Abstract
New approaches for cardiac repair have been enabled by the discovery that the heart contains its own reservoir of stem cells. In Part 1 of this review, we discussed various cardiac stem cell populations, reviewed our own work on cardiosphere-derived cells from human hearts, and outlined large animal preclinical models testing the regenerative potential of cardiac stem cells. Here we continue with a discussion on other adult stem cell sources with clinical potential. We summarize the critical safety issues associated with stem cell therapy and present the possible proarrhythmic and antiarrhythmic effects of stem cell transplantation. We discuss the outcomes of clinical stem cell trials and identify the technical, ethical, and practical issues facing the clinical application of cardiac stem cells.
Keywords: Cardiac stem cells, Myocardial infarction, Myocardial regeneration, Arrhythmia
Introduction
Heart disease, encompassing cardiomyopathies, acute ischemic syndromes, chronic heart failure, arrhythmias, and sudden cardiac death, is the number one killer in Western society.1 Current treatment options for patients living with heart disease include lifestyle changes and drug regimens to prevent disease advancement, angioplasty and stenting to open blocked coronary arteries, bypass surgery to restore blood flow to a compromised portion of the heart, pacemakers and implantable cardioverter defibrillators to regulate heart rhythms, and left ventricular assist devices and heart transplantation to aid or replace a failing organ. Progression of heart disease generally involves loss of myocardium by necrosis or apoptosis, formation of scar tissue, and remodeling of the remaining tissue. Cell therapy is rapidly advancing as a treatment option, in the midst of some concern within the scientific community that this may be occurring too quickly and too uncritically. Cell therapies, if implemented, should improve heart function, create healthy cardiac muscle and vasculature, not create tumors (a very real possibility that has been observed after the delivery of undifferentiated embryonic stem cells [ESCs] to the heart),2 not induce arrhythmias (a well-documented risk of skeletal muscle myoblast [SKMM] delivery to the heart),3-5 not elicit an immune response (an issue also associated with undifferentiated ESC delivery),2 and circumvent societal ethical concerns. An adult-derived autologous cell therapy would minimize many of these general concerns.
We have limited the discussion herein to adult cell sources readily translatable for clinical application. Remarkable advances are also being made generating pluripotent embryonic-like stem cells from somatic cells by viral expression of specific ESC-related genes6-9 and by somatic cell nuclear transfer most recently successful in primates.10 These techniques make the possibility of patient-specific ESCs an imaginable reality. Safety issues regarding the use of viral vectors, the sustained activation or reactivation of particular genes, and the potential for immune rejection caused by the presence of allogeneic mitochondrial DNA after nuclear transfer limit their current applications. Recent success has also been reported using cardiomyocyte-differentiated human ESCs for myocardial repair.11-13 Directed differentiation (and a pro-survival cocktail in one study11) allowed for efficient engraftment of purified ESC-derived cardiomyocytes and an improvement in global heart function in an infarcted rat model without the errant formation of teratomas. Meanwhile, several populations of adult-derived, minimally manipulated, autologous stem cells have established a promising record as therapeutic agents for heart disease. These populations are reviewed here.
Adult stem cell populations with clinical potential
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) contain multiple functionally and phenotypically distinct populations. Mouse HSCs are often defined as c-Kit+Sca-1+Lin− cells capable of giving rise to all blood lineages.14 Mouse HSCs manifested inwardly rectifying potassium currents when co-cultured with neonatal cardiomyocytes, indicative of partial functional cardiogenic differentiation.15 HSCs have a hotly debated ability to transdifferentiate into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Positive results have been presented in animal models after direct cell delivery to the myocardium16-18 or after cell homing to the heart after injury or cytokine treatment.19,20 Negative results show complete lack of transdifferentiation to the cardiac lineages21,22 or acquisition of cardiac phenotypes only via cell fusion.23 Human HSCs can traditionally be defined as rare CD34+ cells capable of reconstituting all blood lineages.24 CD34+ human HSCs have a demonstrated ability to form new cardiomyocytes, endothelial cells, and smooth muscle cells in vivo via transdifferentiation and cell fusion (assessed by detection of human X chromosomes in transplanted mice).25,26 Evidence of extracardiac progenitor cells forming cardiomyocytes, endothelial cells, and smooth muscle cells in the transplanted human heart also exists (identified by expression of the Y chromosome in male patients receiving a female heart), although the extent of formation of each of those cell types is again highly varied and controversial (estimates range from 0.04% to 10% of cardiomyocytes and up to 24% of endothelial cells).27-31 HSCs, as part of a total bone marrow mononuclear cell (MNC) fraction, have been shown to improve angiogenesis and enhance regional heart function in large animal models of acute32 and chronic myocardial infarction (MI).33,34
Endothelial progenitor cells
Circulating endothelial progenitor cells (EPCs), originally defined as mobilized CD34-expressing cells,35 are now often defined as CD133+VEGFR-2+ cells or functionally defined as cells capable of neovascularization.36 Human EPCs isolated from peripheral blood were shown to adopt biochemical and functional features (calcium transients and gap junction–mediated dye transfer) of cardiomyocytes after co-culture with neonatal cardiomyocytes.37 The therapeutic potential of human CD34+ EPCs has also been shown in models of acute MI, in which cell administration resulted in vasculogenesis, angiogenesis, an enhancement of cardiomyocyte survival in the at-risk area, cardiomyogenesis via transdifferentiation and cell fusion, and an improvement in multiple measures of heart function.38-40 One study even noted a dose-dependent response in all of these parameters.40 Human CD34+ EPCs similarly resulted in improved capillary density and heart function in a model of chronic MI when compared with a CD34− fraction of circulating MNCs.41 Delivery of autologous CD31+ EPCs (for lack of a CD34 porcine antibody) in a porcine model of chronic MI also yielded the same beneficial effects.41 A direct comparison of CD34-selected EPCs and an unfractionated population of total circulating MNCs, showed a higher therapeutic potential in the CD34+ fraction,42 providing the first data in support of MNC selection for applications of myocardial repair. The investigators showed increased potency of CD34+ EPCs compared with a low dose of total MNCs (consisting of the same number of cells as in the CD34+ group), and show a detrimental effect of a high dose of total MNCs (containing the same number of CD34+ cells as in the CD34+ group). The animals receiving the high-dose total MNCs had a higher incidence of moderate to severe hemorrhagic infarction, a sign of irreversible damage, and histology revealed high numbers of CD45+ human cells in these animals (presumably undifferentiated hematopoietic cells or inflammatory cells).
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) can be roughly defined as CD105+ CD90+ cells, isolated by preferential adherence in tissue culture, that are capable of osteogenic, chondrogenic, adipogenic, and stromal differentiation.43 Human MSCs isolated from umbilical cord blood or bone marrow have been shown to acquire some biochemical features of cardiomyocytes after treatment with 5-azacytidine44,45 or cultivation in a defined medium containing dexamethasone,46 and to adopt features of endothelial cells after exposure to vascular endothelial growth factor.47 MSCs have shown promise as therapeutic agents for heart disease in animal models. Administration of MSCs has resulted in angiogenesis, cardiomyogenesis, and functional improvement in acute48-50 and chronic infarction models51 and models of dilated cardiomyopathy,52 although the effects have been reported to be transient in some studies,49 and direct regeneration does not seem to be the primary therapeutic effect (paracrine effects on cardiac stem cells [CSCs] and existing myocardium are proposed mechanisms).53 One recent study showed the formation of bone in the heart (in 22 of 43 mice) after MSC delivery,54 highlighting a potential risk of MSC therapy that awaits further exploration. MSCs do have the putative advantage of being immune-privileged55 (and actively immunosuppressive), such that they can be used in allogeneic applications.
Skeletal muscle myoblasts
Organ-specific stem cells with explicit myogenic potential are also being explored for treatment of heart disease. SKMMs, skeletal muscle precursors, have been shown to improve heart function in animal models of ischemia-reperfusion56 and chronic failure.57 Early animal studies did not reveal the tendency of SKMMs to induce lethal arrhythmias such as sustained ventricular tachycardia (VT) or ventricular fibrillation, but did show that SKMM-derived muscle grafts remained functionally isolated from host myocardium.58 An in vitro co-culture model has since been able to reproduce the sustained reentrant arrhythmias observed in patients after SKMM delivery.59 This study showed that expression of connexin-43 in SKMMs, a protein involved in the formation of gap junctions that is naturally lacking in SKMMs, could reduce the incidence of arrhythmias in the co-culture system. Subsequently, a mouse infarct model, in which VT was inducible but not sustained in approximately 96% of control mice, showed a significant increase in susceptibility to sustained arrhythmias in animals treated with SKMMs (approximately 94% inducible and approximately 25% sustained) and a protection against inducibility in animals treated with connexin-43–expressing SKMMs (approximately 38% inducible) or with cardiomyocyte-differentiated ESCs.60 These studies show the importance of electrical coupling in maintaining electrical stability.
Potential complications of stem cell therapy
Arrhythmogenicity
As exemplified by the foregoing discussion of skeletal myoblasts, the creation or exacerbation of arrhythmias after cell therapy is a major concern. Figure 1 shows schematically the various mechanisms whereby stem cell transplantation may influence cardiac electrical stability. The basic cellular arrhythmogenic mechanisms (reentry, abnormal automaticity, and triggered activity) are highlighted within rectangles in the center; the primary effects of cell transplantation appear within highlighted ovals. As the consequences of cell transplantation interact with the basic arrhythmogenic mechanisms, net effects may either be proarrhythmic or antiarrhythmic. Consider the generation of new cardiac tissue strands (top oval, Fig. 1) as a case in point. Such strands, if tenuous and poorly coupled (as they might be in the process of maturation), may create pathways for slow conduction, thus favoring reentry. On the other hand, new, robust, well-coupled myocardial tissue will improve conduction and augment pump function, providing an antiarrhythmic benefit. Likewise, the local secretion of growth factors by stem cells (paracrine effects) will have trophic effects on surrounding myocardium that are likely to suppress rather than favor arrhythmias. Nevertheless, the emphasis here will be on potential proarrhythmic properties of transplanted cells.
Figure 1.
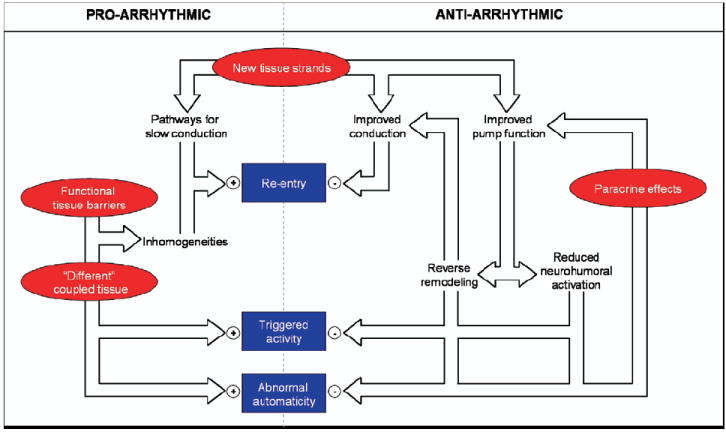
Summary of the interrelated potential effects of stem cell transplantation and how they might contribute to maintaining a proarrhythmic or antiarrhythmic state.
Poor cell–cell coupling (as is the case for unmodified SKMMs), incomplete differentiation of engrafted cells, or a heterogenous distribution of action potential durations in neighboring cardiomyocytes (new and old) could all play a role in generating arrhythmias in vivo. (These potential effects are depicted on the left-hand side of Fig. 1, as functional tissue barriers or different coupled tissue, the latter signifying electrophysiological differences between transplanted and native cells.) Several populations of undifferentiated adult bone marrow–derived stem cell populations (HSCs and MSCs) have been characterized electrophysiologically and have been shown to maintain a relatively depolarized resting membrane potential of −20 mV to −40 mV and express outward calcium and voltage-activated potassium currents, whereas inward sodium, calcium, and inwardly rectifying potassium currents are observed less frequently.15,61,62 This is, of course, in stark contrast to the electrophysiological profile of adult cardiomyocytes, in which large sodium currents drive the rapid firing of action potentials, tightly regulated calcium flux carried primarily by an inward current is crucial to whole-cell electrical and mechanical balance, and an inwardly rectifying potassium current helps maintain a highly negative resting membrane potential near −80 mV in ventricular cells. Transplanted to the damaged heart in an undifferentiated state, stem cells could create islands of electrical heterogeneity if they couple to surrounding cardiomyocytes. An in vitro model successfully produced such a situation when MSCs were co-cultured with neonatal cardiomyocytes.63 At a minimum critical concentration (≥10%), MSCs present in the co-culture reduced wavefront conduction velocity and significantly increased the likelihood of inducing reentrant arrhythmias by rapid pacing. Large focal islands of MSCs present in the co-culture produced delayed activation and repolarization within the island itself that served to anchor spiral wave reentrant arrhythmias. These findings were attributed to the fact that although MSCs do express connexin-43 and can couple to neonatal cardiomyocytes in co-culture, the MSCs remain largely undifferentiated, acting as inexcitable current sinks. This proarrhythmic tendency has been supported by one large animal study in which pigs were given MSCs intravenously and showed shortened epicardial effective refractory periods at all pacing cycle lengths (which combined with an increased dispersion of repolarization can lead to arrhythmias) 3 months after treatment compared with control animals.64 Therefore, careful vigilance for arrhythmias should certainly be a feature of all large-animal preclinical and early-stage clinical stem cell studies.
If transplanted stem cells do couple and do undergo cardiomyogenic differentiation in vivo, stem cell–derived cardiomyocytes might be expected to display a range of electrophysiological phenotypes from embryonic, to neonatal, to adolescent, to adult as the process of differentiation occurs and presumably parallels normal cardiomyocyte development. A gradual and continual course of adult stem cell differentiation is supported in one in vitro study in which a time-dependent increase in connexin-43 expression, an increase in conduction velocity, and a decrease in resting membrane potential was shown in MSCs existing in culture with neonatal cardiomyocytes.65 A recent study in the adolescent feline characterized small, proliferative, mononucleated cardiomyocytes as having T-type calcium currents reminiscent of neonatal cardiomyocytes, as well as prolonged calcium transients and reduced transient outward potassium currents compared to large, binucleated cardiomyocytes,66 thus providing important electrophysiological information regarding normal mammalian cardiomyocyte maturation and insight into what we might expect of adult stem cells as they undergo cardiomyogenic differentiation. Differences (relative to a normal adult cardiomyocyte phenotype) in transient and repolarizing potassium currents and calcium handling will lead to differences in action potential duration in neighboring cells, which can predispose the heart to reentrant and triggered activity, which can lead ultimately to VT. Certainly, new stem cell–derived cardiomyocytes are a potential source of electrical mismatch or heterogeneity. Most reports of in vivo CSC-derived cardiomyocytes show small (often mononucleated) new cardiomyocytes within the infarct.67-70 No study has yet examined the electrophysiological properties of in vivo CSC-derived cardiomyocytes, but given their morphological appearance, we might expect them to have a neonatal-like profile. CSCs have recently been shown to be capable of coupling to host cardiomyocytes (evidenced by synchronous calcium transients) when examined 3 weeks after delivery of cells into the infarct area.71 Therefore, careful assessment of the proarrhythmic potential of CSCs is advisable as this field moves forward.
It is imaginable that stem cells might impart an antiarrhythmic effect in the setting of MI. The VT mouse study discussed above not only illustrates the fundamental importance of electrical coupling, but also shows that when a delivered stem cell population is well coupled within the heart, electrical stability can result.60 Ex vivo optical recordings of calcium transients showed that activation proceeded across the infarct region of animals treated with cardiomyocyte-differentiated ESCs in contrast to control mice, in which calcium waves largely bypassed the infarct. Notably, this occurred even in animals whose stem cell grafts were physically isolated from the native myocardium. Activation was carried across infarct areas composed presumably of myofibroblasts and perhaps endogenous CSCs undergoing differentiation and successfully transferred to the stem cell graft. Conduction delays did exist and uncoupled activity originating from the stem cell grafts was observed, but these factors did not contribute to an increased incidence of arrhythmia in this model. In fact, whole-heart optical mapping studies showed a decrease in incidence of conduction block at the infarct border zone, a decrease in incidence of ectopic activity (approximately 38% of control mice vs approximately 14% of treated mice), and a decrease in the incidence of reentrant activity inducible by a premature ventricular stimulus in animals receiving cardiomyocyte-differentiated ESCs. In addition to creating new and improved pathways for conduction, transplanted stem cells might exert numerous paracrine effects that allow for improved pump function and directly effect arrhythmogenesis. A curious and unexpected benefit observed at a 6-month midpoint in a small phase I MSC clinical trial was a decrease in incidence of arrhythmia in patients receiving cells compared with those receiving a placebo,72 although the small sample size undermines the conclusions. More importantly, no excessive adverse arrhythmic events or sudden deaths have occurred in the hundreds of patients treated to date with bone marrow MNCs.73
Cell fusion
The occurrence of cell fusion would suggest that the delivered cells are incapable of true cardiogenic differentiation and speaks to the plasticity of a cell source. Cell fusion has been implicated in the formation of hepatocytes and Purkinje neurons by HSCs,74,75 but cell fusion in general is not inherently detrimental. An in vitro model of cell fusion between human ESCs and fibroblasts revealed an ability of hybrid somatic cells to undergo genomic reprogramming and adopt a human ESC phenotype.76 Hybrid cells showed a level of plasticity associated with ESCs, offering insight into what might be a unique means of generating progenitors. Fusion with noncardiomyocytes (including fibroblasts, endothelial cells, and HSCs) has also been shown to induce cardiomyocytes to reenter the cell cycle in vitro and in vivo.77,78 The mechanism of HSC cell fusion in the heart is only beginning to be understood. Recent evidence implicates α4β1 integrin and vascular endothelial cell adhesion molecule-1 interactions that ultimately lead to cardiomyocyte proliferation in vivo.78 Interestingly, HSC-derived endothelial cells in the same study were not the result of cell fusion, hinting toward cell-specific differences. Although the mechanisms of cell fusion are beginning to be understood, very little is known regarding the long-term consequences of stem cell fusion with a myocardial cell in vivo.79 It is the unknown that makes this a less-than-desirable occurrence.
Clinical stem cell trials
Concurrent with ongoing efforts to elucidate the mechanisms underlying their therapeutic benefits and drawbacks, and to identify potent subpopulations, autologous blood-derived and bone marrow–derived stem cells and SKMMs isolated from thigh biopsies are being tested in clinical trials. The trajectory of SKMMs is instructive and argues against premature enthusiasm. Several early small trials delivering SKMMs to patients with ischemic cardiomyopathy showed a long-term functional benefit, albeit with a high incidence of arrhythmia3,4,80 (a problem that does not seem to be associated with delivery of blood and bone marrow–derived cells).73 Preliminary results from the first placebo-controlled SKMM trial Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) showed a trend toward improved remodeling at 6 months81; however, this trial was recently discontinued for lack of efficacy apparent in an interim analysis.81,82 Other early studies have examined the safety and efficacy of unfractionated bone marrow and circulating MNCs as opposed to selected HSCs or EPCs. Delivery of autologous bone marrow or circulating MNCs was associated with favorable left ventricular remodeling over a 1-year follow-up period in one early study Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI),83 establishing some evidence of safety and potential efficacy. Follow-up studies comparing delivery of bone marrow MNCs with a placebo control group have yielded varied results. One study Reinfusion of Enriched Progenitor Cells and Infarct Remodelling in Acute Myocardial Infarction (REPAIR-AMI) showed a modest improvement in left ventricular ejection fraction in a 1-year follow-up,84 whereas a similarly designed study Autologous Stem Cell Transplantation in Acute Myocardial Infarction (ASTAMI) showed no functional benefit of treatment,85 a difference that has since been attributed to the quality of the cells delivered as measured by an in vitro cell migration assay.86 A separate study Bone Marrow Transfer to Enhance ST-elevation Infarct Regeneration (BOOST) examined patients after 1.5 years and concluded that the functional benefit seen at 6 months87 was not sustainable for 18 months.88 Overall clinical outcomes have been positive, although primary end points have not always been met. An excellent safety profile has been established, whereas sustained functional benefits remain in doubt. Emerging data from a small phase 1 MSC clinical trial (Prochymal) hint at an improvement in heart and lung function in treated patients compared with a placebo control group at a 6-month midpoint.72 Promising results have also been reported with transplantation of bone marrow MNCs in small trials of patients with chronic cardiomyopathy.1,89-92
A recent meta-analysis of 10 clinical trials involving patients with acute MI who received intracoronary infusion of MNCs evaluated the overall benefit of cell therapy in the setting of acute MI.93 This examination considered 698 patients receiving cells within 14 days of acute MI and revealed multiple modest benefits of cell therapy. Patients receiving cell therapy had an improvement of left ventricular ejection fraction by 3.0% compared with placebo-treated patients. Infarct size and end-systolic volume were significantly reduced in cell-treated patients by 5.6% and 7.4 ml, respectively. Incidence of recurrent acute MI was also significantly reduced, although low in all studies regardless. left ventricular ejection fraction and injected cell volume tended to a positive correlation (P = .066), suggestive of a dose-response relationship. Cell therapy in general is proving to be a viable means of improving overall heart function, although benefits to date have been small and difficult to reproduce. A stem cell source with a high propensity to regenerate myocardium, directly and indirectly, with minimal associated risks, might increase the benefits to patients.
Issues facing the clinical application of CSCs
In Part 1 of this review, we discussed data that show that CSCs isolated from the heart and delivered to ischemic areas will regenerate the heart and maintain global cardiac function in small and large animal models. Furthermore, we presented evidence that CSC can be isolated from adult patients and expanded to theoretically significant numbers ex vivo. CSCs are a logical cell source to use in the treatment of heart disease. We stop now to consider the issues that the scientific community should ask themselves immediately, as the clinical application of CSCs is on the visible horizon.
Do we understand enough about the basic biology of CSCs to utilize them for cardiac repair?
On the one hand, we do not even understand the basic relationships among the populations of CSCs described by different groups in different species (summarized in Part 1 of this review). We know virtually nothing about the biological function of each of the reported markers in terms of CSC self-renewal and differentiation. Remarkable advances are being made identifying cytokines to which CSCs respond (reviewed by Torella et al94), target genes whose altered expression enhances cell engraftment and performance (reviewed by Penn et al95), and the normal and pathological responses of resident CSCs to various disease states (reviewed by Ellison et al96). We know really very little about the undoubtedly many effects of transplanted CSCs in the postinfarct environment. Our ignorance becomes even more evident when we start quantifying the number of cells that actually survive our delivery procedures and the number of cells that actually do differentiate, both of which can be extremely low even in cases in which a functional benefit is observed. The successful “kitchen sink” approach taken to enhance the survival of ESCs recently accurately reflects the state of the art in terms of improving engraftment.11 Further basic understanding will eventually allow us to enhance the processes that already occur naturally in and with these cells. On the other hand, each of the various purported CSC populations is being defined by a functional capacity for repair, albeit incomplete repair, in animal models. We know that these cells are capable of forming new muscle and vessels, even if we know little about the cues that initiate the differentiation process. Risks of unknown effects are compounded the more the cells are manipulated before clinical use. Thus, the use of autologous, minimally manipulated cells (i.e., cells that have not been genetically altered or antigenically selected) may be advisable in early clinical studies.
Have the prior and ongoing clinical studies aimed at repairing the heart with various stem cell populations established a path worth following?
On the whole, stem cell therapy is producing a modest beneficial effect in treated patients, with a generally favorable safety profile. Modest benefits are certainly not the eventual goal: we seek nothing less than complete repair and regeneration. Several avenues are available now thanks to the knowledge already gained. We can abandon pursuit of stem cell therapy altogether, although trends in the right direction in patients, and the often remarkable benefits seen in animal models, would make it hard not to look back and wonder “what if.” We can change fundamental aspects about our approach: patient cohort, cell processing methods, cell delivery methods, cell sources. In this review, we have put forth the case that CSCs might be a more effective cell source compared with others previously tested, but this remains to be proven.
Are the risks of arrhythmia sufficiently severe as to delay clinical trials?
There are two ways to approach this tricky question, the first being empirical. The overwhelming evidence from studies of EPCs and bone marrow MNCs in, collectively, thousand of patients worldwide has not shown any clinical evidence of enhanced arrhythmogenesis. If anything, adverse events, including sudden death, have tended to decrease in cell-treated patients relative to control patients. Even in the case of skeletal myoblasts, where a notorious increase in ventricular arrhythmias was seen in some early uncontrolled clinical studies,3,4,80 such an effect was not the reason for cessation of the only prospective randomized clinical trial of such cells (the MAGIC trial). Instead, that study was apparently halted prematurely for an expected failure to reach prespecified efficacy end points.81,82 The second way to approach the arrhythmia question is from first principles. Figure 1 shows that cell transplantation can be predicted to have a multitude of electrical effects, some potentially destabilizing, but others clearly beneficial. Given the reality that cell therapy studies have begun and will proceed, no matter what naysayers may advocate, we propose that a balanced ethical pathway of thorough preclinical safety assessments precede any clinical studies. Furthermore, such clinical studies should be designed with arrhythmias in mind as a central safety consideration in the case of cell transplantation into the heart. We must do our best to protect patients, and we must proceed carefully, but we cannot withhold from society the potentially revolutionary benefits of regenerative therapeutics.
Acknowledgments
Supported by the National Institutes of Health (Dr. Marbán), the Donald W. Reynolds Foundation (Dr. Marbán), and the Pasteur-Foundation Cenci Bolognetti to the Department of Experimental Medicine, University of Rome La Sapienza (Dr. Giacomello). Capricor has licensed cardiac stem cell technology from Johns Hopkins University and the University of Rome. Dr. Smith is an employee of Capricor. Dr. Marbán holds equity in Capricor. Capricor has provided no funding for any research.
References
- 1.Bolli R, Jneid H, Dawn B. Bone marrow cell-mediated cardiac regeneration a veritable revolution. J Am Coll Cardiol. 2005;46:1659–1661. doi: 10.1016/j.jacc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 3.Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 4.Siminiak T, Kalawski R, Fiszer D, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Smits PC, van Geuns RJ, Poldermans D, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 10.Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 11.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 12.Dai W, Field LJ, Rubart M, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–516. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspi O, Huber I, Kehat I, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 15.Lagostena L, Avitabile D, De Falco E, et al. Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes. Cardiovasc Res. 2005;66:482–492. doi: 10.1016/j.cardiores.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 17.Kajstura J, Rota M, Whang B, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 18.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawn B, Guo Y, Rezazadeh A, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 22.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 23.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 24.Civin CI, Gore SD. Antigenic analysis of hematopoiesis: a review. J Hematother. 1993;2:137–144. doi: 10.1089/scd.1.1993.2.137. [DOI] [PubMed] [Google Scholar]
- 25.Yeh ET, Zhang S, Wu HD, et al. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Wang D, Estrov Z, et al. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 27.Hruban RH, Long PP, Perlman EJ, et al. Fluorescence in situ hybridization for the Y-chromosome can be used to detect cells of recipient origin in allografted hearts following cardiac transplantation. Am J Pathol. 1993;142:975–980. [PMC free article] [PubMed] [Google Scholar]
- 28.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 29.Laflamme MA, Myerson D, Saffitz JE, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 30.Glaser R, Lu MM, Narula N, et al. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–19. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 31.Minami E, Laflamme MA, Saffitz JE, et al. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 32.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 33.Tomita S, Mickle DA, Weisel RD, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 34.Waksman R, Fournadjiev J, Baffour R, et al. Transepicardial autologous bone marrow-derived mononuclear cell therapy in a porcine model of chronically infarcted myocardium. Cardiovasc Radiat Med. 2004;5:125–131. doi: 10.1016/j.carrad.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Badorff C, Brandes RP, Popp R, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 38.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 39.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 41.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 42.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 44.Kadivar M, Khatami S, Mortazavi Y, et al. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Zhang X, Qian H, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 46.Shim WS, Jiang S, Wong P, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 47.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 48.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 49.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 50.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makkar RR, Price MJ, Lill M, et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–233. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 52.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 53.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 54.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 56.Jain M, DerSimonian H, Brenner DA, et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. doi: 10.1161/01.cir.103.14.1920. [DOI] [PubMed] [Google Scholar]
- 57.He KL, Yi GH, Sherman W, et al. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant. 2005;24:1940–1949. doi: 10.1016/j.healun.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 58.Leobon B, Garcin I, Menasche P, et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 60.Roell W, Lewalter T, Sasse P, et al. Engraftment of connexin-43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 61.Heubach JF, Graf EM, Leutheuser J, et al. Electrophysiological properties of human mesenchymal stem cells. J Physiol. 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li GR, Sun H, Deng X, et al. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells. 2005;23:371–382. doi: 10.1634/stemcells.2004-0213. [DOI] [PubMed] [Google Scholar]
- 63.Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832–1841. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 64.Price MJ, Chou CC, Frantzen M, et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–239. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 65.Pijnappels DA, Schalij MJ, van Tuyn J, et al. Progressive increase in conduction velocity across human mesenchymal stem cells is mediated by enhanced electrical coupling. Cardiovasc Res. 2006;72:282–291. doi: 10.1016/j.cardiores.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Wilson RM, Kubo H, et al. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 67.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 68.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 69.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiospherederived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 70.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osiris Therapeutics Announces Positive Results in Groundbreaking Stem Cell Trial to Treat Heart Disease. [3/25/07]; Available at: http://investor.osiris.com/releasedetail.cfm?ReleaseID=235227.
- 73.Katritsis DG, Sotiropoulou P, Giazitzoglou E, et al. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9:167–171. doi: 10.1093/europace/eul184. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 75.Weimann JM, Johansson CB, Trejo A, et al. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- 76.Cowan CA, Atienza J, Melton DA, et al. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 77.Matsuura K, Wada H, Nagai T, et al. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–363. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S, Shpall E, Willerson JT, et al. Fusion of human hematopoietic progenitor cells and murine cardiomyocytes is mediated by alpha 4 beta 1 integrin/vascular cell adhesion molecule-1 interaction. Circ Res. 2007;100:693–702. doi: 10.1161/01.RES.0000260803.98329.1c. [DOI] [PubMed] [Google Scholar]
- 79.Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369–373. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 80.Hagege AA, Marolleau JP, Vilquin JT, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114(Suppl):I108–113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 81.Menasche P. First randomized placebo-controlled Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial. Circulation. 2006;114:2426. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 82.MG Biotherapeutics, LLC. Clinical MAGIC Results. [May 7, 2008];2007 Available at: http://www.mgbiotherapeutics.com/research/MAGIC/mgb_en_p_rd_magicresults.asp.
- 83.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 85.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 86.Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 87.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 88.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 89.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 90.Perin EC, Dohmann HF, Borojevic R, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110(Suppl 1):II213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 91.Dohmann HF, Perin EC, Takiya CM, et al. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: postmortem anatomicopathologic and immunohistochemical findings. Circulation. 2005;112:521–526. doi: 10.1161/CIRCULATIONAHA.104.499178. [DOI] [PubMed] [Google Scholar]
- 92.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 93.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 94.Torella D, Ellison GM, Karakikes I, et al. Growth-factor-mediated cardiac stem cell activation in myocardial regeneration. Nat Clin Pract. 2007;4(Suppl 1):S46–51. doi: 10.1038/ncpcardio0772. [DOI] [PubMed] [Google Scholar]
- 95.Penn MS. Cell-based gene therapy for the prevention and treatment of cardiac dysfunction. Nat Clin Pract. 2007;4(Suppl 1):S83–88. doi: 10.1038/ncpcardio0733. [DOI] [PubMed] [Google Scholar]
- 96.Ellison GM, Torella D, Karakikes I, et al. Myocyte death and renewal: modern concepts of cardiac cellular homeostasis. Nat Clin Pract. 2007;4(Suppl 1):S52–59. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]


