Abstract
BACKGROUND:
Patients with hypertension often do not adhere to their medications.
OBJECTIVE:
To improve medication adherence in patients with essential hypertension by modifying their behaviours.
PATIENTS AND METHODS:
From general practice settings, 4864 patients with essential hypertension were recruited and randomly assigned to receive the angiotensin receptor blocker irbesartan (Avapro) with (intervention group) or without (nonintervention group) a behavioural modification program (Avapromise) based on a model of change. Patients were followed up for 12 months. Patients were subgrouped based on their stage of change in the behavioural change continuum, and the intervention was tailored to address the needs of the particular subgroup. The primary efficacy measure was rate and time to discontinuation with irbesartan.
RESULTS:
At the end of the study, there was no significant difference in the discontinuation rates between the intervention (25.4%, 95% CI 23.7 to 27.2) and nonintervention (25.5%, 95% CI 23.8 to 27.3) groups (P=0.94). The time to discontinuation (P=0.87) and the extrapolated rate of discontinuation estimated from the Kaplan-Meir curve (intervention 23.1%, 95% CI 21.3 to 24.8; nonintervention 23.5%, 95% CI 21.8 to 25.3) were not different between the groups.
CONCLUSIONS:
This behavioural modification intervention based on a model of change was not efficacious at increasing rates of adherence in patients with essential hypertension in this setting. More individualized interventions may be required to increase adherence in this population.
Keywords: Angiotensin, Behaviour modification, Hypertension
Hypertension is an established cardiovascular risk factor and can lead to stroke, myocardial infarction and premature death (1,2). However, despite widespread availability of therapy, hypertension is inadequately controlled and poses a significant risk (3). A Canadian Heart Health survey showed that hypertension was controlled (blood pressure less than 140/90 mmHg) in only 16% of those treated for hypertension and uncontrolled in 23%, despite receiving therapy (4). Suboptimal adherence to therapy is a common cause of uncontrolled hypertension (5). An estimated 16% to 50% of patients with hypertension discontinue their therapy within the first year (6,7).
In a recent overview of randomized, controlled trials (RCTs) of interventions used to improve patient adherence to medication in hypertensive populations, the interventions with positive effects were complex and intensive (8). Also, measurement of adherence in an RCT setting could preclude generalizability of the ‘treatment’ effect because of the nature of the study design (9). It would, therefore, be desirable to examine the effects of an intervention that would be relatively simple and conducted in a usual care setting. Moreover, it would be useful to use an intervention that combined both lifestyle modification and reinforcement, and could potentially affect adherence to therapy by modifying behaviour.
Irbesartan (Avapro, Bristol Myers Squibb/Sanofi-Synthelabo, Canada) is a long acting angiotensin II receptor blocker (ARB). In controlled clinical trials comprising patients with mild to moderate hypertension, seated systolic and diastolic blood pressure reductions achieved with irbesartan were either equal or superior to those achieved with concomitantly used agents. Irbesartan has demonstrated efficacy relative to hydrochlorothiazide, losartan and enalapril (10–12). It has also demonstrated an excellent safety and tolerability profile. Irbesartan can be used alone or in combination with other agents such as thiazides in patients whose hypertension is inadequately controlled by single-drug therapy.
A randomized, open-label, 12-month, phase IV trial comprising patients with essential hypertension was conducted to study the effectiveness of Avapromise, a behavioural modification intervention to increase the adherence of patients receiving treatment with irbesartan in the usual care setting. It is our understanding that this is the largest study conducted with the primary aim of influencing adherence to therapy.
PATIENTS AND METHODS
Patient recruitment and follow-up were conducted by Innovus Inc (Canada), and the data were analyzed by BTB Associates (Canada).
Patients
The trial was designed as a randomized, multicentre, open-label, two-arm study comprising patients with essential hypertension. General practitioners who were willing to participate in the study recruited patients from within their practices based on who, in their opinion, would benefit from therapy with irbesartan. Patients who satisfied the inclusion and exclusion criteria (Table 1), and gave their informed consent were entered into the study.
TABLE 1.
Inclusion and exclusion criteria for a randomized, clinical trial designed to increase adherence to hypertension medications through behavioural modification
| Inclusion criteria |
| History of diastolic blood pressure higher than 90 mmHg and/or systolic blood pressure higher than 140 mmHg, and untreated; or current hypertension treatment requiring alteration in the opinion of the physician |
| Aged 18 to 79 years and, if female, unable to become pregnant |
| Willingness to give informed consent |
| Exclusion criteria |
| If female, pregnant or breast-feeding, or of childbearing potential |
| Taking any investigational drug given within 30 days of initiation of therapy, and participation in other clinical studies while enrolled in this protocol |
| Undergoing peritoneal dialysis |
| Presence of any of the following conditions: |
Cardiovascular disorders
|
Allergies/hypersensitivity
|
Other
|
Patients were randomly assigned to receive a once daily dose of irbesartan 150 mg that could be increased to 300 mg, with or without the intervention Avapromise. The Avapromise intervention was designed to modify behaviour by medication adherence through reinforcement and lifestyle modification. It is made up of two elements that are delivered in unison. The first element attempts to reinforce medication adherence behaviours by using medication reminder letters, blood pressure diaries and telephone nurse counselling sessions. The second element addresses issues of lifestyle management through educational brochures dealing with topics such as healthy living, nutrition, physical fitness and stress management. Patients randomly assigned to receive Avapromise were mailed the material at one, two, three, four, six and 12 months. The receipt schedule of the different components of this intervention is highlighted in Table 2. Patients in the control arm received usual care educational materials in their physicians’ offices.
TABLE 2.
AVAPROMISE receipt schedule
| Month | Stage of change |
|---|---|
| 1 | Enrolment package |
| 2 | Blood pressure: Know the numbers by heart (diary) |
| Hypertension – A Self-Management Approach (book) | |
| 3 | Medication reminder letter |
| Where Fitness Fits in Your Life (brochure/poster) | |
| Telephone nurse counselling session #1 | |
| 4 | Medication reminder letter |
| How to Enjoy Eating Well (brochure/poster) | |
| Telephone nurse counselling session #2 | |
| 6 | Medication reminder letter |
| Taking Charge of Managing Stress (brochure/poster) | |
| Telephone nurse counselling session #3 | |
| 8 | Medication reminder letter |
| The Good News on…Communication (Doctor/Patient Communication) | |
| 10 | Medication reminder letter |
| The Good News on…Fitness (Walking) | |
| Telephone nurse counselling session #4 | |
| 12 | Letter |
| Patient satisfaction survey |
Prescription of additional antihypertensive medications was permitted. Randomization to Avapromise was done by site (recruiting physicians’ offices), such that all the patients within one site were randomly assigned to the same treatment regimen to avoid contamination and minimize investigator bias. Due to the nature of the intervention, blinding was not possible. Randomization was done using a computer-generated algorithm.
Patients were telephoned at two, five, eight and 12 months to estimate their adherence to irbesartan as well as to note the incidence of adverse events and the use of related health services. After the enrolment visit, subsequent visits were decided between patient and physician. The duration of the study was 12 months.
The primary effectiveness measure was patient discontinuation with their irbesartan treatment regimens following up to 12 months of treatment for essential hypertension. The impact of the Avapromise intervention on patient compliance with irbesartan was assessed by comparing the rate and time to discontinuation between these two groups of patients. The ‘time to discontinuation’ was defined as a negative response to a telephone follow-up question “Are you taking your Avapro (irbesartan) every day?”, asked as part of a normal patient follow-up at two, five, eight and 12 months.
Enrolment began on December 19, 1998, and the sponsor decided to terminate the study on November 30, 2000, after the required number of discontinuations had been observed. At that time, the patients still enrolled in the study were contacted a final time by telephone.
Statistical considerations
Sample size:
In the absence of pre-existing data on 12 month discontinuation rates with irbesartan, it was assumed that at least 50% of the required number of patient discontinuations would occur within that period. A difference in the number of discontinuations between treatment groups of 4% was considered clinically relevant. If it is assumed that 20% of patients assigned to receive the medication without the intervention, compared with 16% assigned to receive the medication with Avapromise, would discontinue their medication within one year (an absolute difference of 4%), 2561 discontinuations would have to be observed. A sample size of 5000 patients was considered adequate (90% power) to detect the difference using a two-sided test of statistical significance (logrank test) at the 5% level. The total number of patient discontinuations was monitored throughout the study to determine when the required number of discontinuations had occurred. The study was terminated when 50% of the required discontinuations (1281) were observed by 12 months, and there was adequate power to note statistical differences.
Analysis:
Descriptive statistics were used for the analysis of continuous (mean and standard deviation) and categorical (frequency) variables. The primary effectiveness variable was compared between treatment regimens using a combination of categorical and survival analysis techniques. Time to discontinuation was analyzed using survival analysis techniques. Kaplan-Meir survival curves were estimated for each treatment regimen and compared using a logrank test.
RESULTS
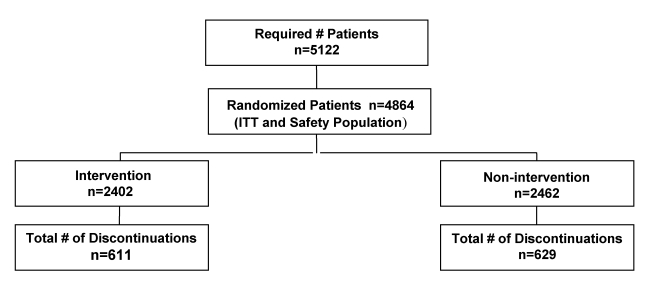
The overall trial profile is displayed in Figure 1. A total of 4864 patients were randomly assigned – 2402 to the intervention group and 2462 to the nonintervention group – and were included in the intent-to-treat population. The two groups were similar in terms of their baseline characteristics, including medical history and baseline antihypertensive medication (Tables 3,4,5). The mean age of the patients was 58 years (range 16 to 89 years) in both the arms; 51% of those enrolled were female. Patients were enrolled at a total of 397 centres across Canada.
Figure 1).
Profile of a trial designed to improve adherence to medication by patients with essential hypertension. ITT Intent to treat
TABLE 3.
Baseline characteristics of patients assigned to receive irbesartan with (Intervention) or without (Nonintervention) the behavioural modification program AVAPROMISE
| Variable | Intervention (n=2402) | Nonintervention (n=2462) | Total (n=4864) |
|---|---|---|---|
| Age, years (range) | 57.6 (22–86) | 57.8 (16–89) | 57.7 |
| Age group, n (%) | |||
| Missing data | 59 (2) | 41 (2) | 100 (2) |
| 10–17 years | 2 (<1) | 0 | 2 (<1) |
| 18–29 years | 13 (1) | 16 (1) | 29 (1) |
| 30–39 years | 125 (5) | 113 (5) | 238 (5) |
| 40–49 years | 421 (18) | 443 (18) | 864 (18) |
| 50–59 years | 755 (31) | 751 (31) | 1506 (31) |
| 60–69 years | 625 (26) | 709 (29) | 1334 (27) |
| 70–79 years | 381 (16) | 375 (15) | 756 (16) |
| >80 years | 21 (1) | 14 (1) | 35 (1) |
| Sex, n (%) | |||
| Missing data | 101 (4) | 81 (3) | 182 (4) |
| Female | 1257 (52) | 1231 (50) | 2488 (51) |
| Hypertension duration, years | 5.8 | 5.4 | 5.6 |
| Systolic blood pressure, mmHg (SD) | 160 (16) | 160 (17) | 160 (16.7) |
| Diastolic blood pressure, mmHg (SD) | 95 (9) | 95 (9.6) | 95 (9.3) |
| Missing data, n (%) | 163 (7) | 118 (5) | 281 (6) |
| Newly diagnosed, n (%) | 218 (9) | 265 (11) | 483 (10) |
| Previously diagnosed, n (%) | 2021 (84) | 2079 (84) | 4100 (84) |
| 0–5 years | 1210 (50) | 1283 (52) | 2493 (51) |
| 6–10 years | 327 (14) | 358 (15) | 685 (14) |
| 11–15 years | 207 (9) | 186 (8) | 393 (8) |
| 16–20 years | 145 (6) | 119 (5) | 264 (5) |
| >20 years | 132 (6) | 133 (5) | 265 (5) |
TABLE 4.
Medical history of patients assigned to receive irbesartan with (Intervention) or without (Nonintervention) the behavioural modification program AVAPROMISE
| Variable | Intervention (n=2402) | Nonintervention (n=2462) | Total (n=4864) |
|---|---|---|---|
| Diabetes | 296 (12) | 316 (13) | 612 (13) |
| Asthma | 131 (5) | 147 (6) | 278 (6) |
| Angina | 85 (4) | 102 (4) | 187 (4) |
| Congestive heart failure | 19 (1) | 27 (1) | 46 (1) |
| Myocardial infarction | 57 (2) | 62 (3) | 119 (2) |
| Peripheral vascular disease | 28 (1) | 41 (2) | 69 (1) |
| Stroke | 32 (1) | 54 (2) | 86 (2) |
| Other cardiovascular disease | 45 (2) | 47 (2) | 92 (2) |
TABLE 5.
Oral antihypertensive medications used by patients assigned to receive irbesartan with (Intervention) or without (Nonintervention) the behavioural modification program AVAPROMISE in the six months preceding the study
| Variable | Intervention (n=2402) | Nonintervention (n=2462) | Total (n=4864) |
|---|---|---|---|
| Beta-blockers, n (%) | 399 (17) | 378 (15) | 777 (16) |
| Diuretics, n (%) | 624 (26) | 539 (22) | 1163 (24) |
| ACEIs, n (%) | 720 (30) | 718 (29) | 1438 (30) |
| CCBs, n (%) | 396 (17) | 536 (22) | 932 (19) |
| ARBs, n (%) | 135 (6) | 212 (9) | 347 (7) |
| Other, n (%) | 87 (4) | 100 (4) | 187 (4) |
| Diuretic plus ACEI, n (%) | |||
| Neither | 1273 (53) | 1420 (58) | 2693 (55) |
| Either | 906 (38) | 821 (33) | 1727 (36) |
| Both | 219 (9) | 218 (9) | 437 (9) |
ACEI Angiotensin-converting enzyme inhibitor; ARB Angiotensin receptor blocker; CCB Calcium channel blocker
The treatment groups were similar with respect to history of hypertension, and 84% of patients had chronic hypertension. The mean duration of hypertension was approximately six years (SD=8.1), and 51% of patients had been hypertensive for five years or less. The mean baseline systolic blood pressure was 160 mmHg (SD=16) and the mean diastolic blood pressure was 95 mmHg (SD=9). Approximately 92% of patients were prescribed a starting dose of irbesartan 150 mg once daily, and 7% were prescribed a starting dose of 300 mg once daily.
A total of 1240 (25% of 4864) patients discontinued their medications – 611 (25.4%, 95% CI 23.7 to 27.2) from the intervention group and 629 (25.5%, 95% CI 23.8 to 27.3) from the nonintervention group, resulting in a difference of −0.1% (−2.6 to 2.3) between the two groups (P=0.94, Table 6).
TABLE 6.
Rate of and time to discontinuation in the intent-to-treat patient population*
| Time point | Intervention, % (95% CI) | Nonintervention, % (95% CI) | Difference† | |
|---|---|---|---|---|
| Patient withdrawal rate | End of study | 25.4 (23.7–27.2) | 25.5 (23.8–27.3) | −0.1% (−2.6–2.3) |
| P=0.948 | ||||
| Cumulative withdrawal rate§ | 6 months | 11.9 (10.6–13.2) | 12.0 (10.7–13.3) | P=0.8770‡ |
| 9 months | 17.6 (16.0–19.1) | 17.9 (16.4–19.5) | ||
| 12 months | 23.1 (21.3–24.8) | 23.5 (21.8–25.3) | ||
| >12 months | 28.5 (26.4–30.5) | 27.8 (25.8–29.7) |
Based on the intent-to-treat patient population after imputing of missing or extreme patient outcomes (ie, duration of irbesartan compliance);
Difference between the intervention and the nonintervention groups;
Logrank P value for comparison survival curves between treatment groups: χ2 test with one degree of freedom;
Based on Kaplan-Meier estimates taken at time points immediately after day 180 (six months), day 270 (nine months), 365 (12 months) and last day (more than 12 months) from the Kaplan-Meier curve
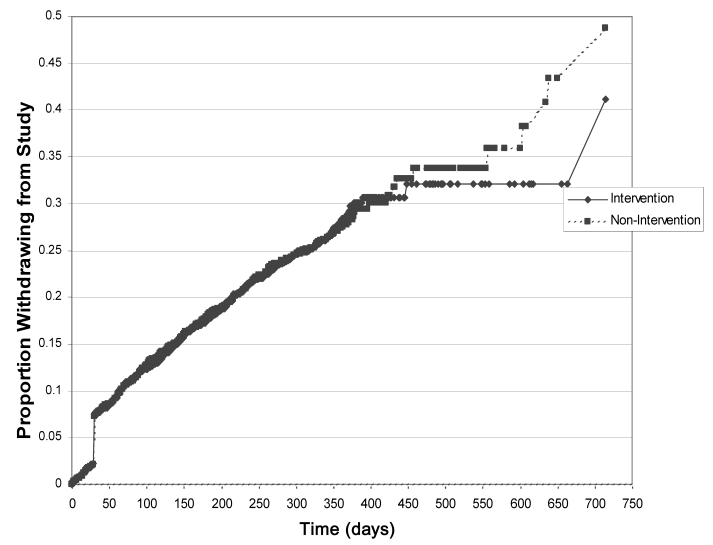
There was no statistically significant difference in the duration of irbesartan compliance between the treatment groups. Overall, the average duration of irbesartan compliance was 267 days (SD=127) and was similar between treatment groups (265 days for the intervention group and 269 days for the non-intervention group). The estimated rates of discontinuation, based on the Kaplan-Meier estimates at 12 months, were 23.1% in the intervention group and 23.5% in the nonintervention group. The patterns of the times to discontinuation, as illustrated in the Kaplan-Meier plot (Figure 2), were not significantly different between treatment groups (P=0.877, logrank test). A small proportion of patients were followed up for more than 12 months because of their earlier enrolment, and there was a separation between the two survival curves beyond 450 days.
Figure 2).
Kaplan-Meier plot of the time to discontinuation
Fourteen per cent (179 of 1240) of patients prematurely terminated the study. In the two study arms combined, personal preference (34%, 61 of 179), investigator decision (22%, 39 of 179), serious adverse drug reactions (19%, 34 of 179) and other reasons (24%, 44 of 179) were cited as primary reasons for the termination. Five deaths were reported during the study. Fifty-four per cent (668 of 1240) of patients who discontinued irbesartan during the study cited side effects (47%, 311 of 688), lack of efficacy (16%, 109 of 688) and physician instruction (14%, 96 of 688) as some of the primary reasons for termination.
DISCUSSION
A randomized, open label, 12-month study was conducted to determine patients’ adherence to antihypertensive therapy with irbesartan by using a behavioural modification intervention called Avapromise in a clinical practice setting. The rate of or time to discontinuation was not different between the two groups. The overall adherence rate was 75% (ie, approximately 25% of patients discontinued medication use) in both arms. On the basis of these observations, it can be stated that the Avapromise intervention did not improve adherence. There are three possible reasons for this lack of improvement.
First, Avapromise was a relatively low resource-intensive intervention, which probably contributed to its lack of efficacy. As noted in the overview by Haynes et al (8), RCTs of interventions that were very intensive and complex tended to yield positive results. Furthermore, in studies designed to improve the adherence of hypertensive patients to medications, these measures included medical care provided at the work site, special pill containers, support groups, feedback, reinforcement, etc (13,14). For the Avapromise study, protocol-defined physician office visits could have added an element of reinforcement during the duration of the study but would have precluded its generalizability to usual care settings.
Second, the literature demonstrates that the success rate for demonstrating statistically significant results in adherence studies is not very high. Once again, the overview by Haynes et al (8) noted that, among the studies that met their eligibility criteria, a little more than one-half (10 of 19) demonstrated a significant result, possibly because adherence itself, as a behavioural phenomenon, requires more detailed study. By extension, studies designed to demonstrate enhanced adherence would require a rethink of the paradigms and methods that have been used so far to measure or improve it.
Third, because adherence in the control group was quite high, interventions to increase it further would perhaps have a lower likelihood of success.
Finally, AVAPROMISE was designed to influence behaviour as a whole rather than to influence medication adherence per se. Providing patients with a broader perspective on the tools required to manage their lifestyles was deemed important enough to influence medication adherence indirectly through behavioural modification. Although these are important issues, interventions to improve adherence in hypertension management by lifestyle modification may not affect medication adherence – a different behaviour that may require specific interventions.
A limitation of this study is that the effect of adherence on blood pressure control was not a predefined end point. However, it is being investigated in a substudy. In this study, two estimates of blood pressure control using a 24 h ambulatory blood pressure monitoring technique (at enrolment and at study termination or discontinuation) are being used. Also, because physicians determined patient eligibility for the study, it is not possible to determine whether there was a selection bias in the nature of enrolment. Involving sequential enrolment may perhaps better benefit future studies of similar design but not affect usual clinical practice.
This study also had some unique strengths. First, to our knowledge, this was the largest prospective study on adherence that has ever been conducted. Second, because it was conducted in a usual care setting, the inclusion and exclusion criteria were fairly nonrestrictive and physicians had wide discretionary powers. As a result, physicians could titrate, or add or switch medication class (except converting enzyme inhibitors) or dose, which enhances the external validity or generalizability of the study.
The literature shows that ARBs are associated with relatively higher rates of adherence than other antihypertensive classes. A recent analysis of the Saskatchewan database revealed higher adherence to ARBs at the end of two years (70%, P<0.01) than to all other classes of antihypertensive medications (15). Also, when patients were prescribed ARBs as initial therapy, adherence rates were higher. In a retrospective cohort analysis using a large pharmaceutical benefits management organization database, patients who received ARBs as initial therapy were significantly more adherent (64%, P<0.05) than other therapies (16). The adherence rates found in this study are significant given that irbesartan was not initial therapy for a majority of patients. The reasons for higher adherence may be attributed to increased tolerability and efficacy, requiring fewer discontinuations.
CONCLUSIONS
Adherence to ARBs seems to be well documented. The Avapromise study is thus far the biggest clinical trial designed and conducted for increasing adherence to medication in a hypertensive population. For increasing adherence, the trans-theoretical model of change offers interesting insights, and therapies with better adaptability and intensity may offer further validation in this setting. The effect of interventions on adherence may be better evaluated using methods specifically designed to change adherence-related behaviour through a better understanding of its causal mechanisms and may thus provide a better validation of the hypothesis tested in this study. However, a more ‘patient-specific’ intervention based on individual needs may prove to be more effective and mandates further research.
Acknowledgments
This study was sponsored by Bristol-Myers Squibb Canada and Sanofi Canada
APPENDIX 1
AVAPROMISE study participants
Dr Adele Adjami, Montreal, Quebec
Dr Walter Ah Now, Pickering, Ontario
Dr Réjean Alarie, Cap-de-la Madeleine, Quebec
Dr HC Alexis, Ottawa, Ontario
Dr Roy C Allison, Thunder Bay, Ontario
Dr Robert Ames, Bolton, Ontario
Dr Shabbir F Amin, Redwater, Alberta
Dr MJ Aniol, Sudbury, Ontario
Dr Chris Annandale, Regina, Saskatchewan
Dr Laval MY Ahpin, Windsor, Ontario
Dr Jacques Auger, Princeville, Quebec
Dr Murray Awde, London, Ontario
Dr Norman Peter Azar, Vita, Manitoba
Dr Terry Babick, Winnipeg, Manitoba
Dr AR Baker, Mississauga, Ontario
Dr Ginette Barrière, Sainte-Catherine, Quebec
Dr JL Barrow, Calgary, Alberta
Dr Garry B Barrs, Verdun, Quebec
Dr Michel Barry, Sainte-Julie, Quebec
Dr J Bart, North York, Ontario
Dr K Bayly, Saskatoon, Saskatchewan
Dr Cyril Beaumont, St-Jean Chrysostome-de-Levis, Quebec
Dr Ronald W Beazley, Dartmouth, Nova Scotia
Dr Phyllis Bedder, Winnipeg, Manitoba
Dr André Belanger, Courcelette, Quebec
Dr Jasmin Belle-Isle, Courcelette, Quebec
Dr Adele Belliveau, Dartmouth, Nova Scotia
Dr Daniel Benoit, Mascouche, Quebec
Dr Maria Berjat, Montreal, Quebec
Dr I Berstein, Toronto, Ontario
Dr Cassim Bhabha, Toronto, Ontario
Dr Ranbir Bhatia, Ottawa, Ontario
Dr Gunuant Bhatt, Prescott, Ontario
Dr Robert Boies, Laval, Quebec
Dr Raynald Boily, St Catherines, Ontario
Dr Emilia Bordalba, Vancouver, British Columbia
Dr R Bornstein, Thornhill, Ontario
Dr G Boudreau, Loretteville, Quebec
Dr Alain Boudrias, Ste-Julienne, Quebec
Dr Ian Bridger, Victoria, British Columbia
Dr Robert Brown, Edmonton, Alberta
Dr DJL Brown, Sherwood Park, Alberta
Dr Jerzy Brzeski, Calgary, Alberta
Dr Daniela Bucur, Montreal, Quebec
Dr Denis Busque, Grand-Mère, Quebec
Dr Sylvie Cadet, Varennes, Quebec
Dr Gilles Campeau, Drummondville, Quebec
Dr David Carswell, Harrow, Ontario
Dr James Casserly, Nepean, Ontario
Dr John Castiglione, Toronto, Ontario
Dr Guy Chamberland, Shawinigan, Quebec
Dr Chan-San-Hoi Chan-Tai-Kong, Lloydminster, Alberta
Dr S David Chapman, Neepawa, Manitoba
Dr Jacques Charbonneau, Sainte-Julie, Quebec
Dr Michel Charest, Montreal, Quebec
Dr Chawla, London, Ontario
Dr Léandre Chénard, Tracy, Quebec
Dr R Chernoff, Saskatoon, Saskatchewan
Dr John Chiasson, Antigonish, Nova Scotia
Dr Martyn Chilvers, Sarnia, Ontario
Dr Maxwell Chin, North York, Ontario
Dr Sylvester Chiu, Simcoe, Ontario
Dr John Chiu, Edmonton, Alberta
Dr Betty Choi-Fung, Toronto, Ontario
Dr Albert Choong, Toronto, Ontario
Dr Robert BW Choptiany, Winnipeg, Manitoba
Dr Guy Chouinard, Charlesbourg, Quebec
Dr W Chow, Victoria, British Columbia
Dr Peter Chung, Port Coquitlam, British Columbia
Dr Norman N Chychota, Taber, Alberta
Dr John Collingwood, St John’s, Newfoundland
Dr Stephen Cord, Toronto, Ontario
Dr Guildo Cote, Cabano, Quebec
Dr Donald Craswell, Middleton, Nova Scotia
Dr Steven Cusimano, Hamilton, Ontario
Dr Daniel Cusson, Laval, Quebec
Dr Claude Cyr, Perce, Quebec
Dr Monika Czarnecka, Winnipeg, Manitoba
Dr Anthony D’Angelo, Whitby, Ontario
Dr Laurindo M Da Silva, Winnipeg, Manitoba
Dr Michel Dagenais, Sainte-Julie, Quebec
Dr Alain Daigle, Bic, Quebec
Dr Frank Dallison, Calgary, Alberta
Dr Pierre Dauth, Cowansville, Quebec
Dr John M Dawson, Richmond Hill, Ontario
Dr S Decena, North York, Ontario
Dr R Decker, Mill Bay, British Columbia
Dr Eric Deernsted, Kanata, Ontario
Dr Jacques Delorme, St Etienne Des Gres, Quebec
Dr S Devi, North York, Ontario
Dr Luc Déziel, Saint-Constant, Quebec
Dr Dininno, Medicine Hat, Alberta
Dr Langis Dionne, Montreal, Quebec
Dr Michele Dionne, Pointe-Claire, Quebec
Dr M DiPaolo, Lachute, Quebec
Dr Patrick Doorly, Barrie, Ontario
Dr Jean-François Dorval, Rimouski, Quebec
Dr Bernard Doyon, Beloeil, Quebec
Dr Gilles Dube, Notre-Dame-du-Lac, Quebec
Dr Helena Duchowska, Richmond, British Columbia
Dr Alain Dupont, Cap-de-la-Madeleine, Quebec
Dr T Echlin, Windsor, Ontario
Dr M El-Tair, Winnipeg, Manitoba
Dr Sylvie Emond, Charlesbourg, Quebec
Dr Dan Ezekiel, Vancouver, British Columbia
Dr A Faiers, Toronto, Ontario
Dr Bernard Farber, Pickering, Ontario
Dr Andrea Faught, Trenton, Ontario
Dr Craig Ferguson, Brighton, Ontario
Dr W Fetherston, Aurora, Ontairo
Dr Nigel Flook, Edmonton, Alberta
Dr LA Flores, Scarborough, Ontario
Dr Dennis Forrester, Mississauga, Ontario
Dr Norman L Fox, Montreal, Quebec
Dr MT Franklyn, Sudbury, Ontario
Dr Evelyne Fraser, St-Jean Chrysostome-de-Levis, Quebec
Dr André Fréchette, Quebec, Quebec
Dr M Frenette, Ste-Agathe, Quebec
Dr Guy Gagné, Loretteville, St-Emile, Quebec
Dr Louise Gagnon, Montreal, Quebec
Dr Solly Gardee, Kanata, Ontario
Dr C Gaudet, Saint-Calixte, Quebec
Dr Gilles Gaudreau, Sorel, Quebec
Dr Shiva Gaur, Scarborough, Ontario
Dr Jean Gauthier, Amqui, Quebec
Dr Daniel Gelinas, St Etienne Des Gres, Quebec
Dr Rosemarie Geonzon, Calgary, Alberta
Dr Pam Gill, Mississauga, Ontario
Dr Sylvie Gill, Sorel, Quebec
Dr Paul-Emile Godin, Beauport, Quebec
Dr Corrina Golding, Saint-John, New Brunswick
Dr Grant Goodine, Lawrencetown, Nova Scotia
Dr Jean Granger, Aylmer, Quebec
Dr Peter Greidanus, Lethbridge, Alberta
Dr Louis Grenier, St-Agapit, Quebec
Dr Gilles Grenier, Iberville, Quebec
Dr Denis Grenon, Vaudreuil, Quebec
Dr Emil Grigore, West Lorne, Ontario
Dr R Grimwood, Victoria, British Columbia
Dr Joseph Anthony Grogan, Winnipeg, Manitoba
Dr Louis Grondin, L’Isletville, Quebec
Dr Suzanne Guillet, Montreal, Quebec
Dr Magdi Habra, Bois-Des-Filion, Quebec
Dr Ken Hahlweg, Teulon, Manitoba
Dr Yousri Hanna, Anjou, Quebec
Dr Claude Hemond, Bromptonville, Quebec
Dr James Hii, Vancouver, British Columbia
Dr Ho-Sen Chen, Montreal, Quebec
Dr Jacob Holub, Sudbury, Ontario
Dr Gaetan Houle, Montreal, Quebec
Dr Don Hunsberger, Owen Sound, Ontario
Dr Hutchinson, Toronto, Ontario
Dr TK Idicula, Wetaskiwin, Alberta
Dr Alfred W Illescas, Montreal, Quebec
Dr Angel Ip, Winnipeg, Manitoba
Dr M Jackson, Hamilton, Ontario
Dr Renée Jacob, Beauport, Quebec
Dr Raymond Jacques, Sudbury, Ontario
Dr Klaus Jakelski, Val Caron, Ontario
Dr Marry Ellen James, Calgary, Alberta
Dr Brett Jamieson, Marbank, Ontario
Dr HP Janssen, Woodstock, Ontario
Dr Anthony F Jeraj, Medicine Hat, Alberta
Dr Tom Johnson, Burlington, Ontario
Dr Anthony Jong, Victoria, British Columbia
Dr Carole Joubert, Ste-Adele, Quebec
Dr Al Karmali, Maple Ridge, British Columbia
Dr P Kelly, Victoria, British Columbia
Dr I Keltz, North York, Ontario
Dr Ian Kendal, Calgary, Alberta
Dr A Keshvara, Medicine Hat, Alberta
Dr Raymond Kevork, Toronto, Ontario
Dr MC Khurana, North Battleford, Saskatchewan
Dr Felix Klajner, Toronto, Ontario
Dr Donald W Korzenowski, Edmonton, Alberta
Dr Joseph H Kozak, Etobicoke, Ontario
Dr Nicholas Krayacich, Windsor, Ontario
Dr Andrew Kuchtaruk, Sudbury, Ontario
Dr Kushner, Weston, Ontario
Dr Carson C Kwok, Cobourg, Ontario
Dr Nabil Labateya, Montreal, Quebec
Dr J Roger Laberge, Chateauguay, Quebec
Dr Robert Labrie, Montagny, Quebec
Dr Louise Lacombe, Shawinigan, Quebec
Dr Jacques Laforest, Angus, Ontario
Dr M Gilles Lafrance, Charlesbourg, Quebec
Dr Patrick Lai, Calgary, Alberta
Dr Anne Laliberte, St-Anselme, Quebec
Dr Sy Lam, Calgary, Alberta
Dr Roch Lambert, St-Lambert-de-Levis, Quebec
Dr Hian Lampoyuen, Lacolle, Quebec
Dr Linda Landry, Notre-Dame-du-Lac, Quebec
Dr James Lane, Stayner, Ontario
Dr Clement Lang, Winnipeg, Manitoba
Dr Louis Langlois, Brossard, Quebec
Dr Michel Lapalme, St-Saveur, Quebec
Dr Claude Lapointe, Ste-Rosalie, Quebec
Dr Mario Lapointe, Chicoutimi, Quebec
Dr Claude Laroche, Montreal, Quebec
Dr Yves Larochelle, Longueuil, Quebec
Dr Howe Leam, Lethbridge, Alberta
Dr Mario Lebel, St-Pascal, Alberta
Dr Marcel Leclair, Anjou, Quebec
Dr JI Leeson, Sauble Beach, Ontario
Dr Jacques Lefebvre, Laval, Quebec
Dr Jack Lefkowitz, North York, Ontario
Dr Paul Lefort, Montreal, Quebec
Dr Jacques Legrand, Quebec, Quebec
Dr D Leung, Strathroy, Ontario
Dr Alex D Leung, Kamloops, British Columbia
Dr Charles Levesques, Plessisville, Quebec
Dr Gary Lindsay, Brandon, Manitoba
Dr Edmond Kam-Hung Liu, Bassano, Alberta
Dr R Lloyd, Pembroke, Ontario
Dr Eddie Lo, Mississauga, Ontario
Dr David Lowe, Missisauga, Ontario
Dr Paul Lu, Winnipeg, Manitoba
Dr Luton, London, Ontario
Dr Tony Lynch, Calgary, Alberta
Dr C Lytle, Maple Ridge, British Columbia
Dr John MacDonald, Dartmouth, Nova Scotia
Dr G Magee, Thornhill, Ontario
Dr Jamuna Makhija, Vancouver, British Columbia
Dr Paul Malette, Hanmer, Ontario
Dr Susan Malloy, Halifax, Nova Scotia
Dr R Mansour, Ottawa, Ontario
Dr Michel Marchand, St-Damien-de-Buckland, Quebec
Dr Laurent Marcoux, St Denis sur Richeliu, Quebec
Dr Douglas J Mark, Scarborough, Ontario
Dr Dennis Mark, Scarborough, Ontario
Dr Marcel Marsolais, Beloeil, Quebec
Dr Murray McCrossin, Amherst, Nova Scotia
Dr William McMullen, Sudbury, Ontario
Dr Luc Meagher, St-Charles-Borromee, Quebec
Dr Maurice Mercier, Thetford Mines, Quebec
Dr F Mishriki, St-Eustache, Quebec
Dr Rajesh Mohan, Etobicoke, Ontario
Dr Charles Monk, Point Edward, Ontario
Dr Champaklal Morar, Cambridge, Ontario
Dr Pierre Morissette, Paspebiac, Quebec
Dr Daniel Nadeau, Rimouski, Quebec
Dr M Naim, Hull, Quebec
Dr Y Nataraj, Wadena, Saskatchewan
Dr L Newman, Chomedey, Laval, Quebec
Dr Barbara Newton, Mississauga, Ontario
Dr J Ng, Burnaby, British Columbia
Dr Ken Ng, Markham, Ontario
Dr Daniel Noël, Sherbrooke, Quebec
Dr C Nunes-Vaz, Toronto, Ontario
Dr M O’Mahony, Sarnia, Ontario
Dr Daniel Ozimok, Barrie, Ontario
Dr Kenneth Park, St Catherines, Ontario
Dr Michael Partridge, Barrie, Ontario
Dr Dinu Patel, Regina, Saskatchewan
Dr Praful Patel, Winnipeg, Manitoba
Dr Sunil V Patel, Gimli, Manitoba
Dr Marilyn Paterson, Grande Prairie, Alberta
Dr Pierre Payer, Ile-Perrot, Quebec
Dr Laura Penava, Windsor, Ontario
Dr Francois Perreault, St-Anne De Bellevue, Quebec
Dr Jean-Claude Philibert, Shawinigan, Quebec
Dr Peter Phillips, Collingwood, Ontario
Dr Jean-Pierre Picard, Sorel, Quebec
Dr Bogdian Z Pietraszek, Toronto, Ontario
Dr Daniel Poitras, Laval, Quebec
Dr Pierre Prévost, Beloeil, Quebec
Dr Clément Proulx, Cap-St-Ignace, Quebec
Dr Denis Proulx, Beloeil, Quebec
Dr Leo P Quinn, Oshawa, Ontario
Dr Allatif Raghavji, Airdrie, Alberta
Dr Chris Ragonetti, Burlington, Ontario
Dr MM Rahman, Winnipeg, Manitoba
Dr Rajendranath Ramgoolam, Winnipeg, Manitoba
Dr Yves Raymond, Rivière-du-Loup, Quebec
Dr Jameel K Razack, Scarborough, Ontario
Dr Irma Reich, Winnipeg, Manitoba
Dr Alain Renzo, Chandler, Quebec
Dr Richard L Richards, North York, Ontario
Dr Andre Rivard, St-Jean Rchelleu, Quebec
Dr P S Robbins, Lockeport, Nova Scotia
Dr Roger Robin, Les Saules, Quebec
Dr David S Rosenberg, Scarborough, Ontario
Dr Gillian Rosenthal, Victora, British Columbia
Dr André Roy, Quebec, Quebec
Dr Bruno Roy, Beauceville-Est, Quebec
Dr Maurice Roy, Grand Falls, New Brunswick
Dr Surendra Ruparelia, Oshawa, Ontario
Dr Alan D Russell, Leamington, Ontario
Dr L Sadinski, Etobicoke, Ontario
Dr WR Salmaniw, Victoria, British Columbia
Dr Mike Sangani, Greenfield Park, Quebec
Dr William B Sara, Bellevue, Alberta
Dr S Scala, Toronto, Ontario
Dr Schacter, London, Ontario
Dr Earl J Schwartz, Toronto, Ontario
Dr R Seeley, Grimsby, Ontario
Dr M Shack, North York, Ontario
Dr Stephen Shore, Langley, British Columbia
Dr Stewart James Silagy, Winnipeg, Manitoba
Dr Coeliflor D Silva, Toronto, Ontario
Dr G Skory, Richmond Hill, Ontario
Dr M Slobodzian, Saskatoon, Saskatchewan
Dr EJ Smith, Vanier, Ontario
Dr Wayne Smith, West Vancouver, British Columbia
Dr LF Smith, Vita, Manitoba
Dr Smosarski, Caledonia, Ontario
Dr Harvey Solomon, Bridgetown, Nova Scotia
Dr Mohunlall Soowamber, Montreal, Quebec
Dr G Paul Stephan, Scarborough, Ontario
Dr Darryl S Stewart, Beaumont, Alberta
Dr James Stewart, Moncton, New Brunswick
Dr John Strang, Burlington, Ontario
Dr Hélène Strobach, Montreal, Quebec
Dr Didacus Su, Ottawa, Ontario
Dr Ziad Subai, Anjou, Quebec
Dr Salim Sunderji, Lambeth, Ontario
Dr Edison Susman, Scarborough, Ontario
Dr J Sussman, Downsview, Ontario
Dr Peter Sy, Mississauga, Ontario
Dr Joseph Sylvain, Grand-Mere, Quebec
Dr Denys F Symons, Mississauga, Ontario
Dr Grace Szczerbowski, London, Ontario
Dr John Taliano, St Catherines, Ontario
Dr Thuang K Tan, Toronto, Ontario
Dr Rose Tannous, Niagara Falls, Ontario
Dr Robert Tautkus, Brampton, Ontario
Dr Alain Thibert, Salaberry-de-Valleyfield, Quebec
Dr Caroll Thivierge, Quebec, Quebec
Dr EC Tillotson, Scarborough, Ontario
Dr Martin Toussaint, L’Isletville, Quebec
Dr Hugues Tremblay, Mantane, Quebec
Dr Line Trepanier, Thetford Mines, Quebec
Dr Pierre Trudeau, Beauceville, Quebec
Dr Khue Tu, Longueil, Quebec
Dr Frances Tung, Trenton, Ontario
Dr Peter Twiss, Edmonton, Alberta
Dr Cornelius Van Zyl, Canora, Saskatchewan
Dr AR Vance, Sudbury, Ontario
Dr Astghik Vartanian, Montreal, Quebec
Dr Johan Viljoen, Medicine Hat, Alberta
Dr Curt Vos, Sherwood Park, Alberta
Dr Dimitrios Voutsis, Regina, Saskatchewan
Dr Marvin Waxman, Toronto, Ontario
Dr Charles Webb, Vancouver, British Columbia
Dr Weicker, Bolton, Ontario
Dr Diana M White, Victoria, British Columbia
Dr Zeph Wiesenthal, Gimli, Manitoba
Dr CL Williams, Victoria, British Columbia
Dr Paul C Woo, Richmond Hill, Ontario
Dr S Wu, North York, Ontario
Dr Molino Yam, Vancouver, British Columbia
Dr John Yang, Saint-John, New Brunswick
Dr PC Yau, Toronto, Ontario
Dr Gordon Yee, Lasalle, Quebec
Dr Jean-Pierre Yelle, Salaberry-Valleyfield, Quebec
Dr Peter Yong, Vancouver, British Columbia
Dr Ricardo Zarruk, Pierrefonds, Quebec
Dr F Zitnansky, Toronto, Ontario
Dr John Zubis, Calgary, Alberta
REFERENCES
- 1.Hennekens C. Lessons from hypertension trials. Am J Med. 1998;104:50S–3S. doi: 10.1016/s0002-9343(98)00188-0. [DOI] [PubMed] [Google Scholar]
- 2.Dawber T. The Framingham study: The epidemiology of atherosclerotic disease. Cambridge: Harvard University Press; 1980. [Google Scholar]
- 3.Collins R, Peto R, MacMohan S, et al. Blood pressure, stroke and coronary heart disease. Part 2. Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 4.Joffres M, Ghadirian P, Fodor G, et al. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10:1097–102. doi: 10.1016/s0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 5.Waeber B, Burnier M, Brunner H. How to improve adherence with prescribed treatment in hypertensive patients? J Cardiovasc Pharmacol. 2000;35(Suppl 3):S23–6. doi: 10.1097/00005344-200035063-00006. [DOI] [PubMed] [Google Scholar]
- 6.Flack J, Novikov S, Ferrario C, et al. Benefits of adherence to anti-hypertensive drug therapy. Eur Heart J. 1996;17:16–20. doi: 10.1093/eurheartj/17.suppl_a.16. [DOI] [PubMed] [Google Scholar]
- 7.Feldman R, Bacher M, Campbell N, et al. Adherence to pharmacologic management of hypertension. Can J Public Health. 1998;89:116–8. doi: 10.1007/BF03404494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes R, Montague P, Oliver T, et al. Interventions for helping patients to follow prescriptions for medications (Cochrane Review) The Cochrane Library; Issue 4 2000 [DOI] [PubMed] [Google Scholar]
- 9.Hennekens C, Buring J. Validity versus generalizability in clinical trial design and conduct. J Card Fail. 1998;4:239–41. doi: 10.1016/s1071-9164(98)80014-6. [DOI] [PubMed] [Google Scholar]
- 10.Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in Mild-to-Moderate Hypertension. Am J Hypertens. 1999;12:797–805. doi: 10.1016/s0895-7061(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 11.Kassler-Taub K, Littlejohn T, Elliott W, et al. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan, in mild-to-moderate hypertension. Am J Hypertens. 1998;11:445–53. doi: 10.1016/s0895-7061(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 12.Mimran A, Ruilope L, Kerwin L, et al. A randomized, double blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose of enalapril for the treatment of mild-to-moderate hypertension. J Hum Hypertens. 1998;12:203–8. doi: 10.1038/sj.jhh.1000591. [DOI] [PubMed] [Google Scholar]
- 13.Haynes R, Sackett D, Gibson E, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;i:1265–8. doi: 10.1016/s0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 14.Logan A, Milne B, Achber C, et al. Work-site treatment of hypertension by specially trained nurses. Lancet. 1979;ii:1175–8. doi: 10.1016/s0140-6736(79)92397-3. [DOI] [PubMed] [Google Scholar]
- 15.Chaput A. Persistency with angiotensin receptor blockers (ARB) versus other antihypertensives (HT) using the Saskatchewan database. Can J Cardiol. 2000;16(Suppl F):194F. [Google Scholar]
- 16.Bloom B. Continuation of initial antihypertensive therapy medication after 1 year of therapy. Clin Ther. 1998;20:671–81. doi: 10.1016/s0149-2918(98)80130-6. [DOI] [PubMed] [Google Scholar]