Abstract
Vitamin A (retinol) plays a key role in the regulation of cell growth and differentiation, and has been studied as a potential chemopreventive agent for prostate cancer. However, findings from epidemiologic studies of the association between circulating retinol concentrations and risk of prostate cancer are inconsistent. We examined whether serum concentrations of retinol were associated with risk of prostate cancer in a nested case-control study using 692 prostate cancer cases and 844 matched controls from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. We estimated risk of prostate cancer using multivariate, conditional logistic regression to calculate odds ratios and 95% confidence intervals for overall prostate cancer and aggressive disease (stage 3 or 4 or Gleason 7+; n=269). Serum retinol concentrations were not associated with overall prostate cancer risk; however, the highest versus lowest concentrations of serum retinol were associated with a 42% reduction in aggressive prostate cancer risk (Ptrend=0.02), with the strongest inverse association for high-grade disease (Gleason Sum7+; OR, 0.52; 95%CI, 0.32–0.84; Ptrend=0.01). Our results suggest that higher circulating concentrations of retinol are associated with a decreased risk of aggressive prostate cancer. Further research is needed to better understand the significance of elevations in serum retinol concentrations and the possible biologic mechanisms through which retinol affects prostate cancer.
INTRODUCTION
Vitamin A is a fat-soluble vitamin that plays an essential role in the visual cycle and is required in the normal growth of bone, reproduction, embryonic development, and in differentiation of epithelial tissues(1). There has also been a great deal of interest in the cancer-preventive potential of vitamin A (retinol), much of which stems from in vitro and animal studies demonstrating a key role for retinol in regulating the growth, differentiation and apoptosis of normal and malignant cells.
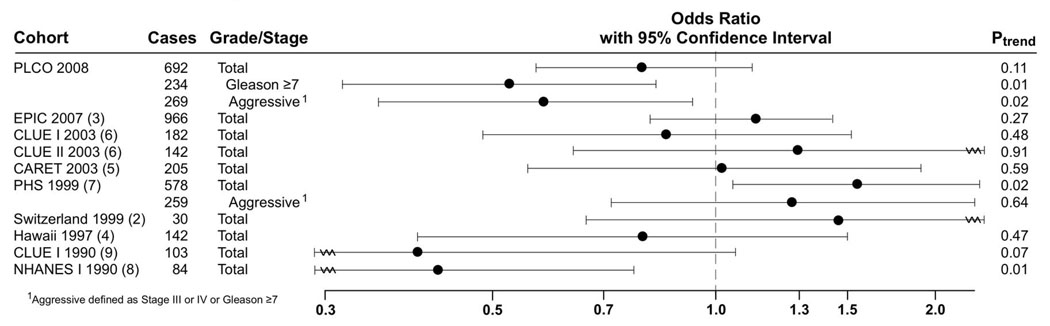
Several observational epidemiological studies and one randomized clinical trial have examined the associations of vitamin A and prostate cancer, although no consistent association with prostate cancer risk has been established. Prospectively designed nested case-control studies have reported no association between circulating concentrations of retinol at baseline and subsequent prostate cancer risk (2–6), while others have reported an increased(7) or decreased(8, 9) risk associated with higher retinol concentrations(Figure 1). However, many of the previous studies had small sample sizes, and few examined the associations for retinol concentration and prostate cancer risk by stage or grade of disease.
Figure 1.
Summary of Prospective Studies on Serum or Plasma Retinol and Prostate Cancer Risk
This brief report examined whether serum retinol concentrations were associated with prostate cancer incidence in a large nested case-control study within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, based on healthy men aged 55 years and older who were screened for prostate cancer regularly, following a standardized protocol.
MATERIALS AND METHODS
Study Setting and Population
The study setting and population has been described previously(10). In brief, this nested case-control study was limited to men randomized to the screening arm of the PLCO trial, who were offered prostate cancer screening by serum prostate-specific antigen (PSA) at entry and annually for 5 years and digital rectal examination (DRE) at entry and annually for 3 years. Men with a positive screening result (PSA >4 ng/mL or DRE suspicious for prostate cancer) were referred to their medical care providers for diagnostic evaluation and follow-up for diagnosis of cancer was conducted by annually mailed questionnaires. Medical and pathologic records related to prostate cancer diagnoses were acquired for all men suspect for prostate cancer by screening examination or annual questionnaire.
Men in this study had no prior history of prostate cancer and at least one blood collection and one valid prostate cancer screen prior to October 1, 2001 (the censor date). All men were followed from their initial prostate cancer screen (PSA and/or DRE), to occurrence of prostate cancer, loss-to-follow-up, death (by National Death Index), or censor date, whichever came first. Cases were men diagnosed with adenocarcinoma of the prostate. Aggressive cancers were defined as clinical stages III or IV (regionally invasive or distant metastatic disease) or biopsy Gleason Sum≥7 based on the pathologic report.
1,320 prostate cancers were identified, from which non-Hispanic Black cases and cases diagnosed in the first year after blood draw were excluded, leaving a total of 803 cases. Controls were frequency-matched by age at entry (5-year intervals), time since initial screening (1-year intervals), year of blood draw, and race/ethnicity using incidence-density sampling(11) with a case-control ratio of 1:1.2. Serum collected at study entry was available for 692 (86.2%) cases and 844 (88.9%) controls.
Assessment of Questionnaire-Based Covariates
At enrollment, all participants were asked to complete a questionnaire about sociodemographic factors, medical history, and risk factors for cancer. Usual dietary intake over the 12 months prior to enrollment was assessed with a 137-item food frequency questionnaire including 14 additional questions about intake of vitamin and mineral supplements and 10 additional questions on meat cooking practices(12). Daily dietary nutrient intake was calculated by multiplying the daily frequency of each consumed food item by the nutrient value of the sex-specific portion size, using the nutrient database from the U.S. Department of Agriculture(13). Total vitamin and mineral intakes were calculated using the sum of dietary and supplement intakes.
Laboratory Analysis
Nonfasting blood specimens collected at the clinical centers were processed and frozen within 2 hours of blood draw and stored at −70°C. Serum concentrations of total retinol (bound and unbound) were determined using reversed-phase high-performance liquid chromatography, with UV detection(14). Cases and their matched controls were analyzed in the same batch. Blinded quality control samples were randomly inserted into each batch. The coefficient of variation estimated from 171 blinded duplicates was 5.1%. To investigate the reproducibility of serum retinol concentrations over time we also included a second serum sample drawn 1 year after study entry in the same sample batch as the subject-paired serum sample collected at study entry for a subset of 46 controls. Serum concentrations of selenium and cholesterol were measured for another nested case-control study, and the methods have been described previously(15). Serum cholesterol was measured enzymatically by a standard procedure at 37°C on a Hitachi 912 analyzer.
Statistical Analysis
Conditional logistic regression was used to estimate ORs and 95% CIs of prostate cancer, with serum retinol concentrations categorized as quintiles, based on the distribution among controls. Tests for linear trend were based on quintile-specific median values expressed as a continuous variable. Analyses were conditioned on matching factors (age, time since initial screening, and year of blood draw), and adjusted for study center. In addition, we evaluated potential confounders based on a priori hypotheses for prostate cancer risk factors (PSA, DRE screening, family history of prostate cancer, educational attainment, physical activity, body mass index (BMI), aspirin and ibuprofen use, history of diabetes, smoking, intakes of alcohol, energy, fat, red meat, heterocylic amines from meat (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP), fruits, vegetables, cruciferous vegetables, vitamin E, calcium, serum selenium, serum cholesterol, month of blood draw); however, none were included in the analyses because the factors, either separately or together, did not change the risk estimates by more than 10%. To assess effect modification we performed stratified analyses and evaluated the statistical significance of multiplicative interaction terms by comparing the −2 log-likelihood statistics of the main effect model and the model including the interaction term. Spearman correlation coefficients were calculated to measure the correlation between serum retinol concentrations obtained one year apart in 46 controls and between serum concentrations and dietary vitamin A intake in all controls. Correlation coefficients were adjusted for month of blood draw, serum cholesterol concentration, smoking, body mass index, age, and energy intake. All P values are two-sided and significance set at the 0.05 level.
RESULTS
The distribution, overall and by retinol concentrations, of demographic and health-related covariates possibly linked to prostate cancer risk is shown in Table 1. The average age at study entry among controls was 64.7 years and did not vary by retinol concentration. Compliance with the PLCO screening protocol was very high and did not differ by serum retinol concentrations, as shown by the average number of cancer screens (PSA or DRE; Table 1). Men with higher serum retinol concentrations were less likely to have a personal history of diabetes, and had higher serum cholesterol concentrations, were less obese, more likely to smoke, more physically active and more likely to use aspirin. Men with higher serum retinol concentrations also had a lower intake of fat and red meat, and higher intakes of PhIP, vitamin D, supplemental vitamin E, and alcohol. The average time between blood collection and prostate cancer diagnosis (among cases) was 2.4 + 1.4 years. The correlation between serum retinol concentrations measured in 46 men at study entry and 1 year later was modest (r=0.38; p=0.009), and the correlation between serum retinol and vitamin A intake from diet and supplements among controls was weak (r=0.08, p=0.06).
Table 1.
Description of Baseline Characteristics Overall and According to Quintiles of Serum Retinol 1
| Characteristic | Quintile of Serum Retinol |
Overall | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Controls, n | 168 | 169 | 168 | 169 | 168 | 842 |
| Quintile range (ug/dl) | 27.4–54.7 | 54.8–64.3 | 64.4–72.8 | 72.9–85.3 | 85.4–262.6 | 27.4–262.6 |
| Mean age at study entry, years (SD) | 65.1 (0.4) | 64.6 (0.4) | 64.5 (0.4) | 64.6 (0.4) | 64.8 (0.4) | 64.7 (0.2) |
| Average no. of screens/yr 2 | 0.96 | 0.96 | 0.97 | 0.95 | 0.97 | 0.96 |
| Family history of prostate cancer, % | 5.6 | 3.4 | 5.7 | 4.5 | 6.3 | 5.3 |
| History of diabetes, % | 11.2 | 10.6 | 6.9 | 8.2 | 5.8 | 8.3 |
| Cholesterol, mmol/l (SD) | 5.4 (0.1) | 5.7 (0.1) | 5.7 (0.1) | 6.2 (0.1) | 7.8 (0.1) | 6.1 (0.1) |
| Mean current BMI, kg/m2 (SD) | 27.8 (0.3) | 27.9 (0.3) | 27.3 (0.3) | 26.9 (0.3) | 26.9 (0.3) | 27.3 (0.1) |
| Smoking history, % | ||||||
| Never | 34.3 | 25.0 | 27.1 | 27.8 | 30.7 | 30.0 |
| Current | 8.8 | 9.3 | 9.7 | 8.9 | 9.4 | 9.1 |
| Former | 46.7 | 52.8 | 46.1 | 56.9 | 51.6 | 51.5 |
| Pipe/Cigar | 8.7 | 11.6 | 15.9 | 4.9 | 6.8 | 9.4 |
| Mean physical activity, hours/week (SD) | 2.7 (0.1) | 2.8 (0.1) | 2.8 (0.1) | 3.2 (0.1) | 3.1 (0.1) | 2.9 (0.1) |
| Aspirin use, ≥1 times/week % | 42.6 | 46.4 | 44.9 | 52.0 | 53.7 | 47.6 |
| Mean intake (SD) | ||||||
| Energy, kcal/day | 2361 (73) | 2333 (72) | 2439 (73) | 2284 (73) | 2350 (72) | 2347 (32) |
| Total fat, g/day | 82.0 (1.3) | 81.7 (1.3) | 79.4 (1.4) | 77.2 (1.3) | 78.0 (1.3) | 79.2 (1.4) |
| Fruit, servings/2000 kcal/day | 3.2 (0.2) | 3.4 (0.2) | 3.5 (0.2) | 3.4 (0.2) | 3.5 (0.2) | 3.5 (0.1) |
| Vegetables, servings/2000 kcal/day | 5.5 (0.2) | 5.2 (0.2) | 5.5 (0.2) | 5.5 (0.2) | 5.4 (0.2) | 5.5 (0.1) |
| Red meat, g/day | 114.8 (4.5) | 100.5 (4.5) | 101.4 (4.5) | 95.7 (4.5) | 96.0 (4.5) | 97.7 (2.6) |
| PHIP, ng/day | 236 (43) | 228 (43) | 226 (43) | 243 (43) | 284 (43) | 220 (19) |
| Calcium, mg/day | 1125 (35) | 1160 (35) | 1132 (35) | 1215 (35) | 1184 (35) | 1166 (21) |
| Vitamin D, IU/day | 350 (25) | 428 (25) | 406 (25) | 448 (25) | 463 (25) | 420 (12) |
| Supplement vitamin E use, % ever 3 | 36.5 | 46.1 | 49.8 | 56.7 | 58.9 | 48.6 |
| Alcohol % | ||||||
| >=0 and <3 g/d | 50.2 | 50.0 | 46.2 | 38.3 | 39.6 | 45.7 |
| >=3 and <15g/d | 22.1 | 28.2 | 23.2 | 19.6 | 18.0 | 21.5 |
| >=15g/d | 27.7 | 21.6 | 31.0 | 41.9 | 42.5 | 32.8 |
All values other than age were directly standardized for age. Intakes of total fat, fruit, vegetables, red meat, PhIP, calcium, vitamin D, and supplement vitamin E were also standardized for energy intake.
Average number of prostate cancer screening examinations (PSA or DRE) up to diagnosis of prostate cancer (cases) or selection as a control. Maximum period is limited to period of active screening (years 0–5).
Includes both single supplement and multivitamin use
Median serum retinol concentrations did not differ significantly between cases and controls (67.11 versus 68.52 ug/dl, p=0.22). In multivariate analyses comparing men in the highest to lowest quintiles, serum retinol was associated with a non-significant 20% reduction in risk of prostate cancer with no indication of a linear trend (Ptrend 0.11, Table 2). When restricted to aggressive prostate cancers (Gleason Sum≥7 or clinical stage III or IV), high concentrations of serum retinol were associated with a significant reduction in risk (OR, 0.58; 95% CI, 0.36–0.92 for highest versus lowest quintile; Ptrend=0.02; Table 2), with the strongest association for highgrade disease (Gleason Sum≥7; OR, 0.52; 95% CI, 0.32–0.84 for highest versus lowest quintile; Ptrend=0.01; Table 2). For both aggressive and high-grade prostate cancers, the greatest decrease in risk occurs between the first and second quintiles, with risk being similar for quintiles 2 through 5.
Table 2.
Odds Ratio (OR) of Prostate Cancer According to Quintile of Serum Retinol and Stage of Disease Progression at Diagnosis
| Serum Retinol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q11 | Q2 | Q3 | Q4 | Q5 | p-trend | ||||||
| Cases | OR (95% CI)4 | Cases | OR (95% CI)4 | Cases | OR (95% CI)4 | Cases | OR (95% CI)4 | Cases | OR (95% CI)5 | ||
| All Cases 2 | 155 | 1.00 | 154 | 0.97(0.71,1.34) | 124 | 0.77 (0.55,1.07) | 130 | 0.79 (0.57,1.10) | 129 | 0.80 (0.57,1.11) | 0.11 |
| Non-Aggressive 3 | 94 | 1.00 | 96 | 1.22 (0.84,1.79) | 71 | 0.86 (0.58,1.29) | 79 | 0.95 (0.64,1.41) | 83 | 0.97 (0.66,1.43) | 0.53 |
| Aggressive | 68 | 1.00 | 57 | 0.69 (0.44,1.07) | 52 | 0.64 (0.40,1.00) | 49 | 0.57 (0.36,0.91) | 43 | 0.58 (0.36,0.92) | 0.02 |
| Stage III & IV | 18 | 1.00 | 20 | 0.87 (0.42,1.79) | 19 | 0.83 (0.40,1.73) | 19 | 0.74 (0.35,1.56) | 14 | 0.69 (0.32,1.51) | 0.33 |
| Gleason Sum >=7 | 66 | 1.00 | 48 | 0.58 (0.37,0.92) | 42 | 0.50 (0.31,0.82) | 39 | 0.45 (0.27,0.73) | 39 | 0.52 (0.32,0.84) | 0.01 |
CI confidence interval
Reference category
Numbers of Non-Aggressive and Aggressive do not add up to All Cases due to missing information on stage or grade
Aggressive defined as Gleason Sum ≥7 or stage III or IV
Adjusted for age, time since initial screening, year of blood draw, and study center
Similar associations between serum retinol and overall prostate cancer and aggressive disease were observed for subgroups characterized by year of diagnosis (<4 and > 4 years), age (<65 and ≥65 years), smoking (ever versus never), family history of prostate cancer (yes versus no), alcohol (0–2.9, 3–14.9 and ≥15g/day), and serum vitamin D (<49.9, 50.0–68.7 and ≥68.8nmol/L) (data not shown). Furthermore, ORs did not vary when these analyses were limited to the years of active screening (years 1–5) or when the second year of follow-up was excluded (the first year of follow-up was already excluded by design; data not shown).
DISCUSSION
In this prospective analysis of 692 incident cases, we observed a decreased risk of aggressive prostate cancer, particularly high-grade disease, with increased concentrations of serum retinol. This association was not modified by age, smoking, family history, alcohol intake, or vitamin D.
Our findings of a decreased risk of aggressive prostate cancer with increased concentrations of serum retinol are supported by results from two earlier cohort studies; in the National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-up Study, high retinol concentrations were associated with a decreased risk of prostate cancer(8) and in CLUE I a non-significant inverse association between serum retinol and prostate cancer was reported(9), although this finding was not confirmed on further follow-up(6).
Analyses in many of these studies were limited to total prostate cancer, and therefore, differences in associations according to stage or grade of disease may have been obscured(2, 4–6, 8, 9). For prostate cancer, this is particularly important since the disease is heterogeneous and the significance of early-stage (PSA-detected) disease is unclear(16). Two larger prospective studies (n=578 and n=966 cases) analyzed non-aggressive and aggressive cancers separately, although neither found an association between retinol concentrations and risk of aggressive (Stage III or IV or Gleason sum≥7)(7) or advanced (T3 or T4, N1+, M1 or metastatic)(3) disease, and one reported an elevated risk for high levels of retinol that was limited to non-aggressive(Stage I or II and Gleason sum<7) disease(7). In the current study, the association of a decreased risk with increasing retinol concentrations was limited to aggressive disease and was strongest for high-grade disease. This association did not differ by year of diagnosis (<4 versus > 4), suggesting that a reverse-causality effect on retinol is unlikely. These findings could indicate that retinol has a specific effect on disease progression(17), although it is also possible that this association is due to chance as our study is the only one reporting an inverse association with aggressive disease specifically.
Our findings are supported by several in vitro and animal models which demonstrate a key role for retinol in regulating the growth, differentiation and apoptosis of normal and malignant prostate cells(18, 19). In experimental models, retinoids suppress transforming effects of carcinogens, inhibit growth of premalignant cells, enhance differentiation of malignant cells and induce apoptosis(20). These regulatory effects are mediated through the activation of two families of retinoid nuclear receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Prostate cancer tissue has lower levels of retinoic acid(21), and low retinol levels could also directly alter the expression of retinoic acid and retinoid X receptors(22), which have been shown to be selectively reduced in prostate cancers(23).
It is unlikely these findings can be solely attributed to differences in dietary or supplemental retinol intake, given that serum retinol concentrations are under tight homeostatic control(24), and neither dietary intake nor supplements is strongly correlated with retinol concentrations(25, 26). However, it should be noted that the range of retinol concentration in this study population is wide (27.4 to 262.6 ug/dl), and other factors such as BMI, physical activity, and dietary fat and alcohol intake are correlated with retinol concentrations, suggesting that in a well-nourished population, these factors influence retinol concentrations to some degree. Lower concentrations of retinol may reflect insufficient vitamin A stores in the liver, although this is unlikely given the healthy study population. Alternatively, low circulating retinol concentrations could result from underlying aberrations in cellular retinol binding protein (CRBP) metabolism(27). CRBP plays an essential role in the maintenance of hepatic retinol stores and the synthesis of retinoic acid from retinol(24), and the reduction or loss of CRBP1 expression has been demonstrated in prostate carcinomas(28).
A limitation of this study and previous observational studies is the use of a single baseline measure of serum retinol. Although one examination of the repeatability of serum retinol concentrations 15 years apart showed moderate correlation (r=0.58; 95%CI:0.46–0.67)(29); the correlation between serum retinol concentrations 1 year apart in this study was somewhat lower (r=0.38; p=0.009) and thus, a single measure of serum retinol may not be representative of long-term retinol exposure. Nonetheless, such non-differential measurement error in serum retinol generally leads to an attenuation of the estimated association, and the true protective effect should be stronger than that found in our study. A further limitation is the relatively short follow-up of 8 years.
The strengths of this study include standardized procedures for prostate cancer screening and very high compliance with the screening protocol, availability of pre-diagnostic serum samples, a large sample size, and detailed diagnostic data which allowed stratification by stage and grade and simultaneous adjustment for multiple confounders.
In summary, our results support a protective association between serum retinol concentrations and risk of aggressive prostate cancer. Further research to better understand the significance of elevations in serum retinol concentrations and possible effects of aberrations in CRBP metabolism on prostate cancer risk would be useful.
Contributor Information
Jeannette M. Schenk, Fred Hutchinson Cancer Research Center, Cancer Prevention Program, PO Box 19024, 1100 Fairview Avenue N, M4-B402, Seattle, WA 98109-1024, phone: 206-667-6860, jschenk@fhcrc.org
Elio Riboli, Nutrition and Cancer Unit, International Agency for Research on Cancer, 150, Cours Albert Thomas, 69372 Lyon Cedex 08, FRANCE, phone: (33) 04.72.73.84.11, eriboli@imperial.ac.uk.
Nilanjan Chatterjee, National Cancer Institute, NIH, Division of Cancer Epidemiology and Genetics, 6120 Executive Blvd, Bethesda, MD 20892, phone: 301-402-7933, chattern@mail.nih.gov.
Michael F. Leitzmann, Institute of Epidemiology and Preventive Medicine, University of Regensburg, Franz-Josef-Strauss-Allee 11, 93053 Regensburg, Germany, michael.leitzmann@klinik.uni-regensburg.de
Jiyoung Ahn, National Cancer Institute, NIH, Division of Cancer Epidemiology and Genetics, 6120 Executive Blvd, Room, Bethesda, MD 20892, phone: 301-451-9581, ahnj@mail.nih.gov.
Demetrius Albanes, National Cancer Institute, NIH, Division of Cancer Epidemiology and Genetics, 6120 Executive Blvd, Bethesda, MD 20892, phone: 301-594-2869, email: daa@nih.gov.
Douglas J Reding, Marshfield Clinic Research Foundation, 1000 N Oak Ave, Marshfield, WI 54449, (715)387-5416, reding.douglas@mcrf.mfldclin.edu.
Yinghui Wang, Fred Hutchinson Cancer Research Center, Cancer Prevention Program, PO Box 19024, 1100 Fairview Avenue N, M2-B500, phone: 206-667-7888, Seattle, WA 98109-1024, yinghuiw@fhcrc.org.
Marlin D. Friesen, Johns Hopkins Bloomberg School of Public Health, Dept. of Environmental Health Sciences, 615 N. Wolfe St. Room E7032, Baltimore, MD 21205 USA, phone: 410-955-4235, mfriesen@jhsph.edu
Richard B. Hayes, National Cancer Institute, NIH, Division of Cancer Epidemiology and Genetics, 6120 Executive Blvd, Bethesda, MD 20892, phone: 301-435-3973, hayesr@mail.nih.gov
Ulrike Peters, Fred Hutchinson Cancer Research Center, Cancer Prevention Program, PO Box 19024, 1100 Fairview Avenue N, M4-B402, Seattle, WA 98109-1024, phone: 206-667-2450, upeters@fhcrc.org, Department of Epidemiology, School of Public Health, University of Washington, 1959 NE Pacific Street, Health Sciences F-262D, Seattle, WA 98195.
REFERENCES
- 1.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.Eichholzer M, Stahelin HB, Ludin E, Bernasconi F. Smoking, plasma vitamins D, E, retinol, and carotene, and fatal prostate cancer: seventeen-year follow-up of the prospective Basel study. Prostate. 1999;38:189–98. doi: 10.1002/(sici)1097-0045(19990215)38:3<189::aid-pros3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Key TJ, Appleby PN, Allen NE, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2007;86:672–81. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 4.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. 1997;6:487–91. [PubMed] [Google Scholar]
- 5.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from β-carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12:518–26. [PubMed] [Google Scholar]
- 6.Huang H-Y, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157:335–44. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 7.Gann P, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30. [PubMed] [Google Scholar]
- 8.Reichman ME, Hayes RB, Ziegler RG, et al. Serum vitamin A and subsequent development of prostate cancer in the first national health and nutrition examination survey epidemiologic follow-up study. Cancer Res. 1990;50:2311–15. [PubMed] [Google Scholar]
- 9.Hsing AW, Comstock GW, Abbey H, Polk BF. Serologic precursors of cancer. Retinol, carotenoids, and tocopherol and risk of prostate cancer. J Natl Cancer Inst. 1990;82:941–6. doi: 10.1093/jnci/82.11.941. [DOI] [PubMed] [Google Scholar]
- 10.Peters U, Leitzmann MF, Chatterjee N, et al. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16:962–8. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]
- 11.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia (PA): Lippincott Raven; 1998. [Google Scholar]
- 12.Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. 2007 Available at http://www.cancer.gov/prevention/plcoDQX.pdf.April2007.
- 13.Tippett KS, Cypel YS. Design and operation: the continuing survey of food intakes by individuals and the diet and health knowledge survey, 1994–96. U.S. Department of Agriculture, Agriculture Research Service; Continuing Survey of Food Intakes by Individuals 1994–1996, Nationwide Food Surveys Rep. No. 96-1. 1997
- 14.Steghens JP, van Kappel AL, Riboli E, Collombel C. Simultaneous measurement of seven carotenoids, retinol and α-tocopherol in serum by high-performance liquid chromatogrphy. Journal of Chromatography B: Biomedical Sciences and Applications. 1997;694:71–81. doi: 10.1016/s0378-4347(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 15.Peters U, Foster CB, Chatterjee N, et al. Serum selenium and risk of prostate cancer - a nested case-control study. Am J Clin Nutr. 2007;85:209–17. doi: 10.1093/ajcn/85.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platz EA, De Marzo AM, Giovannucci E. Prostate cancer association studies: pitfalls and solutions to cancer misclassification in the PSA era. J Cell Biochem. 2004;91:553–71. doi: 10.1002/jcb.10700. [DOI] [PubMed] [Google Scholar]
- 17.Peehl DM, Feldman D. The role of vitamin D and retinoids in controlling prostate cancer progression. Endocr Relat Cancer. 2003;10:131–40. doi: 10.1677/erc.0.0100131. [DOI] [PubMed] [Google Scholar]
- 18.Pili R, Kruszewski MP, Hager BW, Lantz J, Carducci MA. Combination of phenylbutyrate and 13-cis retinoic acid inhibits prostate tumor growth and angiogenesis. Cancer Res. 2001;61:1477–85. [PubMed] [Google Scholar]
- 19.Richter F, Huang HFS, Li M-t, Danielpour D, Wang S-L, Irwin RJ. Retinoid and andgrogen regulation of cell growth, epidermal growth factor and retinoic acid receptors in normal and carcinoma rate prostate cells. Mol Cell Endocrinol. 1999;153:29–38. doi: 10.1016/s0303-7207(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 20.Trump DL. Retinoids in bladder, testis and prostate cancer: epidemiologic, pre-clinical and clinical observations. Leukemia. 1994;8:S50–4. [PubMed] [Google Scholar]
- 21.Pasquali D, Thaller C, Eichele G. Abnormal level of retinoic acid in prostate cancer tissues. J Clin Endocrinol Metab. 1996;81:2186–91. doi: 10.1210/jcem.81.6.8964849. [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Mano H, Kumazawa T, Yoshizawa Y, Kojima R, Masushige S. Effect of retinoid status on α, β and γ retinoic acid receptor mRNA levels in various rat tissues. Biochem J. 1992;286:755–60. doi: 10.1042/bj2860755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotan Y, Xu XC, Shalev M, et al. Differential expression of nuclear retinoid receptors in normal and malignant prostates. J Clin Oncol. 2000;18:116–21. doi: 10.1200/JCO.2000.18.1.116. [DOI] [PubMed] [Google Scholar]
- 24.Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB Journal. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 25.Hallfrisch J, Muller DC, Singh VN. Vitamin A and E intakes and plasma concentrations of retinol, beta-carotene, and alpha-tocopherol in men and women of the Baltimore Longitudinal Study of Aging. Am J Clin Nutr. 1994;60 doi: 10.1093/ajcn/60.2.176. [DOI] [PubMed] [Google Scholar]
- 26.Nierenberg DW, Dain BJ, Mott LA, Baron JA, Greenberg ER. Effects of 4 y of oral supplementation with beta-carotene on serum concentrations of retinol, tocopherol, and five carotenoids. Am J Clin Nutr. 1997;66:315–9. doi: 10.1093/ajcn/66.2.315. [DOI] [PubMed] [Google Scholar]
- 27.Cheng L, Quian S, Rothschild C, et al. Alteration of the Binding Specificity of Cellular Retinol-binding Protein II by Site-directed Mutagenesis. The Journal of Biological Chemistry. 1991;266:24404–12. [PubMed] [Google Scholar]
- 28.Jerónimo C, Henrique R, Oliveira J, et al. Aberrant cellular retinol binding protein 1 (CRBP1) gene expression and promoter methylation in prostate cancer. J Clin Pathol. 2004;57:872–6. doi: 10.1136/jcp.2003.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comstock GW, Burke AE, Hoffman SC, Norkus EP, Gross M, Helzlsouer KJ. The Repeatability of Serum Carotenoid, Retinoid, and Tocopherol Concentrations in Specimens of Blood Collected 15 Years Apart. Cancer Epidemiol Biomarkers Prev. 2001;10:65–8. [PubMed] [Google Scholar]