Abstract
Ischemic preconditioning (PC) preserves myocardial high-energy phosphate metabolites and intracellular pH during subsequent sustained ischemia. Generation of reactive oxygen species may be required to mediate PC, as seen in vitro. In the present study, the effects of inhibiting reactive oxygen species generation during a PC protocol in vivo using an open-chest porcine model were examined. Myocyte ultrastructural changes assessed by electron microscopy were correlated with phosphorus nuclear magnetic resonance spectroscopy data. Open-chest pigs underwent 60 min of left anterior descending coronary artery occlusion. PC was elicited by a single episode of 5 min occlusion and 5 min reperfusion. The cell-diffusible hydroxyl radical and superoxide radical scavenger, N-2-mercapto-propionyl glycine (MPG, 20 mg/kg), or placebo saline were infused for 40 min, starting 30 min before PC (PC plus MPG group, n=10; and PC group, n=9). After PC, ATP and intracellular pH were significantly preserved through 25 min of ischemia (control versus PC, 46±3% versus 55±5% of baseline [P<0.05]; and control versus PC, 6.18±0.08 versus 6.42±0.03 [P<0.05], respectively). Phosphocreatine was significantly preserved through 20 min of ischemia (control versus PC, 0±0% versus 7±2% of baseline [P<0.05]). The preservation of high-energy phosphate metabolites and intracellular pH was abolished by inhibiting the generation of reactive oxygen species with MPG. Preservation of high-energy phosphate metabolites with PC was associated with reduced ultrastructural damage, as seen by electron microscopy, including less myocyte swelling, myofibrillar disruption and nuclear chromatin margination. The present study demonstrates the importance of reactive oxygen species generation in mediating PC preservation of myocyte ultrastructure and high-energy phosphate metabolites during prolonged ischemia in vivo.
Keywords: Electron microscopy, Ischemic preconditioning, Myocardial metabolism, Phosphorus nuclear magnetic resonance spectroscopy, Pig, Reactive oxygen species
Ischemic preconditioning (PC), brief episodes of ischemia and reperfusion, limits infarct size following sustained ischemia (1–4). Proposed mechanisms of PC protection include activation of adenosine A1 receptors (5), alpha-adrenergic receptors (6) and protein kinase C (7), opening of mitochondrial ATP-sensitive potassium channels (8), p38 mitogen-activated protein kinase (9), and generation of reactive oxygen species (10). Several studies support the role of reactive oxygen species generation during PC in mediating PC cardioprotection during subsequent ischemia. Murry et al (11) found that the infarct size-limiting effect of PC was partially attenuated in dogs treated with superoxide dismutase (SOD) plus catalase. Tanaka et al (12) found that N-2-mercaptopropionyl glycine (MPG) or SOD attenuated the PC infarct size-limiting effect in rabbits. Ambrosio et al (13) and Tritto et al (14) showed that pretreatment with exogenous oxygen free radicals generated by the xanthine and xanthine oxidase system improved functional recovery and limited infarct size in isolated rabbit hearts. Recently, Pain et al (15) found that mitochondrial ATP-sensitive potassium channels may function as a trigger that sets the myocardium into a preconditioned state by the generation of oxygen-derived free radicals. The present study was undertaken to evaluate whether MPG, a cell-diffusible hydroxyl radical and superoxide radical scavenger, could attenuate the PC energy-sparing effect in vivo in a porcine heart. Ultrastructural changes examined by electron microscopy were correlated with high-energy phosphate metabolites and intracellular pH monitored with 31P-nuclear magnetic resonance (NMR) spectroscopy during PC and subsequent ischemia.
MATERIALS AND METHODS
Animal preparation
These experiments conformed to the guiding principles of the American Physiological Society regarding the use of laboratory animals.
These methods were previously described in detail (16). Briefly, 61 pigs (12 kg to 17 kg) were sedated (ketamine 15 mg/kg), anesthetized (sodium pentobarbital 15 mg/kg) and mechanically ventilated (18 breaths/min; tidal volume 13 to 17 mL/kg; gas: oxygen 24%, nitrogen 75%, carbon dioxide 1%). Anesthesia was maintained with fluothane (0.5% to 1.5%). A 7F catheter-tipped micromanometer (Catheter Tip Pressure Sensor, Nihon Kohden Inc, Tokyo) was advanced via the left carotid artery into the left ventricle (LV) for measurement of LV pressure and to obtain LV pressure-gated NMR spectra. Aortic pressure and heart rate were recorded via the right femoral artery on a polygraph system (Nihon Kohden Inc, Tokyo). The heart was exposed by midline thoracotomy and suspended in a pericardial cradle. A 3 mm segment of the middle portion of the left anterior descending (LAD) coronary artery was dissected free from the surrounding tissue to position a preformed air occluder. A 19 mm surface coil was positioned in the centre of the area at risk for phosphorus-31 magnetic resonance spectroscopy (31P-MRS) measurements.
Experimental protocol
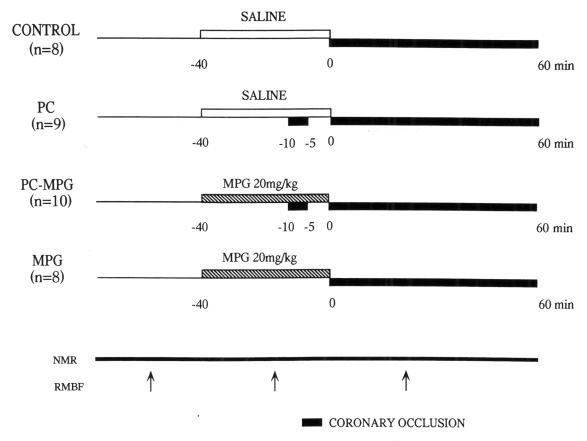
The experimental protocol is shown in Figure 1. Group 1 (control group, n=8) was subjected to 60 min LAD occlusion. Group 2 (PC group, n=9) was preconditioned with a single 5 min occlusion and 5 min reperfusion before 60 min LAD occlusion. In these groups, saline (30 mL) was infused through the left external jugular vein over 40 min until the initiation of sustained ischemia. In groups 3 (PC plus MPG group, n=10) and 4 (MPG group, n=8), animals were subjected to the same protocol as in groups 2 and 1, respectively, but MPG (20 mg/kg, Santen Pharmaceutical Co, Japan) was infused instead of saline. MPG is a highly diffusible low molecular weight compound that scavenges superoxide and hydroxyl radicals produced both intra- and extracellularly. This MPG dose has been shown to attenuate the infarct size-limiting effect of PC in rabbit hearts (12). Other studies (17–19) found that this dose afforded protection in in vivo models of oxygen free radical-mediated damage. Bolli et al (20) demonstrated that removal of hydroxyl radicals is the major mechanism of the beneficial effects of MPG at the dose of an intracoronary infusion (8 mg/kg/h) in open-chest dogs.
Figure 1).
Experimental protocol: All pigs underwent 60 min occlusion of the left anterior descending coronary artery. Ischemic preconditioning (PC) was elicited with 5 min of occlusion followed by 5 min of reperfusion. Saline (control) or N-2-mercaptopropionyl glycine (MPG, 20 mg/kg) was infused over 40 min until the initiation of sustained ischemia. Phosphocreatine, ATP and intracellular pH were serially measured using 31-phosphorus nuclear magnetic resonance (NMR) spectroscopy. The arrows indicate the administration of coloured microspheres for determining regional myocardial blood flow (RMBF)
31P-NMR spectroscopy
The methods of 31P-NMR spectroscopy have been previously described in detail (16). Briefly, 31P-NMR spectra were obtained using a BEM-250/80 in vivo spectrometer (Otsuka Electronics [USA] Inc, USA) with a 1.9-T, 31 cm bore superconducting magnet. A two-turn surface coil inner diameter of 19 mm, tuned to 32.336 MHz, was used. Respiration- and arterial pressure-gated spectra were obtained at end-inspiration and peak systole, accumulating 90 degrees free induction decays over 5 min. Tissue levels of ATP, phosphocreatine (Pcr) and inorganic phosphate (Pi) were estimated by integrating the areas under the individual peaks using a computer program (MEAS 1, Graphtec Co, Tokyo) and a digitizer. Intracellular pH was calculated from the chemical shift of the major Pi peak using the Flaherty equation (21). The levels of Pcr and ATP are known to change during the cardiac cycle (22,23). In the present study, blood pressure gating eliminated such cardiac cyclic variation and respiration gating maintained the position of the heart constant in the magnetic field, which enhanced the accuracy of the metabolic information (24,25).
Measurement of regional myocardial blood flow
Regional myocardial blood flow measurements were made with 15 μm coloured polystyrene microspheres (E-Z Trac, USA) as previously described (16). Approximately 107 microspheres were injected into the left atrium at baseline, at 20 min after onset of MPG or saline, and 20 min after LAD occlusion. The extraction of microspheres from the blood and tissue samples was performed as described by Hale et al (26).
where Cm is the microsphere count per gram tissue, and Qr and Cr are, respectively, the withdrawal rate of and microsphere count in a reference blood sample.
Analysis of ischemic area at risk
After the pigs were euthanized, their hearts were removed and the left and right coronary arteries were cannulated as previously described (16). Keeping the LAD occluder inflated, 1% monastral blue dye was injected into the coronary arteries to visualize the ischemic area at risk. Slices of the LV were fixed with 10% formalin, weighed, and the apical surfaces were photographed. Percentage areas at risk were calculated and multiplied by the slice weight. Summed weights of areas at risk were divided by the LV weight to yield the %LV at risk.
Ultrastructural study
MPG effects on the morphological protection afforded by PC were examined using electron microscopy. Additional pigs were sacrificed at 25 min into sustained ischemia for ultrastructural analysis (n=3 per group). Hearts were excised and three trans-mural LV slices were taken from the centre of the ischemic areas and divided into the endocardial and epicardial halves. Tissue from endocardial halves were sliced (1.0 mm thickness) in the plane parallel to the atrioventricular groove and large sections of 2.0×4.0×1.0 mm were cut. In the same way, nonischemic tissue sections were prepared to serve as controls. Sections were fixed in cold 2% glutaraldehyde for 3 h, post-fixed in osmium tetroxide for 8 h, dehydrated in a graded series of ethanol and propylene oxide, embedded in the spurr medium and cut into 1.0 μM thick sections (Ultracut N, Reicher Jung Co, Austria). After toluidine blue staining, approximately 0.08 μM sections were mounted on plain copper grids, stained with uranyl acetate and lead citrate, and examined with an H-600 transmission electron microscope (Hitachi, Japan). A scoring system to semi-quantitatively evaluate morphological damage was used, adapted from Fujiwara et al (27). Five random pictures of each specimen were scored and averaged with ultrastructural damage scored in three grades (1 – mild; 2 – moderate; 3 – severe) in terms of swelling of sarcoplasm, myofibrillar disruption, margination of nuclear chromatin and mitochondrial damage (swelling and disruption).
Statistical analysis
All results were expressed as mean ± SEM. Differences between groups in hemodynamics, regional myocardial blood flow, high-energy phosphate metabolites and intracellular pH were compared by a two-factor ANOVA with repeated measures. When F values indicated significant differences between the groups, differences were analyzed by multiple comparison at each time point with Tukey’s method using statistics analysis software (SAS Institute Japan). P<0.05 was considered statistically significant.
RESULTS
Ultrastructural findings
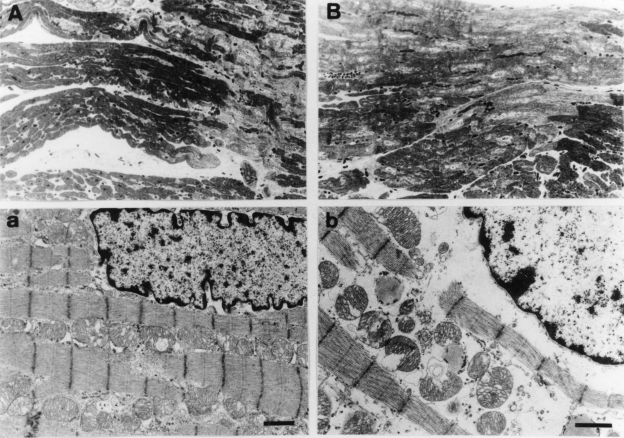
Morphological studies were performed at 25 min of sustained ischemia because this was the last time point where high-energy phosphate metabolite preservation was significantly different between the PC control and MPG groups. The majority of the myocytes from the saline-treated PC group were stained normally with toluidine blue with well-defined rows of mitochondria between myofibrils. A few lightly stained swollen myocytes were seen but they comprised only a minor population scattered among the normal myocytes (Figure 2A). Electron microscopy showed that the majority of myofibrils were not disrupted and the cytoplasm was not edematous. Their nuclei showed only mild margination of chromatin materials, and some mitochondria were slightly swollen and partially disrupted (Figure 2a). A scoring system adapted from the report by Fujiwara et al (27) was used to semiquantitatively evaluate morphological damage. PC reduced the ultrastructural damage score significantly (PC score 1.4±0.1 versus non-PC score 2.5±0.1, P<0.05).
Figure 2).
Light and transmission electron micrographs of the subendocardial tissue obtained from pig myocardium after 25 min of ischemia in preconditioning (PC) (A and a, respectively) and PC plus N-2-mercaptopropionyl glycine (MPG) (B and b) hearts. A The majority of myocytes are stained normally with toluidine blue with well-defined rows of mitochondria between myofibrils. As shown in the right lower side of the panel and scattered among the normal-appearing myocytes, there is a minority of lightly stained swollen myocytes. (Original magnification ×200) a One of the majority of normal-appearing myocytes from a PC heart is shown. The myofibrils are not disrupted and the cytoplasm is not edematous. The nucleus appears nearly normal, except for mild margination of chromatin material and slight swelling and disruption of some mitochondria. Bar indicates 1 μm. (Original magnification ×5000) B Most of the myocytes from a PC plus MPG-treated heart show reduced nonuniform staining of cytoplasmic organelles and clearing of chromatin material in the nuclei. Cellular vacuolation, particularly in the perinuclear space, is evident. The interstitial space is diminished due to myocyte swelling. (Original magnification ×200) b One of the majority of abnormal-appearing myocytes from a PC plus MPG-treated heart is shown. The cytoplasm is markedly edematous. Myofibrils are separated and disrupted, and margination of nuclear chromatin is evident. Mitochondria are severely swollen and cristae are disrupted. Bar indicates 1 μm. (Original magnification ×6000)
MPG abolished the PC preservation of myocyte ultra-structure. The majority of myocytes from MPG-treated hearts showed reduced and nonuniform staining of cytoplasmic organelles and clearing of chromatin materials in their nuclei. Cellular vacuolation, particularly in the perinuclear space, was evident and the interstitial space was diminished due to myocyte swelling (Figure 2B). Electron microscopy showed that the cytoplasm of these cells was markedly edematous. Myofibrils were separated and disrupted, and margination of nuclear chromatin was evident. Mitochondria were severely swollen and cristae were disrupted (Figure 2b). Thus, PC protection was abolished by MPG (MPG plus PC group ultrastructural damage score 2.4±0.1 versus PC group score 1.4±0.1, P<0.05). Ultrastructural damage was similar between the saline- and MPG-treated non-PC groups (2.5±0.1 versus 2.6±0.1, not significant), indicating that MPG was not toxic.
High-energy phosphate metabolites
During 40 min of baseline measurements, ATP, Pcr, Pi and intracellular pH did not change significantly (Figures 3 to 5). During sustained ischemia, changes in ATP, Pcr and Pi were expressed as a percentage relative to baseline values measured 5 min before sustained ischemia. Neither PC nor MPG treatment altered high-energy phosphate metabolites before sustained ischemia, when compared with the control group.
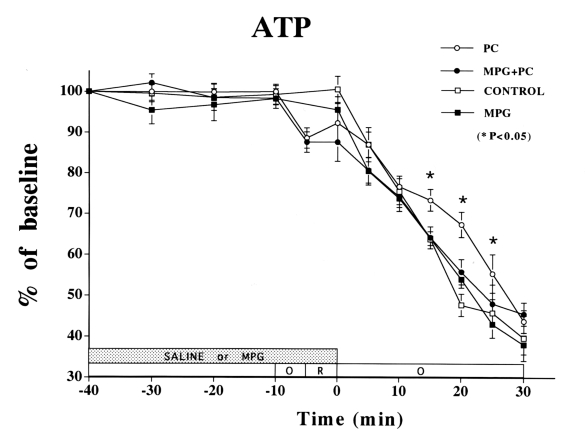
Figure 3).
Changes in ATP in the ischemic myocardium over time. ATP was significantly preserved in the preconditioning (PC) group until 25 min of sustained ischemia (P<0.05 versus control group). ATP preservation was attenuated in the PC plus N-2-mercaptopropionyl glycine (MPG) group (not signficant versus control or MPG group). No difference was observed at any time between the control and MPG groups. There were no significant differences in ATP among the four treatment groups after 30 min of sustained ischemia
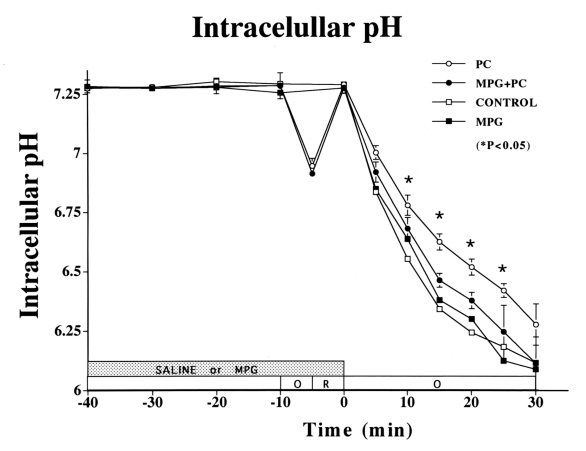
Figure 5).
Changes in intracellular pH in the ischemic myocardium over time. Intracellular pH was significantly higher in the preconditioning (PC) group than in the control group until 25 min of sustained ischemia (P<0.05 versus control group). Intracellular pH preservation was blocked by N-2-mercaptopropionyl glycine (MPG) (not significant for PC plus MPG versus control or MPG group)
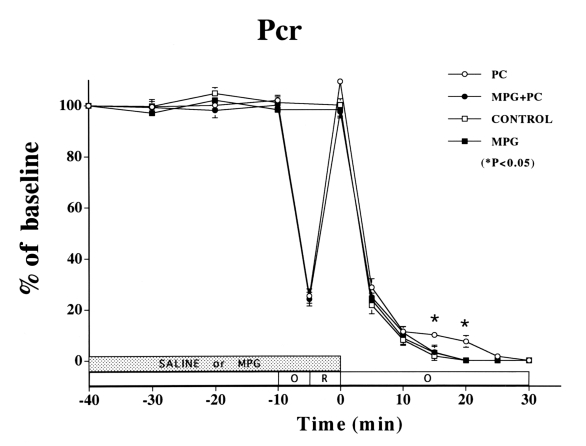
Sustained ischemia caused a fall in ATP (Figure 3). MPG did not affect the fall in ATP in the non-PC group compared with the control group. However, MPG did attenuate the ATP preservation of PC, which was significant through 25 min of sustained ischemia (control versus PC: 46±3% versus 55±5% of baseline, P<0.05). The fall in Pcr was similar in the non-PC control and MPG groups (Figure 4). However, MPG attenuated the PC preservation of Pcr (control versus PC: 0±0% versus 7±2% of baseline, P<0.05) through 20 min of ischemia.
Figure 4).
Changes in phosphocreatine (Pcr) in the ischemic myocardium over time. In the preconditioning (PC) group, a Pcr overshoot was observed after PC, and Pcr was still detectable until 20 min of sustained ischemia (P<0.05 versus control group). No difference was observed at any time between the control and the N-2-mercaptopropionyl glycine (MPG) group. There were no significant differences in Pcr among the four treatment groups after 20 min of sustained ischemia
Baseline intracellular pH did not differ between the four groups (intracellular pH 7.28) (Figure 5). While MPG had no effect on the fall in intracellular pH during sustained ischemia in the non-PC group, MPG attenuated the PC preservation of intracellular pH (control versus PC: 6.18±0.08 versus 6.42±0.03, P<0.05, respectively) through 25 min of ischemia.
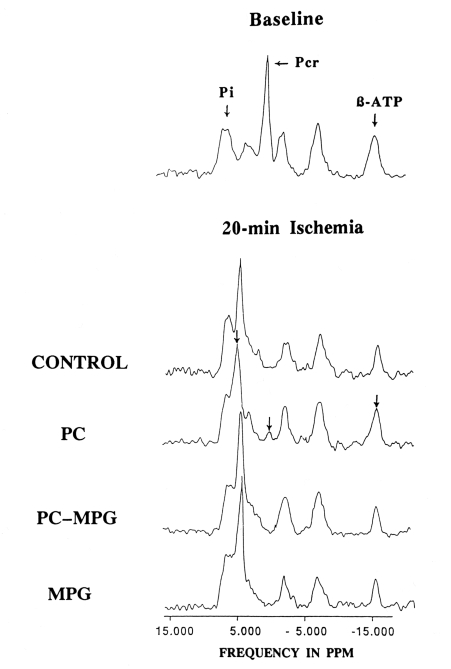
There were no significant differences in Pi among the four groups at baseline or at any point during sustained ischemia. Pi rapidly increased more than three-fold over baseline values during 60 min of sustained ischemia in all four groups. Representative 31P-NMR spectra at 20 min of sustained ischemia from each group are shown (Figure 6).
Figure 6).
Representative 31P-magnetic resonance spectra obtained from the four groups after 15 min of sustained ischemia (compared with the baseline study). Each spectrum is an accumulation of 90 free induction decays taken during peak systolic and end-inspiratory phases over 5 min. In the preconditioning (PC) group, phosphocreatine (Pcr) and ATP were preserved, and the chemical shift of the major inorganic phosphate (Pi) peak was slight, as shown by arrowheads. Note the loss of this beneficial effect of PC in the PC plus N-2-mercaptopropionyl glycine (MPG) group
Hemodynamics, area at risk and regional myocardial blood flow
MPG had no significant effects on hemodynamics or regional myocardial blood flow at baseline compared with the control group. There were no significant differences in systolic blood pressure among the four groups at baseline and throughout ischemia (baseline systolic blood pressures for saline, PC, MPG plus PC, and MPG were 88±4 mmHg, 93±3 mmHg, 92±4 mmHg and 87±6 mmHg, respectively), for diastolic blood pressures (52±2 mmHg, 59±3 mmHg, 57±3 mmHg and 54±6 mmHg, respectively) or LV end-diastolic pressures (5±2 mmHg, 3±1 mmHg, 3±2 mmHg and 4±1 mmHg, respectively). There were no significant differences in the area at risk (as percentage of the LV) among the four groups (22.3±2.3%, 20.5±2.1%, 19.8±1.8% and 21.1±1.6%, respectively).
There were no significant differences in baseline regional myocardial blood flow among the ischemic zone (1.22±0.06, 1.18±0.07, 1.22±0.04 and 1.16±0.08 mL/g/min, respectively) and nonischemic zone (1.11±0.06, 1.10±0.08, 1.08±0.05 and 1.06±0.04 mL/g/min, respectively) or between the subendocardium and subepicardium. MPG did not affect regional myocardial blood flow. Ischemia resulted in negligible regional myocardial blood flow in the ischemic zone (0.01±0.00, 0.02±0.01, 0.00±0.00 and 0.01±0.01 mL/g/min, respectively) and preserved regional myocardial blood flow in the nonischemic zone (1.01±0.06, 0.99±0.07, 0.96±0.06 and 0.98±0.05 mL/g/min, respectively).
Mortality and animal exclusions
Sixty-one pigs were entered into the protocol. Three were excluded due to pericarditis and one because of LAD trauma. Two pigs (one each from the PC and MPG groups) were excluded because the surface coil was out of the area at risk at the end of the experiment. Of the remaining 55 pigs, five from the control group, five from the PC group, six from the PC plus MPG group and four from the MPG group were excluded because they developed ventricular fibrillation during 60 min occlusion (between 25 and 30 min of occlusion in most cases). Data are presented for the 35 pigs in which ventricular fibrillation did not occur and that completed the study successfully.
DISCUSSION
The present study, for the first time in vivo, demonstrates the importance of reactive oxygen species generation in mediating PC preservation of both myocyte ultrastructure and high-energy phosphate metabolites during prolonged ischemia.
PC preserves myocardial high-energy phosphate metabolites and intracellular pH during subsequent sustained ischemia (16,28). Reactive oxygen species generation may be required to mediate PC cardioprotection, as demonstrated in vitro. In the present study, we examined the effects of inhibiting reactive oxygen species generation during a PC protocol in vivo using an open-chest porcine model. Ultrastructural damage was reduced as demonstrated by electron microscopy, and included less myocyte swelling, myofibrillar disruption and nuclear chromatin margination. This reduction of myocyte damage was associated with ATP, Pcr and intracellular pH preservation through 20 to 25 min of ischemia. This myocardial preservation was abolished by inhibiting reactive oxygen species generation with MPG.
PC produces a burst of oxygen free radicals, which appear to play an important role in the cardioprotective effect of PC (13–15). Ovize et al (29) reported that complete reperfusion is mandatory for PC in the canine heart. This indicates that factors generated during reperfusion of PC, such as oxygen free radicals, play a role in mediating cardioprotection. Murry et al (11) found that the PC infarct size-limiting effect was partially attenuated in dogs treated with SOD plus catalase.
Tanaka et al (12) found that MPG or SOD attenuated the PC infarct size-limiting effect in rabbits. Ambrosio et al (13) and Tritto et al (14) showed that pretreatment with exogenous oxygen free radicals generated by the xanthine and xanthine oxidase system improved functional recovery and limited infarct size in isolated rabbit hearts. The present study supports the reactive oxygen species hypothesis that oxygen free radicals play an important role in mediating the cardioprotective effect of PC. Several potential mechanisms could explain the role of reactive oxygen species in mediating PC. Mitochondrial ATP synthetase produces most of the ATP during normoxia in species with a slow heart rate such as pigs (and humans) but, during ischemia, begins to function as an ATP hydrolytic enzyme resulting in ATP depletion (30). Oxygen free radicals produced by PC may inhibit this enzyme, resulting in better ATP preservation during sustained ischemia.
Cohen and Downey (7) proposed that translocation of protein kinase C from the cytosol into the cell membranes is required to activate PC. Reactive oxygen species have been found to initiate hydrolysis of phospholipase C (31) and stimulate endothelial cell phospholipase D (31), both of which are involved in protein kinase C activation.
Finally, Tokube et al (33) demonstrated that reactive oxygen species open ATP-sensitive K+ channels in guinea pig ventricular myocytes. Pain et al (15) also found that reactive oxygen species generation may be related to the opening of mitochondrial ATP-sensitive K+ channels. Conversely, it was reported that opening of mitochondrial ATP-sensitive K+ channels increases oxygen free radical production in isolated rat ventricular myocytes (34). The opening of mitochondrial ATP-sensitive K+ channels has been shown to play an important role in PC cardioprotection.
Study limitations
In vivo 31P-MRS analyses require obtaining spectra from a localized myocardial region with continuous changes in the filling factor (amount of myocardial mass within the region of the surface coil) and potential contamination from the LV cavity. In the present study, signal contamination from the nonischemic myocardium was avoided by reconfirming the position of the surface coil at the end of the experiment. During ischemia, the filling factor depends on the degree of bulging. As we reported previously, the degree of bulging during sustained ischemia is comparable between control and preconditioned hearts (16).
In the present study, we did not directly measure oxygen free radical release. However, Bolli et al (20) directly measured free radical release during reperfusion after a brief period of ischemia (myocardial stunning model) in vivo. They demonstrated a marked increase in radical adducts of alpha-phenyl N-tert-butyl nitrone by electron paramagnetic resonance that was completely blocked by pretreatment with MPG at the current dose (20 mg/kg).
Although we used a PC protocol with a single episode of 5 min occlusion and 5 min reperfusion in the present study, this protocol has shown similar protective features on energy metabolism to PC protocols with multiple occlusion-reperfusion episodes (28). Morphological protection by PC does not seem to decay in parallel with metabolic protection. According to Murry et al (35), at 40 min of sustained ischemia, ultrastructural preservation by PC was still evident despite the fact that metabolic protection had already dissipated. Finally, in the present study, we did not try other antioxidants. However, in our previous study, we demonstrated that SOD and MPG blocked the infarct size-limiting effect of PC in rabbits (12).
CONCLUSIONS
In the present study, we examined the effects of inhibiting reactive oxygen species generation during a PC protocol in vivo using an open-chest porcine model. High-energy phosphate metabolite preservation with PC was associated with reduced ultrastructural damage as demonstrated by electron microscopy, and included less myocyte swelling, myofibrillar disruption and nuclear chromatin margination. PC-induced preservation of myocyte ultrastructural damage and high-energy phosphate metabolites during ischemia were abolished by inhibiting reactive oxygen species generation. This study, for the first time in vivo, demonstrates the importance of reactive oxygen species generation in mediating PC preservation of myocyte ultrastructure and high-energy phosphate metabolites during prolonged ischemia.
Acknowledgments
This study was supported in part by Grant-in-Aid for Scientific Research (C) 12670706, 14571806 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We are grateful to Dr Makoto Ishikawa and Dr Takayuki Sogabe for their expert technical advice on 31P-NMR spectroscopy and to Dr Toyoki Mori for his encouragement and advice. We thank M Yamashita for her secretarial work.
REFERENCES
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990;66:1133–42. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- 3.Downey JM, VanWinkle DM, Pollard JD. Preconditioning in rabbit hearts. FASEB J. 1990;4:852. [Google Scholar]
- 4.Yellon DM, Alkhulaifi AM, Browne EE, Pugsley WB. Ischemic preconditioning limits infarct size in the rat heart. Cardiovasc Res. 1992;26:983–7. doi: 10.1093/cvr/26.10.983. [DOI] [PubMed] [Google Scholar]
- 5.Thornton JD, Liu GS, Olsson RA, Downey JMJ. Intravenous pretreatment with A1- selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–65. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee A, Locke-Winter C, Rogers KB, et al. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an α1-adrenergic mechanism. Circ Res. 1993;73:656–70. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MV, Downey JM. Ischemic preconditioning: Can the protection be bottled? Lancet. 1993;342:6. doi: 10.1016/0140-6736(93)91878-p. [DOI] [PubMed] [Google Scholar]
- 8.Garlid KD, Paucek P, Yarov-Yarovy V, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP sensitive K+ channels: Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–82. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 9.Weinbrenner C, Liu G-S, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383–91. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, O’Rourke B. Opening of mitochondrial K(ATP) channels triggers cardioprotection: Are reactive oxygen species involved? Circ Res. 2001;88:750–2. doi: 10.1161/hh0801.090537. [DOI] [PubMed] [Google Scholar]
- 11.Murry CE, Richard VJ, Jennings RB, Reimer KA. Preconditioning with ischemia: Is the protective effect mediated by free radical-induced myocardial stunning? Circulation. 1988;78(Suppl II):77. (Abst) [Google Scholar]
- 12.Tanaka M, Fujiwara H, Yamasaki K, Sasayama S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischemic preconditioning in rabbits. Cardiovasc Res. 1994;28:980–6. doi: 10.1093/cvr/28.7.980. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosio G, Tritto I, Chiariello M. The role of oxygen free radicals in preconditioning. J Mol Cell Cardiol. 1995;27:1035–9. doi: 10.1016/0022-2828(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 14.Tritto I, D’Andrea D, Eramo N, et al. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–8. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 15.Pain T, Yang X-M, Critz SD, et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–6. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 16.Miyamae M, Fujiwara H, Kida M, et al. Preconditioning improves energy metabolism during reperfusion but does not attenuate myocardial stunning in porcine hearts. Circulation. 1993;88:223–34. doi: 10.1161/01.cir.88.1.223. [DOI] [PubMed] [Google Scholar]
- 17.Mitsos SE, Askew TE, Fantone JC, et al. Protective effects of N-2-mercaptopropionyl glycine against myocardial reperfusion injury after neutrophil depletion in the dog: Evidence for the role of intracellular-derived free radicals. Circulation. 1986;73:1077–86. doi: 10.1161/01.cir.73.5.1077. [DOI] [PubMed] [Google Scholar]
- 18.Mitsos SE, Fantone JC, Gallagher KM, et al. Canine myocardial reperfusion injury: Protection by a free radical scavenger, N-2-mercaptopropionyl glycine. J Cardiovasc Pharmacol. 1986;8:978–88. [PubMed] [Google Scholar]
- 19.Myers ML, Bolli R, Lekich RF, Hartley CJ, Roberts R. N-2-mercaptopropionyl glycine improves recovery of myocardial function after reversible regional ischemia. J Am Coll Cardiol. 1986;8:1161–8. doi: 10.1016/s0735-1097(86)80396-5. [DOI] [PubMed] [Google Scholar]
- 20.Bolli R, Jeroudi MO, Patel BS, et al. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial stunning is a manifestation of reperfusion injury. Circ Res. 1989;65:607–22. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty JT, Weisfeldt ML, Bulkley BH, Gardner TJ, Gott VL, Jacobus WE. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 nuclear magnetic resonance. Circulation. 1982;65:561–71. doi: 10.1161/01.cir.65.3.561. [DOI] [PubMed] [Google Scholar]
- 22.Coffelt JW, Sievers R, Coffelt RJ, et al. The cardiac cycle: Regulation and energy oscillation. Am J Physiol. 1983;245:H354–62. doi: 10.1152/ajpheart.1983.245.2.H354. [DOI] [PubMed] [Google Scholar]
- 23.Fossel ET, Morgan HE, Ingwall J. Measurement of changes in high-energy phosphates in the cardiac cycle by using gated 31P nuclear magnetic resonance. Proc Natl Acad Sci USA. 1980;77:3654–8. doi: 10.1073/pnas.77.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara H, Tanaka M, Ishida M, et al. Ultrastructural and metabolic changes in ischemic porcine hearts using in vivo 31P-NMR spectroscopy. Am J Cardiac Imaging. 1989;3:111–5. [Google Scholar]
- 25.Tanaka M, Fujiwara H, Ishida M, et al. Influence of propranolol on high energy phosphate and tissue acidosis in regional ischemic myocardium of pigs: Assessment with arterial pressure and respiration gated in vivo 31-phosphorus magnetic resonance spectroscopy. Int J Cardiol. 1989;24:165–72. doi: 10.1016/0167-5273(89)90300-8. [DOI] [PubMed] [Google Scholar]
- 26.Hale SL, Alker KJ, Kloner RA. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation. 1988;78:428–34. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara H, Ashraf M, Millard RW, Sato S, Schwartz A. Effect of diltiazem, a calcium channel inhibitor, in retarding cellular damage produced during early myocardial ischemia in pigs: A morphometric and ultrastructural analysis. J Am Coll Cardiol. 1984;3:1427–37. doi: 10.1016/s0735-1097(84)80281-8. [DOI] [PubMed] [Google Scholar]
- 28.Kida M, Fujiwara H, Ishida M, et al. Ischemic preconditioning preserves creatine phophate and intracellular pH. Circulation. 1991;84:2495–503. doi: 10.1161/01.cir.84.6.2495. [DOI] [PubMed] [Google Scholar]
- 29.Ovize M, Przyklenk K, Kloner RA. Partial coronary stenosis is sufficient and complete reperfusion is mandatory for preconditioning the canine heart. Circulation. 1992;71:1165–73. doi: 10.1161/01.res.71.5.1165. [DOI] [PubMed] [Google Scholar]
- 30.Rouslin W, Broge CW, Grupp IL. ATP depletion and mitochondrial functional loss during ischemia in slow and fast heart-rate hearts. Am J Physiol. 1990;259:H1759–66. doi: 10.1152/ajpheart.1990.259.6.H1759. [DOI] [PubMed] [Google Scholar]
- 31.Shasby DM, Yorek M, Shasby SS. Exogenous oxidants initiate hydrolysis of endothelial cell inositol phospholipids. Blood. 1988;72:491–9. [PubMed] [Google Scholar]
- 32.Natarajan V, Taher MM, Roehm B, et al. Activation of endothelial cell phospholipase D by hydrogen peroxide and fatty acid hydroperoxide. J Biol Chem. 1993;268:930–7. [PubMed] [Google Scholar]
- 33.Tokube K, Kiyosue T, Arita M. Openings of cardiac KATP channel by oxygen free radicals produced by xanthine oxidase reaction. Am J Physiol. 1996;271:H478–89. doi: 10.1152/ajpheart.1996.271.2.H478. [DOI] [PubMed] [Google Scholar]
- 34.Forbes R, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–9. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–31. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]