Abstract
OBJECTIVE:
To evaluate the association of three endothelin-1 (ET-1) gene polymorphisms with essential hypertension, as well as with two cardiovascular risk factors: body mass index (BMI) and smoking.
DESIGN:
Three gene polymorphisms and the genotype and allelic distributions were compared between normotensive healthy volunteers and patients with essential hypertension. The genetic association of the three genotypes with BMI and smoking status was calculated.
PATIENTS AND METHODS:
CA/CT dinucleotide repeat polymorphism, G(8002)A polymorphism and −3A/−4A polymorphism (−138 insertion/deletion) were examined in the gene coding for ET-1 (6p21.3) in 398 subjects: 192 normotensives (healthy volunteers) and 206 patients with essential hypertension. Normotension was verified by 24 h ambulatory blood pressure monitoring.
RESULTS:
Significant inner associations were observed between all three polymorphisms, which suggests possible complex interactions inside the gene. The only significant difference in a single gene case control study was in the lengths of allelic variants of CA/CT dinucleotide repeat polymorphism. In hypertensive patients, the alleles of G(8002)A and −3A/−4A ET-1 polymorphisms were found to be significantly associated (G with −3A and A with −4A). None of the ET-1 gene polymorphisms was associated with BMI. A highly significant increase of the −3A allele of the −3A/−4A ET-1 polymorphism was found in hypertensive men who were current smokers or had smoked at least seven cigarettes a week for at least one year at any time in their life compared with hypertensive men who had never smoked (odds ratio 1.54, 95% CI 1.03 to 2.32, P=0.009).
CONCLUSIONS:
Smoking seems to be an independent cardiovascular risk factor genetically codetermined by the ET-1 gene variant.
Keywords: Body mass index, Endothelin-1, Essential hypertension, Gene polymorphism, Smoking
The vascular endothelium is regarded as a vital and dynamic interface, whose functional phenotype is responsive to the biomechanical environment of the vessel wall resulting from the pulsatile blood flow (fluid shear stresses, cyclic strains and hydrostatic pressures) and to humoral substances such as angiotensin II, growth factors and cytokines. Blood flow is a critical modulator of the endothelial phenotype in vivo through integration of biomechanical and biochemical effects (1).
Plasma endothelin-1 (ET-1) is increased in hypertensive patients before the development of end-organ damage (2,3). Although human urotensin II was shown to be at least 10 times more potent as a vasoconstrictor (4), clinical studies are confirming the importance of ET-1 in the regulation of vascular tone in health and in cardiovascular disease. ET-1 seems to be a mediator in many new areas, including inflammation, nociception and arythmogenesis (5). Properties of polymorphic variants of ET-1 gene influencing transcription may be involved in the onset of cardiovascular diseases associated with nonphysiological blood flow characteristics. Surprisingly, few studies have examined the relation between genes coding for endothelin system genes (6–9).
The aim of the present study was to evaluate the association between the allelic and genotype distribution of three ET-1 gene polymorphisms and essential hypertension. The second was to associate alleles and genotypes of the polymorphisms with two risk factors: overweight as assessed by body mass index (BMI) and smoking.
PATIENTS AND METHODS
Subjects
The study groups consisted of 398 subjects, 192 of whom were normotensive (healthy volunteers). Normotension was verified by 24 h ambulatory blood pressure monitoring (90 207, Spacelabs Medical, USA) according to the criterion of pressure overload: in normotensives, fewer than 15% of blood pressure readings are above 140/90 mmHg in the daytime (06:00 to 22:00) and fewer than 15% are above 120/80 mmHg at night (22:00 to 6:00) (10). The patient group was composed of 206 patients with essential hypertension, diagnosed and treated in the 1st Internal Clinic of St Ann’s Faculty Hospital in Brno, Czech Republic.
Overweight and obesity were evaluated according to BMI at the time of clinical examination.
Smoking status was defined as smoking status 1 (smokers who had smoked at least seven cigarettes a week for at least one year at any time in their life) and, alternatively, as smoking status 2 (those who were smokers at the time of clinical examination and DNA blood sampling). Smoking status 1 reflects a possible genetic predisposition (if any) to become a smoker, and smoking status 2 reflects the direct effect of smoking on the vessel wall at the time of the clinical examination and the fact that more hypertensive than normotensive subjects stop smoking according to the recommendation of their doctors.
The study was approved by the Committee for Ethics of Medical Experiment on Human Subjects, Faculty of Medicine, Masaryk University, Brno (no 64/93, 1993). Signed, informed consent was obtained from the subjects.
Genotyping
Genomic DNA was isolated from peripheral leukocytes by a standard technique using proteinase K according to Sambrook et al (11).
Polymerase chain reaction (PCR) was used to amplify CA/CT dinucleotide repeat polymorphism in the Z-DNA region of the gene for ET-1 (6p21.3) with a microsatellite sequence consisting of (CA)17 immediately preceded by (CT)13, according to Pages et al (12). By comparing results of several detection methods (electrophoresis on 4% Metaphore agarose gel [BioWhittaker Molecular Applications ApS, Denmark], polyacrylamide gel electrophoresis, capillary electrophoresis [13] and direct sequencing), the lengths of PCR fragments from 187 to 221 were determined. Ninety subjects, 38 normotensive controls and 52 patients with essential hypertension, were genotyped.
The G(8002)A polymorphism in intron 4 in the ET-1 gene was detected by using this protocol. PCR products (primers 5′-CAA ACC GAT GTC CTC TGT A-3′and 5′-ACC AAA CAC ATT TCC CTA TT-3′) were further analyzed by restriction analysis with Taq I (T↓CGA). On gel electrophoresis with ethidium bromide, three genotypes, GG (150+208 bp), GA (358, 150 and 208 bp) and AA (358 bp), were identified (Figure 1). A total of 380 subjects, 181 normotensive controls and 199 hypertensive patients, were successfully genotyped.
Figure 1).
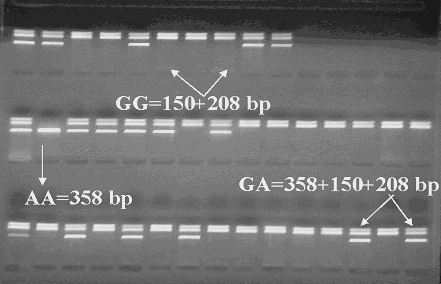
G(8002)A endothelin-1 genotypes
PCR (primers 5′-GCT GCT TTT CTC CCC GTT AA-3′ and 5′-CAA GCC ACA AAC AGC AGA GA-3′) were used to analyze −3A/−4A polymorphisms (−138 insertion/deletion) in the gene coding for ET-1 using the restriction enzyme BsiYI (CCNNNNN↓NNGG). Genotypes were −4A/−4A=195 bp, −4A/−3A=176+195+19 bp and −3A/−3A=176+19 bp (Figure 2). A total of 380 subjects, 181 normotensive controls and 199 hypertensive patients, were successfully genotyped.
Figure 2).
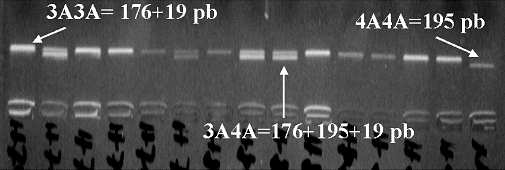
−3A/−4A (−138) endothelin-1 genotypes
Statistical analysis
Differences in allelic frequencies of the polymorphisms were calculated using Fisher’s exact test.
The significance of Hardy-Weinberg disequilibrium for each polymorphism separately was tested from the difference between observed and calculated numbers of genotype carriers using the χ2 test.
The strength of the allelic association was calculated by multiple iterations from observed double genotypes when the strength was presumed to range from 0 (independent coincidence of alleles) to 1 (100% coincidence of alleles).
STATISTICA v3.0 (StatSoft Inc, USA) was used for statistical anlyses.
RESULTS
The 54.4% of hypertensive patients (grade I and grade II [13]) were pharmacologically treated for a sufficient time. Beta-blockers were administrated to 70%, angiotensin-converting enzyme inhibitors to 59%, calcium antagonists to 58%, diuretics to 22% and alpha1-receptor blockers to 3% of patients. A combination of three or more types of antihypertensive agents was given to 37% of treated patients. None of the hypertensive patients was observed to be resistant to treatment.
While a single-peak distribution of the lengths of allelic variants of (CA/CT) dinucleotide repeat polymorphism in the Z-DNA region of the ET-1 gene (about 203 bp) was found in normotensive controls, a double-peak distribution (about 203 and 211 bp, respectively) was observed in hypertensive subjects. There was a significant difference in the lengths of allelic variants of the polymorphism (P=0.03, Mann-Whitney U-test) between normotensive controls and hypertensive subjects (Figure 3).
Figure 3).
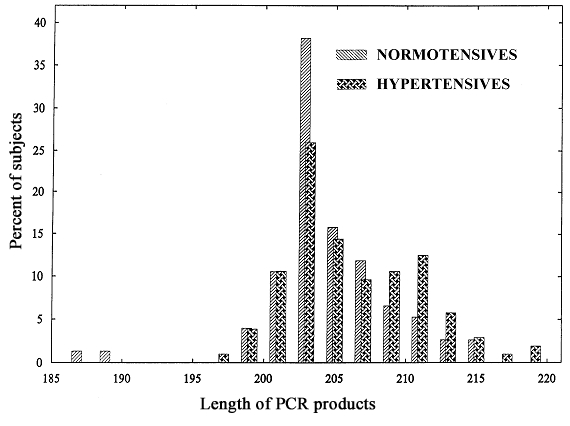
Allelic distribution of CA/CT endothelin-1 gene polymorphisms in normotensive and hypertensive subjects. PCR Polymerase chain reaction
Although no significant association between genotypes or alleles of G(8002)A and −3A/−4A ET-1 polymorphisms was found when they were evaluated separately (Tables 1,2), an allelic association was observed between the alleles of the two polymorphisms (the A allele coincided more often with −3A and the G allele was associated with −4A; P=0.008).
TABLE 1.
G(8002)A endothelin 1 (ET-1) gene (6p21.3) polymorphism and allelic distribution in normotensive and hypertensive groups
| Genotypes and alleles | ||||||
|---|---|---|---|---|---|---|
| GG | GA | AA | G | A | P | |
| Hypertensive group (n=199) | 106 | 82 | 11 | 0.739 | 0.261 | |
| Men (n=112) | 62 | 42 | 8 | 0.742 | 0.258 | |
| Women (n=87) | 44 | 40 | 3 | 0.736 | 0.264 | |
| Control group (n=181) | 109 | 66 | 7 | 0.739 | 0.261 | 0.113 |
| Men (n=81) | 44 | 33 | 4 | 0.747 | 0.253 | 0.497 |
| Women (n=100) | 64 | 33 | 3 | 0.805 | 0.195 | 0.070 |
TABLE 2.
−3A/−4A (−138) endothelin-1 (ET-1) gene (6p21.3) polymorphism and allelic distribution in normotensive and hypertensive groups
| Genotypes and alleles | ||||||
|---|---|---|---|---|---|---|
| 3A3A | 3A4AA | 4A4A | 3A | 4A | P | |
| Hypertensive group (n=199) | 101 | 84 | 14 | 0.696 | 0.304 | |
| Men (n=112) | 54 | 51 | 7 | 0.710 | 0.290 | |
| Women (n=87) | 47 | 33 | 7 | 0.730 | 0.270 | |
| Control group (n=181) | 79 | 85 | 17 | 0.662 | 0.338 | 0.187 |
| Men (n=81) | 36 | 38 | 7 | 0.679 | 0.321 | 0.295 |
| Women (n=100) | 43 | 47 | 10 | 0.665 | 0.335 | 0.106 |
A significant association was observed between the CA/CT dinucleotide repeat polymorphism (expressed as mean allelic length) and G(8002)A ET-1 alleles in all subjects (P=0.0001, Kruskal-Wallis test), independently of sex and hypertension. The −3A/−4A ET-1 polymorphism was correlated with homozygosity in the CA/CT dinucleotide repeat polymorphism (P=0.021).
Table 3 presents cardiovascular risk factor distributions in normotensive and hypertensive groups. Those with hypertension were more often overweight (P=0.001). Normotensive women were leaner than normotensive men (P=0.004) and hypertensive women (P=0.008). None of the evaluated polymorphisms was associated with BMI.
TABLE 3.
Risk factor data in normotensive and hypertensive subjects
| Age, years (mean±SD) | BMI >25 (kg/m2) (%) | Smokers 1 (%) | Smokers 2 (%) | |
|---|---|---|---|---|
| Normotensives (n=192) | 46±3 | 44.9 | 45.8 | 35.7 |
| Men (n=82) | 43±2 | 55.8 | 53.0 | 41.4 |
| Women (n=110) | 48±2 | 35.6* | 39.6 | 30.9 |
| Hypertensives (n=206) | 49±8 | 61.4† | 50.6 | 30.6 |
| Men (n=116) | 47±9 | 65.7 | 59.4 | 34.7 |
| Women (n=90) | 51±7 | 55.4 | 38.4‡ | 23.3 |
P<0.01 versus normotensive men and versus hypertensive women;
P<0.01 versus normotensive group;
P<0.01 versus hypertensive men. Smokers 1 were patients who had smoked at least seven cigarettes a week for at least one year at any time in their life. Smokers 2 were smokers at the time of the clinical examination and DNA blood sampling. BMI Body mass index
Fewer hypertensive women than hypertensive men were smokers 1 (P=0.005). The (−3) A allele of −3A/−4A ET-1 polymorphism was associated with smoking 1. A highly significant increase of −3A allele was detected in hypertensive men who were current or former smokers compared with never-smoking hypertensive men (odds ratio 1.54, 95% CI 1.03 to 2.32, P=0.009), and −3A/−4A was significantly increased in hypertensive, nonsmoking women compared with hypertensive, nonsmoking men (P=0.03) (Table 4). Independently of sex, a marginally significant increase of the −3A allele was found in hypertensive smokers (P=0.048). Independently of hypertension, the frequency of the −3A allele was significantly higher in male smokers than in male nonsmokers (P=0.003).
TABLE 4.
−3A/−4A endothelin-1 (ET-1) genotype and allelic distribution according to smoking status in normotensive and hypertensive groups
| Smokers 1 | Nonsmokers | |||||||
|---|---|---|---|---|---|---|---|---|
| −3A/−4A ET-1 genotype | −3A−3A | −3A−4A | −4A−4A | −3A | −3A−3A | −3A−4A | −4A−4A | −3A |
| Normotensives (n=173) | 34 | 36 | 8 | 0.66 | 43 | 43 | 9 | 0.68 |
| Men (n=78) | 20 | 18 | 2 | 0.73 | 14 | 19 | 5 | 0.62 |
| Women (n=95) | 14 | 18 | 6 | 0.61 | 29 | 24 | 4 | 0.72 |
| Hypertensives (n=196) | 49 | 35 | 4 | 0.76* | 51 | 48 | 9 | 0.69 |
| Men (n=111) | 34 | 24 | 1 | 0.78** | 19 | 27 | 6 | 0.63 |
| Women (n=85) | 15 | 11 | 3 | 0.71 | 32 | 21 | 3 | 0.76† |
P<0.05 versus normotensives smokers 1;
P<0.05 versus male nonsmokers;
P<0.01 versus male nonsmokers. Smokers 1 are those who had smoked at least seven cigarettes a week for at least one year at any time in their life
When the smoking status 1 was requalified to smoking status 2 (patients who were smokers at the time of physical examination and DNA blood sampling), the results changed. A difference in allelic distribution of the −3A/−4A ET-1 polymorphism was observed between normotensive male smokers 2 and nonsmokers (P=0.04). In women, a significant relative risk of the associated AG3A3A genotype of both G(8002)A and −3A/−4A ET-1 gene polymorphisms was seen for hypertensive nonsmoking women compared with normotensive nonsmoking women (odds ratio 3.13, 95% CI 1.31 to 7.48, P=0.007).
DISCUSSION
Evaluating allelic and genotype distributions of three ET-1 gene polymorphisms in normotensive and hypertensive subjects separately, we found that the only significant difference was in the lengths of allelic variants of the CA/CT dinucleotide repeat polymorphism. In hypertensive subjects, the alleles of G(8002)A and −3A/−4A ET-1 polymorphisms proved to be significantly associated (G with −3A and A with −4A). A highly significant increase of the −3A allele of −3A/−4A ET-1 polymorphism was found in hypertensive men who were smokers 1 compared with never-smoking hypertensive men. The polymorphism association changed when smoking status was requalified, and depended on the higher occurrence of former smokers in the hypertensive group.
Only three studies on polymorphisms of −3A/−4A (−138) ET-1 have been published so far (7–9). Our allelic frequencies in both normotensive and hypertensive subjects were a little lower than those published by Stevens et al (7). The study of Lajemi et al (9) does not contain information about the allelic frequency of the polymorphism in never-treated hypertensives. Lajemi et al (9) found an association between endothelin receptor gene polymorphisms and aortic stiffness in women and with radial artery thickness in men. They did not associate insertion/deletion (−138) ET-1 gene polymorphism with any arterial or cardiac parameters in their patients. On the other hand, Brugada et al (14) showed that G(8002)A polymorphisms may influence the median left ventricular hypertrophy score in patients with hypertrophic cardiomyopathy when greater left ventricular mass was found in patients who carried the A allele of the polymorphism.
Cardiovascular risk factors for adults with hypertension such as smoking, dyslipidemia, diabetes mellitus, age, sex and family history of premature cardiovascular disease (15) can be viewed as comparably suitable phenotypic traits potentially associated with frequent gene polymorphisms such as vascular wall and cardiac parameters. The genetic association of the −3A allele of the −3A/−4A ET-1 polymorphism with essential hypertension in male smokers 1 may reflect their inborn susceptibility to become smokers. The finding of a difference in allelic frequency of the same polymorphism between nor-motensive men, smokers and nonsmokers 2 is not in conflict with the results for smokers 1 but rather reflects a different smoking status. The status is influenced by a physician’s recommendation that may modify or ‘mask’ a genetically based trait to become a smoker.
Acknowledgments
The study was supported by grants No 306/96/0099 (Grant Agency of the Czech Republic), VS 96097 “Promotion of Research in Universities” and CEZ J07/98:141100002 from the Ministry of Education, Youth and Physical Education of the Czech Republic.
REFERENCES
- 1.Topper JN, Gimbrone MA. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–6. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 2.Lemne CE, Lundeberg T, Theodorsson E, De Faire I. Increased basal concentrations of plasma endothelin on borderline hypertension. J Hypertens. 1994;12:1069–74. [PubMed] [Google Scholar]
- 3.Schneider MP, Hilgers KF, Klingbeil AU, John S, Veelken R, Schmieder RE. Plasma endothelin is increased in early essential hypertension. Am J Hypertens. 2000;13:579–85. doi: 10.1016/s0895-7061(99)00260-5. [DOI] [PubMed] [Google Scholar]
- 4.Ames RS, Sarau HM, Chambers JK, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–6. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 5.Gray GA, Battistini B, Webb DJ. Endothelins are potent vasocontrictors, and much more besides. Trends Pharmacol Sci. 2000;21:38–40. doi: 10.1016/s0165-6147(99)01431-5. [DOI] [PubMed] [Google Scholar]
- 6.Berge KE, Berg K. No effect of a Taq I polymorphism in DNA at the endothelin 1 (EDN1) locus on normal blood pressure level or variability. Clin Genet. 1992;41:90–5. doi: 10.1111/j.1399-0004.1992.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 7.Stevens PA, Brown MJ. Genetic variability of the ET-1 and the ETA receptor genes in essential hypertension. J Cardiovasc Pharmacol. 1995;26:S9–12. [PubMed] [Google Scholar]
- 8.Brown MJ, Sharma P, Stephens PA. Association between diastolic blood pressure and variants of the endothelin-1 and endothelin-2 genes. J Cardiovasc Pharmacol. 2000;35:S41–3. doi: 10.1097/00005344-200000002-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lajemi M, Gautier S, Poirier O, et al. Endothelin gene variants and aortic and cardiac structure in never-treated hypertensives. Am J Hypertens. 2001;14:755–60. doi: 10.1016/s0895-7061(01)02162-8. [DOI] [PubMed] [Google Scholar]
- 10.Horký K, Widimský J, Cífková R. Hypertenze. Bull Cesk Spol Hypertenz. 1998;1:3–11. [Google Scholar]
- 11.Sambrook J, Fritsch EF, Maniatis T.Molecular Cloning: A Laboratory Manual 2nd ednBook 2Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989 [Google Scholar]
- 12.Pages JC, Drieu C, Blanche H, Beckmann J, Cann HM. A short tandem repeat polymorphism at the endothelin 1 (EDN1) locus. Hum Mol Genet. 1993;2:90. doi: 10.1093/hmg/2.1.90. [DOI] [PubMed] [Google Scholar]
- 13.Kleparnik K, Mala Z, Pribyla L, Blazkova M, Vasku A, Bocek P. Ultrafast detection of microsatellite repeat polymorphism in endothelin 1 gene by electrophoresis in short capillaries. Electrophoresis. 2000;21:238–46. doi: 10.1002/(SICI)1522-2683(20000101)21:1<238::AID-ELPS238>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Brugada R, Kelsey W, Lechin M, et al. Role of candidate modifier genes on the phenotypic expression of hypertrophy in patients with hypertrophic cardiomyopathy. J Investig Med. 1997;45:542–51. [PubMed] [Google Scholar]
- 15.The sixth report of the Joint National Committee on prevention, detection evaluation and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]


