described for the first time that pro-atherosclerotic lipids can specifically phosphorylate Cx43 at unique phosphorylation sites depending on the OxPL species, and that these phosphorylation sites may contribute to VSMC proliferation.
We first identified reductions in VSMC Cx43-T expression in ApoE−/− mice, consistent with studies by others in western-diet, low-density lipoprotein-R−/− atheroprone mice.1 These mice were reported to form discrete aortic atherosclerotic plaques and considered to be a good model of early atherogenesis.40,41 Alterations in the expression and functionality of Cx43 can be controlled by carboxyl terminus phosphorylation events, which have not been previously described in atherogenesis. In ApoE−/− mice, we identified extensive Cx43 phosphorylation at both the pS279/282 and pS368 sites in carotid VSMCs. These phosphorylation sites can be differentially modulated through MAPK and PKC pathways respectively,19 and may be important in the modulation of Cx43 expression and cell proliferation.18,42 It is therefore plausible that phosphorylation at these sites are integral to changes in Cx43 expression and promotion of atherogenesis.
Site-Specific Cx43 Phosphorylation Resulting from OxPL Treatments
OxPAPC is composed of a number of structurally similar lipid species, including PGPC and POVPC, which accumulate at sites of atherosclerotic plaques.43 Previous studies by our lab have demonstrated that the OxPAPC mixture can have dramatic effects on connexin expression, phosphorylation, and function, however the effect of individual OxPAPC species has not been demonstrated.20 To that end, carotids treated with POVPC demonstrated enhanced Cx43 pS279/282, but reduced Cx43-T expression, a result similar to that seen in ApoE−/− mice. However, POVPC treatments did not phosphorylate Cx43 at the S368 site, as was identified in the ApoE−/− mice. PGPC phosphorylated Cx43 at S368, but did not alter levels of total Cx43 or pS279/282. The differences may be explained by the biological activities of the phospholipids, which act through MAPK (POVPC)- and PKC (PGPC)-mediated pathways.11,14 This correlates with our previous findings that OxPAPC enhanced Cx43 phosphorylation of the tyrosine 265 carboxyl terminus site (pY265) in vivo and in vitro,20 which has been shown to be activated through v-src pathways.19 It is therefore possible that POVPC acts to phosphorylate Cx43 S279/282, which occurs primarily by MAPK and that PGPC targets S368 phosphorylation through PKC pathways.4,7,19
One factor that is not accounted for by the phosphorylation status alone is the reduction in total Cx43 expression in POVPC-treated mice (and cells). Expression of Cx43 can be modulated by reduced synthesis,5 degradation before membrane targeting,44 or increased internalization,45,46 all of which can be modulated by its phosphorylated status.47,48 Therefore specific phosphorylation at pS279/282, but not pS368 (or pY26520), may be integral to reduction or internalization of Cx43.
Treatments with POVPC, but not PGPC, reduced Cx43-T in VSMCs, consistent with changes observed in ApoE−/− mice. This correlates with our previous studies using OxPAPC, that not all OxPL isoforms alter total Cx43 expression.20 However, neither POVPC nor PGPC induced changes in any of the other vascular connexins, including Cx37, which is up-regulated in the ApoE−/− mice. Increased Cx37 expression has been reported by Burt et al to reduce the proliferative potential of rat insuloma cells, and may be another important aspect to atherogenic control,49 with several lines of evidence suggesting that in disease states Cx37 may act as a compensatory mechanism for a more sustained loss of Cx43.50,51,52,53 This concept correlates well with our findings that Cx37 is only up-regulated in the ApoE−/− mice. In our studies acute treatments with the OxPLs excluded any form of compensation by Cx37 or any of the other connexins, which suggest the responses are specific to alterations in Cx43.
Correlations between Altered Connexins and Proliferative States in Atherogenesis
Qualitative ultrastructural analysis of ApoE−/− mice demonstrated that cells were in a proliferative state, which correlates well with what is known about changes in the phenotypic states of VSMC during atherogenesis. Increased proliferation was also identified by EDU incorporation in POVPC, but not PGPC treatments in vivo and in vitro, which correlates with other in vitro studies demonstrating POVPC can induce thymidine uptake in smooth muscle cells.15,16 Because EDU (and other thymidine analogs) is taken up by the cell during the early part of the cell cycle (transition from G1 to S phase), and our time scale was on the order of 24 hours, our data indicate that POVPC rapidly induces this cell cycle transition. Because Cx43 is rapidly turned over in the plasma membrane and has been highly associated with proliferation, this appears to be a logical target for cell-cycle initiation.
Treatments with POVPC enhanced VSMC proliferation, which corresponded with reductions in Cx43 expression and enhanced S279/282 phosphorylation. Studies by others have demonstrated that a loss of Cx43,3,18,32,33,34,35 or altered phosphorylation status,3,54 is sufficient to enhance the proliferative state. In addition to the many studies suggesting Cx43 is capable of controlling cell proliferation rates, several lines of evidence suggest that pS279/282 may be critical in this regard. For example, in PDGF-treated cells, proliferation is reduced by approximately 50% in cells transfected with Cx43-256M-truncated mutant, as compared with controls,55 which suggests that Cx43 carboxyl terminal sites between 256 and 382 are integral in proliferation control. Alanine substitutions at the S279/282 sites (Cx43-S279A), preventing its phosphorylation, caused slower growth rates in 3T3 fibroblasts and Neuro2a cells, as compared with controls and other truncated mutants, further indicating this site is important in normal cell growth.56 In clinical studies, mitotic cells in CIN III cervical lesions express high levels of Cx43 pS279/282, which is reported to be integral to the proliferation and differentiation states of cells in these lesions.34 Phosphorylation of S279/282 is also required for resumption of meiosis in arrested oocytes, without a requirement for pS368.32 In ApoE−/− mice the S279/282 and S368 sites are phosphorylated, in keeping with studies demonstrating these sites are capable of co-expression in Cx43,19 although this is not true of all phosphorylation sites.57 The proliferation-associated activity of pS279/282, but not pS368, does not necessarily implicate a dominant function, but more likely differing roles or interactions. Therefore pS279/282 of Cx43 may be an important initiating factor in cellular proliferation or in removal of the cell from quiescence. These previous results, in keeping with our own findings, suggest that sites in the carboxyl terminus are important in the alteration of cellular proliferation. However, from our studies we cannot rule out the participation of reductions in Cx43-T in cellular proliferation.
Summary
We have identified that in the carotids of early atherogenic mice, Cx43 is reduced and specifically phosphorylated and that these changes can be effectively mimicked through applications of the OxPL species POVPC and to a lesser extent PGPC. It is clear that more work is required to elucidate the mechanisms involved between Cx43 phosphorylation and proliferation, but we provide here a definitive link between the two events.
Supplementary Material
Acknowledgments
Sectioning of tissue was performed by University of Virginia Research Histology Core. Electron microscopy images were obtained with the expertise of Jan Redick and Stacey Guillot at the Advanced Microscopy Core. We gratefully acknowledge the technical expertise and assistance provided by the staff of the School of Medicines Flow Cytometry Core Facility at the University of Virginia. Catherine Hedrick and Coleen McNamara provided initial ideas and tissue sections, and Angela Best provided expert technical assistance. We also thank Brian Wamhoff and Pamela Schoppee Bortz for supply of ASMC cells and PDGF.
Footnotes
Address reprint requests to Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, PO Box 801394 Charlottesville VA 29908. E-mail: bei6n@virginia.edu.
Supported by NIH HL088554 (B.E.I.), HL084422-01 (N.L.), AHA 0755457U (N.L.), GM55632 (P.D.L.), and an American Heart Association Scientist Development Grant (B.E.I.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, Mach F. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 2003;107:1033–1039. doi: 10.1161/01.cir.0000051364.70064.d1. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–514. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007;313:4083–4090. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Kidder GM, Caveney S, Naus CC. Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc Natl Acad Sci USA. 1992;89:10218–10221. doi: 10.1073/pnas.89.21.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, Binder BR, Weber C, Leitinger N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, McIntyre TM, Du BN, Fogelman AM, Berliner JA. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995;95:774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willfort-Ehringer A, Ahmadi R, Gessl A, Gschwandtner ME, Haumer A, Lang W, Minar E, Zehetmayer S, Ehringer H. Neointimal proliferation within carotid stents is more pronounced in diabetic patients with initial poor glycaemic state. Diabetologia. 2004;47:400–406. doi: 10.1007/s00125-004-1345-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Berliner JA, Subbanagounder GG, Bhunia AK, Koh S. Identification of a biologically active component in minimally oxidized low density lipoprotein (MM-LDL) responsible for aortic smooth muscle cell proliferation. Glycoconj J. 2004;20:331–338. doi: 10.1023/B:GLYC.0000033629.54962.68. [DOI] [PubMed] [Google Scholar]
- Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Ghosh N. Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology. 1996;6:303–311. doi: 10.1093/glycob/6.3.303. [DOI] [PubMed] [Google Scholar]
- Leitinger N. Oxidized phospholipids as triggers of inflammation in atherosclerosis. Mol Nutr Food Res. 2005;49:1063–1071. doi: 10.1002/mnfr.200500086. [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247. Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Kronke G, Kadl A, Leitinger N, Duling BR. Oxidized phospholipids alter vascular connexin expression, phosphorylation, and heterocellular communication. Arterioscler Thromb Vasc Biol. 2006;26:2216–2221. doi: 10.1161/01.ATV.0000237608.19055.53. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada KA. Effects of diminished expression of connexin43 on gap junction number and size in ventricular myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H1662–H1670. doi: 10.1152/ajpheart.2000.278.5.H1662. [DOI] [PubMed] [Google Scholar]
- Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y, Kim TJ, Yoo HS, Yun YP. Hesperetin, a bioflavonoid, inhibits rat aortic vascular smooth muscle cells proliferation by arresting cell cycle. J Cell Biochem. 2008;104:1–14. doi: 10.1002/jcb.21592. [DOI] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy JP, Roche D, Kirschner D, Tagami H, Nakatani Y, Almouzni G. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 2004;23:3516–3526. doi: 10.1038/sj.emboj.7600362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Chandra A, Angle N. VEGF inhibits PDGF-stimulated calcium signaling independent of phospholipase C and protein kinase C. J Surg Res. 2006;131:302–309. doi: 10.1016/j.jss.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzo JL, Mennecier G, Mesnil M, Hernandez-Blazquez FJ, Fukumasu H, da Silva TC, Rao KV, Dagli ML. Deletion of a single allele of Cx43 is associated with a reduction in the gap junctional intercellular communication and increased cell proliferation of mouse lung pneumocytes type II. Cell Prolif. 2007;40:411–421. doi: 10.1111/j.1365-2184.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff I, Leykauf K, Bleyl U, Durst M, Alonso A. Phosphorylation of the gap junction protein Connexin43 in CIN III lesions and cervical carcinomas. Cancer Lett. 2006;235:291–297. doi: 10.1016/j.canlet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Shao Q, Wang H, McLachlan E, Veitch GI, Laird DW. Down-regulation of Cx43 by retroviral delivery of small interfering RNA promotes an aggressive breast cancer cell phenotype. Cancer Res. 2005;65:2705–2711. doi: 10.1158/0008-5472.CAN-04-2367. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- Hou CJ, Tsai CH, Su CH, Wu YJ, Chen SJ, Chiu JJ, Shiao MS, Yeh HI. Diabetes reduces aortic endothelial gap junctions in ApoE-deficient mice: simvastatin exacerbates the reduction. J Histochem Cytochem. 2008;56:745–752. doi: 10.1369/jhc.2008.950816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Yokode M, Horiuchi H, Yoshida H, Sano H, Kita T. Overexpression of low density lipoprotein receptor eliminates apolipoprotein B100-containing lipoproteins from circulation and markedly prevents early atherogenesis in apolipoprotein E-deficient mice. Atherosclerosis. 2000;153:295–302. doi: 10.1016/s0021-9150(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Berliner JA. MM-LDL and atherogenesis—a major role for phospholipid oxidation products. Keaney JF Jr, editor. New York: Kluwer Academic Publishers,; Oxidative Stress and Vascular Disease. 1999 [Google Scholar]
- VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithe E, Rivedal E. Ubiquitination of gap junction proteins. J Membr Biol. 2007;217:43–51. doi: 10.1007/s00232-007-9050-z. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62:256–267. doi: 10.1016/j.cardiores.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Laird DW, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Burt JM, Nelson TK, Simon AM, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol. 2008;295:C1103–C1112. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibschull M, Magin TM, Traub O, Winterhager E. Cx31 and Cx43 double-deficient mice reveal independent functions in murine placental and skin development. Dev Dyn. 2005;233:853–863. doi: 10.1002/dvdy.20424. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- Yeh HI, Lai YJ, Chang HM, Ko YS, Severs NJ, Tsai CH. Multiple connexin expression in regenerating arterial endothelial gap junctions. Arterioscler Thromb Vasc Biol. 2000;20:1753–1762. doi: 10.1161/01.atv.20.7.1753. [DOI] [PubMed] [Google Scholar]
- Dang X, Jeyaraman M, Kardami E. Regulation of connexin-43-mediated growth inhibition by a phosphorylatable amino-acid is independent of gap junction-forming ability. Mol Cell Biochem. 2006;289:201–207. doi: 10.1007/s11010-006-9162-2. [DOI] [PubMed] [Google Scholar]
- Moorby CD, Gherardi E. Expression of a Cx43 deletion mutant in 3T3 A31 fibroblasts prevents PDGF-induced inhibition of cell communication and suppresses cell growth. Exp Cell Res. 1999;249:367–376. doi: 10.1006/excr.1999.4485. [DOI] [PubMed] [Google Scholar]
- Moorby C, Patel M. Dual functions for connexins: cx43 regulates growth independently of gap junction formation. Exp Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.