Abstract
Prostaglandin E2 (PGE2) is produced at high levels in the injured central nervous system, where it is generally considered a cytotoxic mediator of inflammation. The cellular actions of PGE2 are mediated by G-protein signaling activated by prostanoid receptors termed EP1, EP2, EP3 and EP4. Recent studies have implicated the EP2 prostanoid receptor in apparently conflicting roles promoting neuronal death in some model systems and the survival of neurons in others. Here we show that treatment of immortalized human microglia and CCF-STTG1 astrocytes with either PGE2 or the EP2 selective agonist butaprost stimulates the release of brain-derived neurotrophic factor (BDNF). Both cell lines express mRNA for the EP2 receptor, whereas transcripts for the other subtypes are not detected. Pharmacological studies using PGE2 and modulators of cyclic AMP signaling implicate this pathway in PGE2-stimulated BDNF release. These results indicate that EP2 prostanoid receptor activation induces BDNF secretion through stimulation of cyclic AMP dependent signaling. Our findings provide a mechanism by which endogenous PGE2 might contribute to either neurotoxicity or neuroprotection in the injured brain via the induction of BDNF release from microglial cells and astrocytes.
Keywords: Prostaglandin E2, microglia, astrocytes, BDNF, G-protein coupled receptor, brain derived neurotrophic factor, cyclic AMP
Prostaglandin E2 (PGE2) is a prominent lipid autocrine/paracrine signaling mediator produced by the sequential metabolism of arachidonic acid by cyclooxygenase (COX) and PGE2 synthase. PGE2 is a major agent in local intercellular signaling associated with inflammation, pain, fever and immune responses (reviewed by Matsuoka & Narumiya, 2007; Khanapure et al., 2007; Harris et al., 2002). The cellular actions of PGE2 are mediated by the activation of a group of G-protein coupled receptors that includes subtypes termed the EP1, EP2, EP3 and EP4 prostanoid receptors. These receptor subtypes are distinguished from one another by their selectivity for different ligands and by their unique coupling to intracellular signaling pathways. Thus, activation of the EP1 receptor is associated with intracellular Ca2+ transients (Funk et al., 1993) while stimulation of the EP2 receptor promotes the production of intracellular cyclic AMP (Regan et al., 1994a). Activation of the EP3 receptor, which is expressed as multiple isoforms through alternative mRNA splicing, inhibits cyclic AMP signaling (Regan et al., 1994b) while also promoting the turnover of inositol phosphates (Wada et al. 2007). Stimulation of the EP4 receptor promotes the activities of both the cyclic AMP and phosphatidylinositol-3-kinase pathways (Fujino et al., 2002).
Numerous studies have found that levels of PGE2 in the central nervous system (CNS) increase in various neurological disorders, including ischemic stroke, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis. Conditions such as these are associated with neuronal death resulting from inflammation and glutamate receptor-mediated excitotoxicity (Minghetti, 2004; Klegeris & McGeer, 2005; Lee et al., 2000). Recent studies have suggested that the EP2 receptor is especially important in a number of these pathophysiological processes by virtue of its capacity to affect neuronal death or survival. Thus, activation of EP2 receptors has been shown to induce apoptosis in cell cultures of rat hippocampal neurons (Takadera et al., 2004) and to exacerbate glutamate receptor mediated neurotoxicity in cultured rat cortical neurons (Takadera & Ohyashiki, 2006). Additionally, EP2 receptors expressed in cultured murine microglia have also been shown to be important mediators of neurotoxicity caused by microglial activation (Jin et al., 2007) and inflammatory innate immune responses (Shie et al., 2007). Counterintuitively, mounting evidence suggests that the EP2 receptor also participates in processes that protect neurons from neurotoxic processes. In studies using cell cultures of CNS neurons from a variety of species, selective activation of EP2 receptors was shown to be protective against toxicity induced by glutamate receptor-mediated excitation (Akaike et al., 1994; McCullough et al., 2004), tumor necrosis factor-alpha (Lee et al. 2004), and β-amyloid peptide (Echeverria et al., 2005). These findings have been corroborated by studies that show a protective effect of EP2 receptor activation against glutamate receptor-mediated toxicity in mice (Ahmad et al., 2006) and in organotypic slice cultures from the hippocampus (Liu et al., 2005) and the spinal cord (Bilak et al., 2004).
Whereas studies using cultured neurons suggest that the effects of EP2 receptor activation are at least partially mediated by direct action of PGE2 on the neurons themselves, studies using live animals and organotypic slices leave open the possibility that other CNS cell types may contribute to the induction of neurotoxic and neuroprotective processes. Microglia and astrocytes perform a broad variety of metabolic, immune, and trophic functions that can support the viability and function of neighboring nerve cells in healthy brains, but can also contribute to neurodegenerative processes under certain conditions. One well-characterized function of these cells is the induction of factors belonging to the neurotrophin family, such as brain-derived neurotrophic factor (BDNF). This neurotrophin was initially described as a secreted protein capable of promoting the survival of neurons, but is now appreciated for its ability to induce neuronal apoptosis (reviewed in Binder & Scharfman, 2004). Both microglia and astrocytes have been shown to release BDNF in response to chemical stimulation (Nakajima et al., 2002; Jurič et al., 2008). Toyomoto et al. (2004) showed that prostaglandin treatment of cultured mouse astrocytes stimulates the release of BDNF and nerve growth factor (NGF). Moreover, BDNF reverses the deficits in learning and synaptic plasticity observed in rats treated with ibuprofen, a nonselective COX inhibitor used to block prostaglandin production (Shaw et al., 2003). These findings suggest that PGE2 may regulate of the expression of neurotrophic factors capable of regulating neuronal death and survival in situations of neurological disease or injury.
Experimental Procedures
Materials
Alpha Modification of Eagle’s Medium (AMEM), RPMI 1640, fetal bovine serum (FBS), trypsin, penicillin/streptomycin, and sodium pyruvate were obtained from Mediatech (Manassas, VA). PGE2 and butaprost were from Cayman Chemical (Ann Arbor, MI). Forskolin and H-89 were from Calbiochem (San Diego, CA). Human cytokine antibody array kits (Cat. No. AAH-CYT-5) were from Raybiotech (Norcross, GA). Monoclonal and biotinylated polyclonal anti-human BDNF antibodies were from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)-conjugated streptavidin, dimethylsulfoxide (DMSO), isobutylmethylxanthine (IBMX) and oligonucleotide PCR primers were from Sigma (St. Louis, MO). One-Step Ultra TMB-ELISA solution and the SuperSignal West Pico Enhanced Chemiluminescence (ECL) system were from Pierce (Rockford, IL). TRizol and Opti-MEM were from Invitrogen (Carlsbad, CA). RNase-free DNase I, dNTPs, reverse transcriptase and oligo-dT primers were from Fermentas (Hanover, MD). Taq polymerase and ThermoPol reaction buffer were from New England BioLabs (Ipswich, MA). Antibodies for phospho-CREB (Ser-133) and total CREB were from Cell Signaling Technology (Beverly, MA). FuGENE-6 was from Roche Applied Science (Indianapolis, IN). The pGL3/CRE-Luc reporter plasmid was from Strategene (La Jolla, CA). HRP-conjugated anti-rabbit antibody, pRL-CMV-BActin reporter plasmid and Dual Luciferase Reporter Assay System were from Promega (Madison, WI).
Cell Culture
Immortalized human microglial cells were a generous gift from Dr. Carol A. Colton at Duke University. This cell line was established by SV40 transformation as described by Janabi et al. (1995), and characterization studies showed that the cells retained the macrophage-like functional and antigenic properties of primary microglia. The immortalized cells were grown at 37°C under 91% air and 9% CO2 in media consisting of AMEM with 10% (vol/vol) heat-inactivated FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin. CCF-STTG1 human astrocytoma cells, noted for their similarity to native astrocytes (Mentz et al., 1999) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Glial fibrillary acidic protein (GFAP), a phenotypic marker for astrocytes, has been shown to be expressed in 80% of cultured CCF-STTG1 cells (Barna et al., 1985). These cells were grown at 37°C under 95% air and 5% CO2. Media for the astrocytes were made by supplementing RPMI 1640 with 10% (vol/vol) FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 4.5 g/L glucose and 10 mM HEPES. Both cell lines were passaged in 75 cm2 flasks until transfer to 10-cm or 6-well plates for experiments. In experiments involving treatment of cells with H-89 and PGE2, drugs were prepared as 1000-fold concentrated solutions in DMSO and delivered to the cell culture media appropriately. Forskolin was prepared as a 1000-fold concentrated solution in ethanol and delivered to cells identically. Vehicle treatments were 0.1% (vol/vol) DMSO or 0.1% (vol/vol) ethanol in the culture media.
Antibody Array Screening Assay
Human microglial and astrocyte cells were grown to confluence in 10 cm2 plates. Cells were serum starved for 16 h, then treated with vehicle or 1 μM PGE2 for 24 h. Culture media supernatants were collected from the treated cultures and cleared by centrifugation at 2100 g. Supernatants were incubated 24 h over membrane-based arrays of antibodies against a panel of secreted cytokines and growth factors. The membranes were processed and developed according to the manufacturer’s instructions, using reagents provided in the array kit. Signals were detected by chemiluminescence.
Measurement of BDNF by ELISA
Human microglial and astrocyte cells were grown to confluence in 6-well plates. Cells were serum starved for 16 h and treated with PGE2 using the time and concentration conditions specified in Figures 1 and 4. Culture media supernatants were collected from the treated cultures, cleared by centrifugation at 2100 g, and incubated overnight at 4°C in 96-well polystyrene microtiter plates coated with anti-human BDNF monoclonal capture antibodies. The plates were washed with a solution of phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween-20 and 1% (vol/vol) FBS, then incubated overnight at 4°C with a solution of 50 μg/mL biotinylated anti-BDNF detection antibodies in a blocking buffer of PBS containing 1% (vol/vol) FBS. The plates were washed again and incubated for 1 h at room temperature with 1 mg/mL HRP-conjugated streptavidin for 1 h at room temperature. Detection of BDNF was accomplished by development in 1-Step Ultra TMB-ELISA solution followed 0.5 M H2SO4. Data were acquired by measuring absorbance at 450 nm on a plate spectrophotometer.
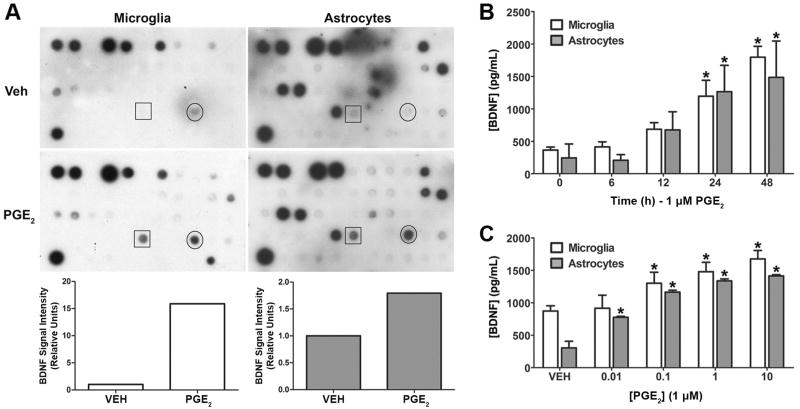
Figure 1. PGE2 stimulates the secretion of BDNF from cultured human microglia and astrocytes.
(A). PGE2 stimulated BDNF release from human microglial cells and astrocytes was initially identified by using an antibody microarray to screen a panel of human cytokines and growth factors. The arrays were incubated in conditioned media supernatants from microglia (left) and astrocytes (right) treated with vehicle or 1 μM PGE2 for 24 h. Signals from the BDNF antibody are shown in the boxes. Signals for VEGF are circled. Bar graphs show the results of densitometry analysis of the scanned autoradiograms for BDNF. BDNF signal values were normalized to the corresponding positive control values derived from the upper left spots on each array. (B). Regulation of BDNF secretion was confirmed by ELISA analysis of conditioned media from PGE2 treated microglial and astrocyte cultures. The graph shows the accumulation of BDNF in supernatants from microglial cells (open bars) and astrocytes (filled bars) treated with PGE2 for the indicated times. (C). ELISA analysis of the effect of PGE2 concentration on the secretion of BDNF from microglia and astrocytes. ELISA data are shown as the mean ± SD of triplicate measurements from a representative experiment that was repeated three times. *p < 0.05 compared to 0 h time point or vehicle control.
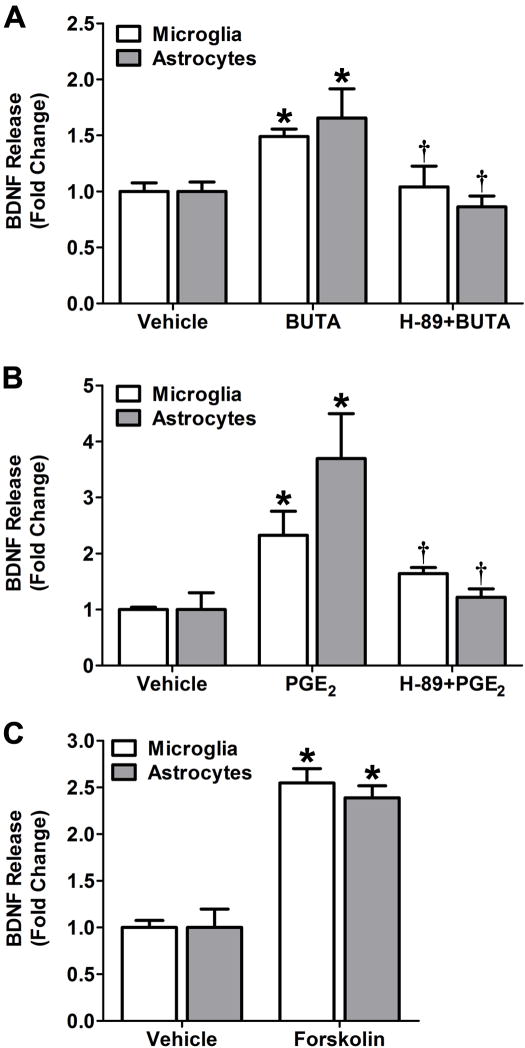
Figure 4. Role of the EP2 receptor, PKA, and adenylyl cyclase in PGE2 stimulated BDNF secretion in microglia.
(A) The roles of the EP2 receptor and PKA in the stimulation of BDNF release were investigated following treatment with the EP2-selective agonist butaprost either under control conditions or following pretreatment with the PKA inhibitor H-89. Serum starved microglia (open bars) and astrocytes (filled bars) were treated with vehicle, 10 μM butaprost alone (BUTA), or butaprost in the presence of 10 μM H-89 for 24 h. (B) Cells were treated with either vehicle, 1 μM PGE2 alone, or PGE2 in the presence of 10 μM H-89 for 24 h. (C) The role of cyclic AMP in PGE2 stimulated BDNF release was determined using forskolin, a direct activator of adenylyl cyclase. Cells were treated with either vehicle or 10 μM forskolin for 24 h. In all experiments, BDNF levels were assessed by ELISA. Data are presented as mean ± SD from triplicate measurements in representative experiments that were repeated three times each. * p < 0.05 compared to vehicle treatment; † p < 0.05 compared to PGE2 treatment.
Detection of EP Receptor mRNAs
Microglial cells and astrocytes were cultured to confluence in 10 cm plates and scraped into TRizol. Total RNA was extracted from the lysates with chloroform, precipitated in isopropanol and incubated with RNase-free DNase I for 30 min at 37°C. RNA was extracted from the DNase reaction using a mixture of phenol, chloroform, and isoamyl alcohol (25:24:1) and purified by two cycles of ethanol precipitation. The purified RNA was reverse transcribed using oligo-dT primers and 5 μL of this reaction solution were used as template material for PCR analysis of the EP prostanoid receptor transcripts. Each reaction contained 1.5 mM MgCl2, 0.2 mM in each dNTP, 0.2 mM each in forward and reverse primers, 1 unit Taq polymerase and 1x ThermoPol reaction buffer. The primer sequences were as follows: EP1 forward 5’-GGTATCATGGTGGTGTCGTG-3’, reverse 5’-GGCCTCTGGTTGTGCTTAGA-3’; EP2 forward 5’-CCACCTCATTCTCCTGGCTA-3’, reverse 5’-CGACAACAGAGGACTGAACG-3’; EP3 forward 5’-CTTCGCATAACTGGGGCAAC-3’, reverse 5’-CTTCGCATAACTGGGGCAAC-3’; EP4 forward 5’-TGGTATGTGGGCTGGCTG-3’, reverse 5’-GAGGACGGTGGCGAGAAT-3’. Plasmids containing the full-length cDNAs for each of the EP receptors were used as positive controls, and template was replaced with water in the negative control reactions. The reactions were run through 30 cycles of 1 min at 94°, 30 s at 55°, and 1 min at 72° followed by a final 10 min extension step at 72°. The products were resolved on 1.2% agarose gels containing 500 ng/mL ethidium bromide.
Measurement of Cyclic AMP Production
Human microglia and astrocytes were cultured to confluence in 10-cm plates and washed once with assay media consisting of 0.1 mg/mL IBMX in Opti-MEM. Cells were detached by treatment with 0.25% trypsin, counted, and distributed in equal numbers among culture tubes containing 1 mL assay media. Cells were then treated in suspension with varying concentrations of PGE2 for 1 h at 37°C. The cAMP content of the cells was determined as described previously (Fujino et al., 2005).
Immunoblot Analysis of CREB Phosphorylation
Microglial cells and astrocytes were grown to confluence on 10-cm plates. Cells were pretreated with either vehicle or 10 μM H-89 for 15 min, then incubated with either vehicle or 1 μM PGE2 for 10 min. The levels of Ser133-phosphorylated of CREB and total CREB were determined by immunoblot analysis as previously described (Fujino et al., 2005).
Transient Transfections and Luciferase Activity Assays
Human microglia and astrocytes were seeded into 6-well plates at approximately 50% confluence. Approximately 24 h later, the cells were transiently co-transfected with 2 μg/well of the firefly luciferase reporter plasmid pGL3/CRE-Luc and 10 ng/well of Renilla luciferase reporter pRL-CMV-BActin using 5 μL FuGENE-6 in 1 mL of Opti-MEM. After 4 h, the transfection media were replaced with 2 mL growth media and the cells were incubated overnight under normal growth conditions. Transfected cultures were pretreated with either vehicle or 10 μM H-89 for 15 min, then incubated for 18 h with either vehicle or 1 μM PGE2. The cells were harvested and luciferase activity in 5 uL of each sample was measured using a Dual Luciferase Reporter Assay System as instructed by the manufacturer. The data were normalized for differences in transfection efficiency by calculating ratios of firefly luciferase scores to the corresponding Renilla luciferase values.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software. For multiple comparisons, data were analyzed by a one-way analysis of variance followed by the Newman-Keuls multiple comparison test. For paired comparisons, data were analyzed by a one-tailed Student’s t-test. The threshold for statistical significance was set at p < 0.05.
Results
PGE2 stimulation of BDNF secretion from human microglia and astrocytes
BDNF was initially identified as an up-regulated secreted product in PGE2 treated human microglia and astrocytes by screening a panel of cytokines and growth factors using an antibody array method (Figure 1A). Media supernatants from cultures of both cell lines, treated with 1 μM PGE2, produced markedly stronger BDNF signals on the arrays than did supernatants from control cultures. The signal for vascular endothelial growth factor (VEGF) was also increased in PGE2-treated cultures of both cell types, whereas, the signals for most of the seventy-seven other cytokines and growth factors tested were not different between the PGE2 treated and control supernatants (e.g., neurotrophins-3 and -4, interleukin-1β, tumor necrosis factor-α, etc.). Based on this preliminary result and the findings of Toyomoto et al. (2004) and Shaw et al. (2003) regarding prostaglandin regulation of other neurotrophins, we were interested in the potential link between PGE2 and BDNF secretion from these cells. To confirm the array findings, we used a two-site ELISA approach to determine the time- and concentration-response characteristics of PGE2-mediated BDNF regulation. When cells were treated with 1 μM PGE2 for varying lengths of time up to 48 h, BDNF accumulated in the culture media of both microglial cells and astrocytes in a time-dependent manner (Figure 1B). This effect achieved statistical significance (p < 0.05) at 24 h after dosing. Cultured microglia and astrocytes treated with increasing concentrations of PGE2 for 24 h released BDNF in a concentration dependent manner (Figure 1C). For microglia the increase in BDNF accumulation became significant in cultures treated with 0.1 μM PGE2, whereas for astrocytes significance was achieved at 0.01 μM. We observed that the basal BDNF secretion level measured in the concentration-response study (VEH control, Figure 1C) was greater than that of the basal level measured in the time course study (0 h time point, Figure 1B), whereas the corresponding astrocyte measurements were equivalent. Because the vehicle control measurements in the concentration response studies were taken from cells that were incubated for the same time period as the PGE2-treated cells, this difference suggests a higher rate of BDNF secretion in unstimulated microglia compared to astrocytes. In contrast, the 0 h time point measurements in the time course studies were taken from samples harvested at the beginning of the experiment, before the PGE2-treated cells were harvested.
EP2 receptor mRNA expression in human glial cells
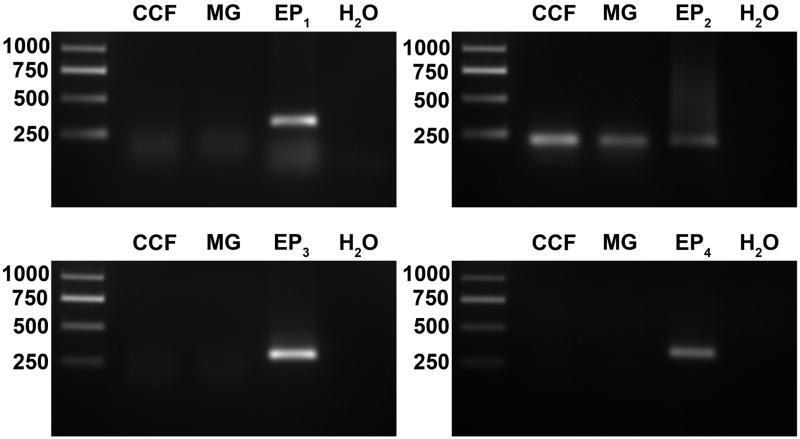
To identify the potential receptors that mediate the effect of PGE2 on BDNF release, total RNAs were extracted from cultured microglia and astrocytes and analyzed by RT-PCR. RT reaction products were amplified by PCR in reactions containing primer pairs selective for each of the human EP1, EP2, EP3 and EP4 prostanoid receptor cDNAs. Positive control reactions which used expression vectors for each of the EP receptors as template material were included for reference. The EP2 transcript was detected in both microglia and astrocytes by the observation of bands that matched the electrophoretic mobility of the EP2 reference band and were of the expected size (Figure 2). Although appropriate sized reference bands were observed for each of the EP1, EP3 and EP4 positive control PCR reactions, we did not detect the transcripts for these receptors in either the microglia or the astrocytes.
Figure 2. Cultured human microglia (MG) and astrocytes (CCF) express the EP2 prostanoid receptor mRNA transcript.
The expression of EP receptor mRNAs was evaluated by RT-PCR analysis using primer pairs selective for each of the EP receptor subtype genes. The lanes marked CCF and MG show the PCR products of reactions that included cDNA templates from astrocytes and microglia, respectively. The reference lanes (marked EP1, EP2, EP3 and EP4) shows the products of positive control reactions using plasmids containing the EP receptor gene cDNAs as templates. The negative control lane (H2O) shows the absence of products from reactions in which water was used in place of a template. Molecular size standards (bp) are shown in the left lane of each panel. Data are representative of four independent experiments.
PGE2 stimulated cyclic AMP production in human glial cells
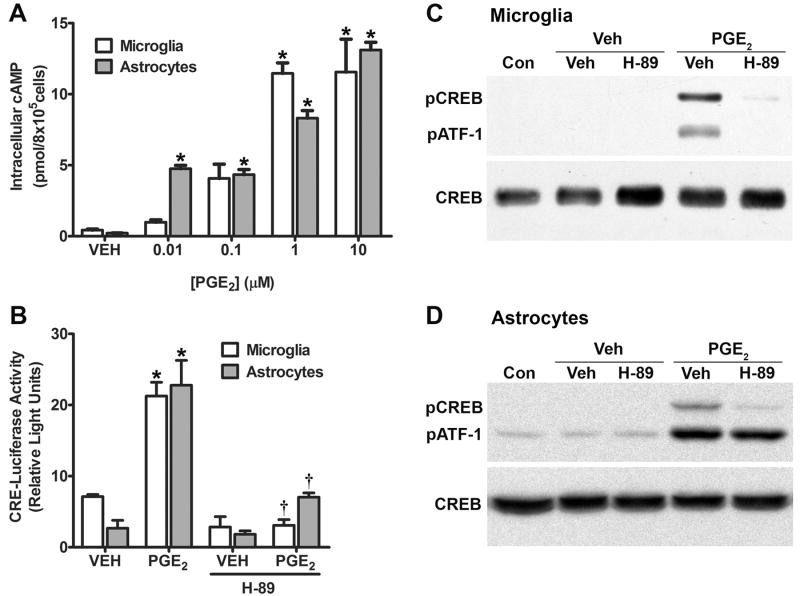
Binding of PGE2 to the Gαs-coupled human EP2 prostanoid receptor leads to the activation of adenylyl cyclase (EC 4.6.1.1) and the conversion of intracellular ATP to cyclic AMP (Regan et al. 1994a). Thus the major second messenger associated with EP2 receptor stimulation is cyclic AMP. To confirm the presence of a functional EP2 receptor in cultured human microglia and astrocytes, we treated cell cultures with increasing concentrations of PGE2 for 1 h. Lysates from these cultures were assayed for cyclic AMP content by competitive binding against a standard amount of 3H-labeled cyclic AMP in reactions containing purified PKA. In both microglia and astrocytes, we observed a concentration dependent increase in cyclic AMP in response to PGE2 stimulation (Figure 3A). In microglial cells, cyclic AMP was significantly increased over the vehicle-treated cells starting with 1 μM PGE2 (p < 0.05), whereas, in astrocytes cyclic AMP was significantly increased over the control cells starting with 0.01 μM PGE2 (p < 0.05).
Figure 3. PGE2 stimulates cyclic AMP signaling in cultured microglia and astrocytes.
(A) Activation of second messenger signaling in PGE2 treated human microglia and astrocytes was evaluated by measuring cyclic AMP production. Cells were treated with the indicated concentrations of PGE2 for 1 h. Data are presented as the mean ± SD from duplicate cultures from a representative experiment that was repeated three times. (B). Cultured human microglia and astrocytes were transiently transfected with luciferase reporter plasmids under the control of a CRE. Cells were treated with either vehicle (VEH) or 1 μM PGE2 with or without 10 μM H-89 for 16 h. Data are presented as the mean ± SD from triplicate measurements cultures from a representative experiment that was repeated three times. (C & D) The activation of CREB in PGE2 treated microglia (C) and astrocytes (D) was analyzed by immunoblotting for Ser133-phosphorylated CREB (pCREB, upper panel). Cultured human microglia were either untreated (Con) or pretreated with either vehicle (Veh) or 10 μM H-89 followed by treatment with either vehicle or 1 μM PGE2 for 10 min. The antibody we used also detects the phosphorylated form of the CREB-related protein ATF-1 (pATF-1). The membrane was stripped and reprobed with an antibody that labels total CREB (lower panels). The blots are representative of three independent experiments for both microglia (C) and astrocytes (D). * p < 0.05 compared to vehicle treatment; † p < 0.05 compared to PGE2 treatment.
PGE2 stimulated CREB/CRE signaling in human glial cells
An important function of cyclic AMP signaling is the induction of genes controlled by the cyclic AMP response element (CRE) through the sequential activation of protein kinase A (PKA, EC 2.7.11.11) and the cyclic AMP response element binding protein (CREB). Cyclic AMP-dependent signaling has also been shown to be specifically important in the regulation of BDNF expression. For instance, BDNF has been shown to be increased in rat astrocytes by treatment of with forskolin, a direct activator of adenylyl cyclase commonly used in experiments to increase intracellular cyclic AMP levels (Jurič et al., 2008). The 5’-promoter region of the BDNF gene has been shown to contain multiple CRE sequences (Tabuchi et al., 2002; Fukuchi et al., 2005) and activation of CREB increases BDNF expression in rat neurons (Tao et al., 1998). To characterize the link between EP2 receptor activation and CRE-mediated transcription, microglial and astrocyte cultures were transiently transfected with a pGL3/CRE-Luc reporter plasmid containing the CRE sequence linked to a downstream firefly luciferase reporter gene. Stimulation of these cells with 1 μM PGE2 produced a threefold increase in the luciferase activity of transfected microglia and a tenfold increase in astrocytes (Figure 3B). These effects were statistically significant in both cell lines (p < 0.05). The presence of 10 μM H-89, an inhibitor of PKA, inhibited the stimulation of CRE reporter activity by PGE2 in both microglia and astrocytes (p < 0.05). The phosphorylation of CREB at Ser133 by a variety of kinases is associated with the activation of this transcription factor. Immunoblotting with antibodies against phospho-CREB (Ser133) can, therefore, be used as a measure of CREB activation. In both microglial (Figure 3C) and astrocyte (Figure 3D) cultures treated with 1 μM PGE2, CREB phosphorylation is strongly induced after 10 min; this effect is completely blocked by pretreatment of the cells with 10 μM H-89. In contrast, these drug treatments left total CREB levels essentially unchanged in both cell lines.
Role of EP2 receptor, PKA, and adenylyl cyclase in PGE2 stimulated BDNF production in microglia
To assess the direct effects of cyclic AMP dependent signaling and EP2 receptor activation on the induction of BDNF release, we measured the secretion of BDNF in cultures of microglial cells and astrocytes treated with pharmacological agents that selectively modulate this pathway. To confirm the link between EP2 receptor activation and the secretion of BDNF, microglial cells and astrocytes were treated with 10 μM butaprost, an agonist that selectively stimulates the EP2 receptor. We chose to use this relatively high concentration because compared to PGE2, butaprost has been shown to have lower potency in activating EP2-mediated second messenger signaling (Regan et al., 1994a). Butaprost significantly enhanced the secretion of BDNF from both cell lines compared to vehicle treatment (Figure 4A; p < 0.05). Furthermore, this stimulation of BDNF secretion by butaprost involved the activation of PKA because pretreatment of the of both cell lines with 10 μM H-89 abolished butaprost stimulated BDNF secretion (p < 0.05). The same concentration of H-89 also significantly inhibits the release of BDNF induced by PGE2 in both cell lines (Figure 4B; p < 0.05). Treatment of microglia and astrocytes with 10 μM forskolin, which induces cyclic AMP production by directly activating adenylyl cylcase also significantly increased the secretion of BDNF from both cell lines compared to vehicle treated cells (Figure 4C; p < 0.5).
Discussion
Over the past several years, the complex contributions of PGE2 to the survival and death of nerve cells have been the subject of great interest. Much of this work has focused on the actions of PGE2 through the EP2 prostanoid receptor and, surprisingly, has linked the activation of this receptor to both neurotoxic and neuroprotective outcomes. Based on the known capacity of glial cells to affect neuronal survival, we became interested in the effect of PGE2 on EP2 receptor activation and the secretion of bioactive factors in cultures of these cells. Our initial studies using antibody array assays and RT-PCR showed that PGE2 induces the secretion of BDNF in cultures of immortalized microglial cells and CCF-STTG1 astrocytes (Figure 1A), and that these cells express the transcript for the EP2 receptor (Figure 2). To confirm this observation of PGE2 dependent BDNF induction, we performed detailed ELISA analyses and showed the effect of PGE2 on BDNF levels to occur in a time- (Figure 1B) and concentration- (Figure 1C) dependent manner in both cell types. Additionally, the capacity of the EP2 receptor to induce BDNF release was verified using the EP2 selective agonist butaprost (Figure 4A). These findings are consistent with published reports that show prostaglandin-stimulated release of neurotrophins from mouse astrocytes (Toyomoto et al., 2004) and studies that show correlations between PGE2 and BDNF levels (Shaw et al., 2003; Ajmone-Cat et al. 2006).
Moreover, the induction of BDNF by EP2 receptor activation constitutes a potential mechanism by which PGE2 may contribute to both neurotoxic and neuroprotective functions. Like other members of the neurotrophin family, BDNF is initially synthesized as a precursor that gives rise to the mature product through intracellular prohormone convertase-mediated cleavage in the regulated secretory pathway (Mowla et al., 1999). Whereas the precursor form of BDNF was once considered to be an inactive pro-protein, it is now understood that both it and the mature product are released from cells and capable of exerting biological effects. The functions of mature BDNF most thoroughly documented in the literature are related to the promotion of neuronal survival, differentiation and synaptogenesis through a high affinity interaction with the tyrosine receptor kinase B (TrkB) receptor. BDNF mediated activation of TrkB receptors has been shown to be important in memory, mood, behavior and the rescue of neurons threatened by injury or disease (reviewed by Binder & Scharfman, 2004). On the other hand, both the precursor and mature forms of BDNF can also bind to the low affinity p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor receptor family. In neurons lacking high affinity TrkB receptors, interactions between neurotrophins, or proneurotrophins, and the p75NTR are capable of inducing apoptosis when co-receptors such as sortilin are available (Frade et al., 1996; Teng et al., 2005). Thus, depending on variations in posttranslational BDNF processing and the receptor expression profile of neighboring neurons, BDNF is capable of promoting neuronal survival in some situations while inducing neuronal death in others. EP2 receptor mediated induction of BDNF secretion may constitute a mechanism by which the EP2 receptor may contribute to either neurotoxicity in pathological situations or neuroprotection during functional recovery.
Our analysis of the mRNA expression of the four EP receptor subtypes indicates that the EP2 receptor is expressed in both microglial and astrocyte cell lines (Figure 2). Based on our observation of PGE2 stimulated cyclic AMP production in both cell lines (Figure 3A), the Gαs-coupled EP4 receptor subtype could be expected to participate in PGE2 mediated BDNF secretion. However, mRNA encoding the EP4 prostanoid receptor was not detected in either the microglial or astrocyte cultures by our RT-PCR analysis. This is also true for the EP1 and EP3 receptor subtypes. While detection of the EP2 receptor in these cell lines are consistent with the findings of other studies on prostanoid receptor expression in human glial cells (Caggiano & Kraig, 1999; Payner et al., 2006), the absence of the other receptors is not. Thus, expression of the mRNAs for the EP1 (Caggiano & Kraig 1999) and EP3 (Kitanaka et al., 1996) receptors have been reported in different microglial cell culture systems; while EP3 (Kitanaka et al., 1996) and EP4 (Payner et al., 2006) receptor transcripts have been found in astrocyte model systems. It should be noted, however, that there is some disagreement among these studies concerning the presence of particular receptor subtypes in different types of microglial and astrocyte cultures. There is even less certainty regarding the expression of EP receptor subtypes in the native microglia and astrocytes of living animals and some evidence suggests that expression patterns in glial cells can be altered by excitotoxic and proinflammatory stimuli (Slawik et al., 2004; Waschbisch et al., 2006). Nevertheless, our findings have clearly established that functional EP2 receptors are expressed in the human glial cell lines used for our studies and that stimulation of these receptors leads to the induction of BDNF release.
Based upon the dual actions of BDNF on neuronal survival, our findings provide a mechanism by which the EP2 receptor could potentially contribute to either neurotoxicity or neuroprotection in the brain. However, identifying the conditions that determine which outcome prevails will require the use of more complex model systems that include both glial cells and neurons, such as co-culture models or organotypic brain slice cultures. Ideally, such a model system will be able to account for the diversity of the brain in terms of cell types, prostanoid receptors and neurotrophin receptors. While our results clearly link EP2 receptor activation to BNDF secretion, the aforementioned variables have the potential to modulate BDNF regulation and its ultimate effect on neuronal survival in vivo.
Activation of the EP2 prostanoid receptor is known to stimulate cyclic AMP-dependent intracellular signaling that involves the activation of PKA and consequent phosphorylation and activation of the transcription factor CREB (Regan, 2003). Activation of CREB leads to the expression of genes under the control of promoters that contain the CRE sequence, including many that block neuronal death (Dawson & Ginty, 2002). The bdnf gene is known to contain multiple CRE sequences in its 5’-upstream promoter region and expression of this gene is increased by cyclic AMP signaling and CREB activation (Tao et al., 1998; Tabuchi et al., 2002; Fukuchi et al., 2005). Our studies now show for the first time that PGE2 activates a cyclic AMP/PKA/CREB signal transduction pathway in cultured human microglia and astrocytes. In both cell lines, we observed increased CRE mediated transcriptional activity (Figure 3B) and CREB activation (Figure 3C & D) in response to PGE2 stimulation. Both of these effects were blocked by H-89 in both cell lines, implicating PKA as an effector of PGE2 stimulated signaling in these cells. Inhibition of PKA in microglia and astrocytes also blocked the secretion of BDNF induced by EP2 receptor stimulation by PGE2 (Figure 4A) and butaprost (Figure 4B). Moreover, treatment of the microglial and astrocyte cells with forskolin also increases BDNF secretion (Figure 4C), showing that direct activation of adenylyl cyclase in both cell lines is sufficient to induce BDNF release. These data support the model shown in Figure 5, in which the increased levels of secreted BDNF observed in our studies are the results of EP2 receptor mediated activation of cyclic AMP/PKA signaling leading to the phosphorylation of CREB and the transcriptional activation of the bdnf gene.
Figure 5. Model for PGE2 stimulated BDNF secretion through the EP2 prostanoid receptor in human microglia and astrocytes.
PGE2 stimulates the secretion of BDNF from both microglial and astrocyte human cell lines through the activation of the EP2 receptor and the cyclic AMP pathway. Both cell types express the mRNA for the EP2 receptor and have a functional cAMP response to PGE2 treatment. Luciferase reporter gene studies indicate that CRE-mediated transcriptional activity is induced by PGE2 in both cell types in response to PGE2. Accordingly, CREB is rapidly phosphorylated following PGE2 stimulation in a PKA-dependent manner. Signal transduction studies confirm the involvement of the EP2 receptor, cAMP signaling, and PKA activity in PGE2 stimulated BDNF secretion.
Acknowledgments
We thank Carol A. Colton (Duke University) for providing the immortalized human microglial cells. We also thank the National Institutes of Health (EY11291) and Allergan Inc. for financial support.
Abbreviations
- PGE2
Prostaglandin E2
- EP
E-type Prostanoid receptor
- COX-2
cyclooxygenase-2
- BDNF
brain-derived neurotrophic factor
- PKA
protein kinase A
- cyclic AMP
3’-5’-cyclic adenosine monophosphate
- CRE
cyclic AMP response element
- CREB
cyclic AMP response element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad AS, Zhuang H, Echeverria V, Doré S. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. Journal of Neurotrauma. 2006;23:1895–1903. doi: 10.1089/neu.2006.23.1895. [DOI] [PubMed] [Google Scholar]
- Ajmone-Cat MA, Iosif RE, Ekdahl CT, Kokaia Z, Minghetti L, Lindvall O. Prostaglandin E2 and BDNF levels in rat hippocampus are negatively correlated with status epilepticus severity: No impact on survival of seizure-generated neurons. Neurobiology of Disease. 2006;23:23–35. doi: 10.1016/j.nbd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor mediated glutamate cytotoxicity. Brain Research. 1994;663:237–243. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- Barna BP, Chou SM, Jacobs B, Raushoff RM, Hahn JF, Bay JW. Enhanced DNA synthesis of human glial cells exposed to human leukocyte products. Journal of Neuroimmunology. 1985;10:151–158. doi: 10.1016/0165-5728(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Bilak M, Wu L, Wang Q, Haughey N, Conant K, St. Hillaire C, Andreasson K. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Annals of Neurology. 2004;56:240–248. doi: 10.1002/ana.20179. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano AO, Kraig RP. Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1b production. Journal of Neurochemstry. 72:565–575. doi: 10.1046/j.1471-4159.1999.0720565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ginty DD. CREB family transcription factors inhibit neuronal suicide. Nature Medicine. 2002;8:450–451. doi: 10.1038/nm0502-450. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Clerman A, Doré S. Stimulation of PGE2 receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following β-amyloid exposure. European Journal of Neuroscience. 2005;22:2199–2206. doi: 10.1111/j.1460-9568.2005.04427.x. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodríguez-Tébar A, Barde Y-A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Molecular Pharmacology. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Journal of Biological Chemistry. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Tabuchi A, Tsuda M. Transcriptional regulation of neuronal genes and its effect on neural functions: Cumulative mRNA expression of PACAP and BDNF genes controlled by calcium and cAMP signaling in neurons. Journal of Pharmacological Sciences. 2005;98:212–218. doi: 10.1254/jphs.fmj05001x4. [DOI] [PubMed] [Google Scholar]
- Funk CD, Furci L, FitzGerald GA, Grygorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. Journal of Biological Chemistry. 1993;268:26767–26772. [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends in Immunology. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Janabi N, Peudenier S, Héron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neuroscience Letters. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- Jin J, Shie F-S, Liu J, Wang Y, Davis J, Schantz AM, Montine KS, Montine TJ, Zhang J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated α-synuclein. Journal of Neuroinflammation. 2007;4:1–10. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurič DM, Lončar D, Kržan MČ. Noradrenergic stimulation of BDNF synthesis in astrocytes: Mediation via α1-and β1/β2-adrenergic receptors. Neurochemistry International. 2008;52:297–306. doi: 10.1016/j.neuint.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Current Topics in Medicinal Chemistry. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Hashimoto H, Gotoh M, Kondo K, Sakata K, Hirasawa Y, Sawada M, Suzumura A, Marunouchi T, Matsuda T, Baba A. Expression pattern of messenger RNAs for prostanoid receptors in glial cell cultures. Brain Research. 1996;707:282–287. doi: 10.1016/0006-8993(95)01256-7. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Current Alzheimer Research. 2005;2:355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- Lee EO, Shin YJ, Chong YH. Mechanisms involved in prostaglandin E2-mediated neuroprotection against TNF-alpha: possible involvement of multiple signal transduction and beta-catenin/T-cell factor. Journal of Neuroimmunology. 2004;155:21–31. doi: 10.1016/j.jneuroim.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Lee J-M, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. Journal of Clinical Investigation. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Annals of Neurology. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. The ScientificWorldJournal. 2007;7:1329–1347. doi: 10.1100/tsw.2007.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. Journal of Neuroscience. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentz S, de Lacalle S, Baerga-Ortiz A, Knauer MF, Knauer DJ, Komives EA. Mechanism of thrombin clearance by human astrocytoma cells. Journal of Neurochemistry. 1999:980–987. doi: 10.1046/j.1471-4159.1999.0720980.x. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain disease. Journal of Neuropathology and Experimental Neurology. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi H, Petrecca K, Fawcett J, Seidah NG, Morris S, Sossin W, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. Journal of Neuroscience. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. Journal of Neurochemistry. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Payner T, Leaver HA, Knapp B, Whittle IR, Trifan OC, Miller S, Rizzo MT. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E2-dependent activation of type II protein kinase A. Molecular Cancer Therapeutics. 2006;5:1817–1826. doi: 10.1158/1535-7163.MCT-05-0548. [DOI] [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Pepperl DJ, Pierce KL, Bogardus AM, Donello JE, Fairbairn CE, Kedzie KM, Woodward DF, Gil DW. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Molecular Pharmacology. 1994a;46:213–220. [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Donello JE, Pierce KL, Pepperl DJ, Zhang D, Kedzie KM, Fairbairn CE, Bogardus AM, Woodward DF, Gil DW. Molecular cloning and expression of human EP3 receptors: Evidence of three variants with differing carboxyl termini. British Journal of Pharmacology. 1994b;112:377–385. doi: 10.1111/j.1476-5381.1994.tb13082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sciences. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. European Journal of Neuroscience. 2003;17:2438–2446. doi: 10.1046/j.1460-9568.2003.02643.x. [DOI] [PubMed] [Google Scholar]
- Shie F-S, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Slawik H, Volk B, Fiebich B, Hüll M. Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochemistry International. 2004;45:653–660. doi: 10.1016/j.neuint.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. Journal of Biological Chemistry. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- Takadera T, Shiraishi Y, Ohyashiki T. Prostaglandin E2 induced caspase-dependent apoptosis possibly through activation of EP2 receptors in cultured hippocampal neurons. Neurochemistry International. 2004;45:713–719. doi: 10.1016/j.neuint.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-apartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sciences. 2006;78:1878–1883. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. Journal of Neuroscience. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomoto M, Ohta M, Okumura K, Yano H, Matsumoto K, Inoue S, Hayashi K, Ikeda K. Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS Letters. 2004;562:211–215. doi: 10.1016/S0014-5793(04)00246-7. [DOI] [PubMed] [Google Scholar]
- Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, Sui DX, Regan JW, Smith WL. Enzymes and receptors of prostaglandin pathways with arachidonic acid-vs. eicosapentaenoic acid-derived substrates and products. Journal of Biological Chemistry. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- Waschbisch A, Fiebich BL, Akundi RS, Schmitz ML, Hoozemans JJM, Candelario-Jalil E, Virtainen N, Veerhuis R, Slawik H, Yrjänheikki J, Hüll M. Interleukin-1 beta-induced expression of the prostaglandin E2-receptor subtype EP3 in U373 astrocytoma cells dependes on protein kinase C and nuclear factor-kappaB. Journal of Neurochemistry. 2006;96:680–693. doi: 10.1111/j.1471-4159.2005.03599.x. [DOI] [PubMed] [Google Scholar]