Abstract
Cardiosphere-derived resident cardiac stem cells (CDCs) are readily isolated from adult hearts and confer functional benefit in animal models of heart failure. To study cardiogenic differentiation in CDCs, we developed a method to genetically label and selectively enrich for cells that have acquired a cardiac phenotype. Lentiviral vectors achieved significantly higher transduction efficiencies in CDCs than any of the nine adeno-associated viral (AAV) serotypes tested. To define the most suitable vector system for reporting cardiogenic differentiation, we compared the cell specificity of five commonly-used cardiac-specific promoters in the context of lentiviral vectors. The promoter of the cardiac sodium-calcium exchanger (NCX1) conveyed the highest degree of cardiac specificity, as assessed by transducing seven cell types with each vector and measuring fluorescence intensity by flow cytometry. NCX1-GFP-positive CDC subpopulations, demonstrating prolonged expression of a variety of cardiac markers, could be isolated and expanded in vitro. Finally, we used chemical biology to validate that lentiviral vectors bearing the cardiac NCX1-promoter can serve as a highly accurate biosensor of cardiogenic small molecules in stem cells. The ability to accurately report cardiac fate and selectively enrich for cardiomyocytes and their precursors has important implications for drug discovery and the development of cell-based therapies.
INTRODUCTION
Cellular (or cell-based) therapy has emerged as a potential new therapeutic option for regenerating the infarcted heart. So far, many different cell types, including cardiac stem cells (CDCs), skeletal myoblasts and bone marrow mononuclear cells have been shown to have beneficial effects in various animal models of heart failure1–4 or in clinical trials.5,6 Cardiosphere-derived adult CDCs have received particular attention, as they can be readily isolated even from small human endomyocardial biopsy specimens and promote cardiac regeneration in the mammalian heart.1,2 Sustained enhancement of myocardial pump function would be facilitated by maximizing differentiation of the transplanted cells into functional cardiomyocytes. Therefore, specific isolation of cardiomyocyte precursors should help to improve any given cellular therapy for myocardial repair. Cardiomyocytes and their precursors can be enriched by transducing stem cells with vectors encoding for selectable markers under the control of a cardiomyocyte-specific promoter.7,8 Lentiviral and adeno-associated viral (AAV) vectors offer many advantages over other vector systems, including low immunogenicity and stable gene transfer,9 making them promising tools for gene transfer to stem cells. However, given the potential interactions between heterologous promoters and cis-sequences of the viral vector, it cannot be predicted which cell-specific promoter performs best in the context of viral vectors. Therefore, the aim of this study was two fold: first, we sought to define which viral vectors (AAV versus lentivirus) transduce human CDCs more efficiently and compared the myocardial specificity of five commonly used cardiac promoters in the context of viral vectors in vitro and in vivo. Second, we tested the utility of this vector system to report the cardiogenic phenotype in CDCs, both under baseline conditions and when induced to differentiate by exposure to synthetic small molecules.
RESULTS
Lentiviral vectors confer higher transduction efficiencies and long-term expression in CDCs compared to AAV vectors
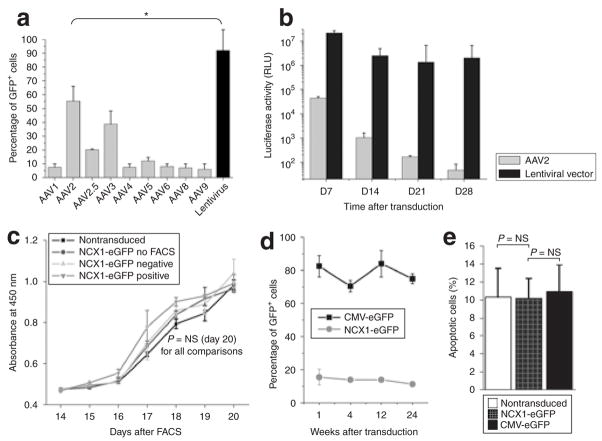
Lentiviral vectors proved to be the most effective of all viral vectors tested, concurrent with low levels of cell toxicity. Using a multiplicity of infection (MOI) of ~35, transduction efficiencies of >90% were achieved for CMV-eGFP (Figure 1a and d) without impairing cell-proliferation kinetics (Figure 1c) or increasing the rate of apoptosis (Figure 1e). Long-term expression of CMV-eGFP was demonstrated in CDCs continuously cultured over a period of 6 months (Figure 1d). In contrast to the high transduction efficiency of vesicular stomatitis virus–pseudotyped lentiviral vectors, only AAV2 achieved transduction efficiencies of ~50%, while the remaining serotypes did not lead to marked expression of the reporter gene in human CDCs (Figure 1a). In addition, direct comparison of AAV2 and lentiviral vectors expressing firefly luciferase under the transcriptional control of the cytomegalovirus (CMV) promoter demonstrated gradual loss of reporter gene expression in AAV2 vectors with luciferase activity falling below background levels (<50 relative light units) over a period of 28 days. In contrast, luciferase activity remained robust in lentiviral-transduced cells for up to 4 weeks, making vesicular stomatitis virus–pseudotyped lentivirus the preferred viral vector for the transduction of CDCs (Figure 1b).
Figure 1. Lentiviral vectors efficiently transduce cardiac stem cells (CDCs).
(a) Human cardiosphere-derived cells (CDCs) were transduced with different adeno-associated virus (AAV) serotypes [multiplicity of infection (MOI) of 250,000; n = 4] and lentiviral vectors (MOI of 35; n= 6) expressing enhanced green fluorescent protein (eGFP) under the control of the cytomegalovirus (CMV) promoter. Vesicular stomatitis virus (VSV)-pseudotyped lentiviral vectors achieved significantly higher transduction efficiencies than any of the nine different AAV serotypes tested. *Denotes P < 0.05. (b) Direct comparison of AAV2 and VSV-pseudotyped lentiviral vectors expressing firefly luciferase revealed significantly higher luciferase activity in cells transduced with lentivirus (n = 4). (c) Porcine CDCs were transduced with NCX1-eGFP lentiviral constructs (MOI of 35, n = 3) and sorted into a GFP-negative and GFP-positive cell population by fluorescence-activated cell sorting (FACS). Colorimetric proliferation assay revealed no slowing of growth curves after viral transduction and FACS (n = 6). (d) Persistent transgene expression over a 6-month period in NCX1-eGFP and CMV-eGFP transduced porcine CDCs as quantified by flow cytometry (n = 3). (e) Percentage of apoptotic cells as determined by flow cytometry (7-amino-actinomycin-negative and annexin-V-APC positive; n = 6). NS, not significant; RLU, relative light units.
Probing the cardiac specificity of five different cardiac-specific promoters in vitro and in vivo
In order to obtain the optimal promoter system for testing cardiac differentiation in stem cells, we generated different lentiviral vectors and compared five promoters with respect to their cardiac specificity in seven different cell lines. Neonatal rat ventricular myocytes (NRVMs) and CMV-eGFP served as the positive control cell line and as the positive control lentiviral construct, respectively. Figure 2a shows fluorescent pictures taken 7 days after lentiviral transduction with an MOI of 35. As expected, lentiviral constructs with cardiac-specific promoters gave the highest signals in NRVMs compared to the six noncardiomyocyte cell lines tested. Whereas the promoters of the three sarcomeric genes (MLC-2v, αMHC3, and TnI) also displayed high activity in vascular smooth muscle and fibroblast cell lines, the promoters of the two cardiac ion channel/transporter genes (α1C and NCX1) seemed to achieve a higher cardiac specificity. This was confirmed by flow cytometry, where the NCX1-promoter showed the lowest expression levels in noncardiomyocyte cell lines. This is indicated by the intensity of the green fluorescent protein (GFP), corresponding to the strength of a given promoter, which was lowest for the NCX1-promoter across all noncardiomyocyte cell lines (Figure 2b).
Figure 2. Comparing cardiac-specificity of different promoters.
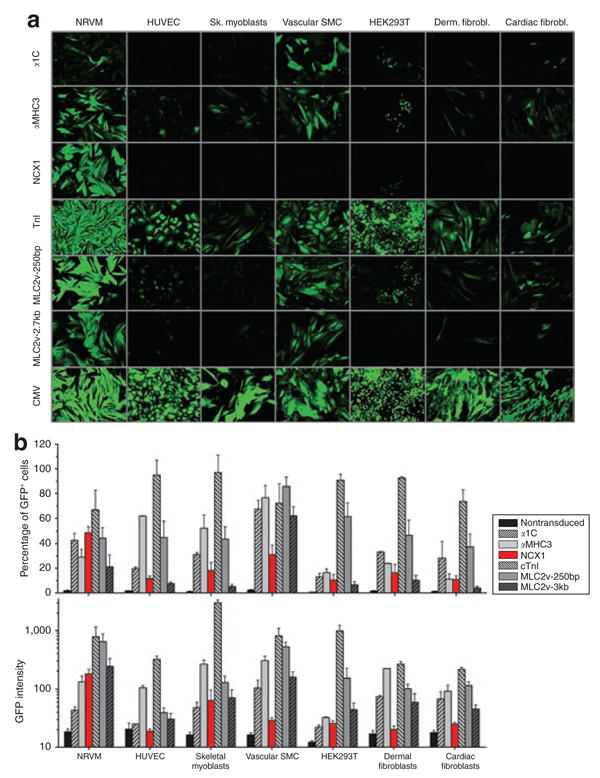
(a) Fluorescent images of seven different cell lines transduced with lentiviral vectors bearing one of the following cardiac-specific promoters: (i) α-myosin heavy chain, αMHC3; (ii) myosin light chain, ventricular (long), MLC-2v-3kb; (iii) myosin light chain, ventricular (short), MLC-2v-250bp; (iv) cardiac troponin I, TnI; (v) cardiac sodium calcium exchanger, NCX1; (vi) cardiac L-type calcium channel, α-1c. Pictures were taken 7 days after transduction when expression of green fluorescent protein (GFP) was considered to have achieved plateau. Exposure time was 2 seconds except for neonatal rat ventricular myocytes (NRVMs) transduced with MLC-2v-250bp (1 second) and all cells transduced with cytomegalovirus (CMV) (200 ms). Representative images are shown (cell confluency >90%). ×10 Magnification was used for all images. (b) Quantitative assessment of promoter activity by flow cytometry, indicating the percentage of GFP-positive cells and the GFP intensity across the seven cell lines examined. NCX1-eGFP lentiviral constructs were the most cardiac specific, overall showing the lowest GFP intensity in noncardiomyocyte cell lines (n = 4–12). HUVEC, human umbilical vein endothelial cell.
The performance of the most cardiac-specific promoter identified by in vitro testing was further assessed in vivo. NCX1-luciferase activity was ~6× higher in myocardium compared to skeletal muscle after direct injection (n = 3 each; Figure 3a), confirming the myocardial specificity of NCX1-promoter lentiviral constructs. In vivo bioluminescence imaging after direct myocardial injections of NCX1-luciferase showed that signals decayed ~50% in the first month, but remained essentially stable thereafter (n = 4; Figure 3c). In vitro luciferase assays performed after 6 months, demonstrated that the luciferase activity originated from the heart only (Figure 3b).
Figure 3. In vivo specificity of NCX1-Luc constructs.
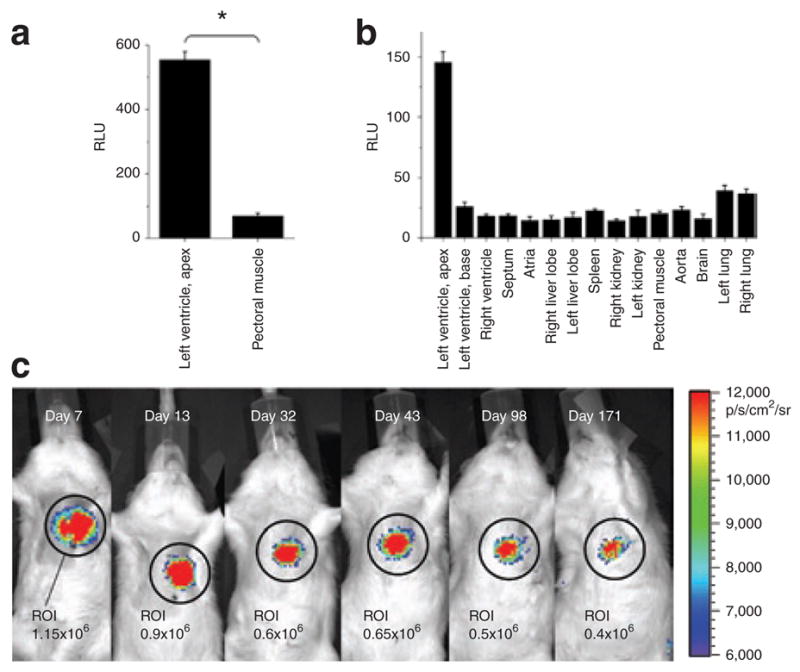
(a) Direct injection into heart and pectoral muscle revealed ×6 higher luciferase activity in the myocardium compared to skeletal muscle (n = 4; * denotes P < 0.05). (b) In vitro luciferase assays 6 months after direct injection into the myocardium demonstrate that luciferase activity is confined to the site of injection. (c) In vivo bioluminescence imaging shows that the cardiac NCX1 promoter is sufficient to drive robust myocardial long-term expression. The luminescent signal is quantified in the region of interest (ROI). RLU, relative light units.
The endogenous viral 3′-self-inactivating long-terminal repeat retains promoter/enhancer activity
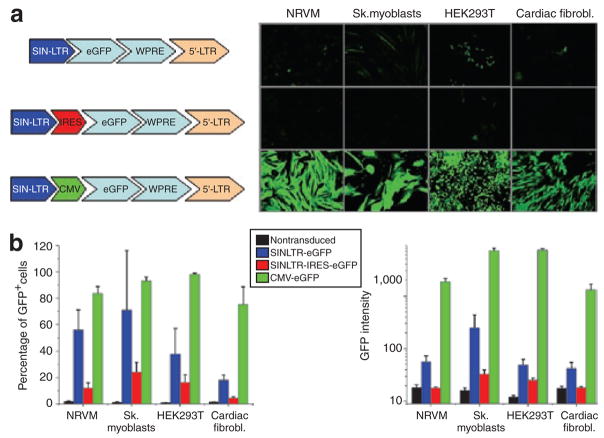
Data from transgenic animal models carrying a reporter gene under the transcriptional control of cardiac-specific promoters suggest no expression in extra-cardiac tissue.10,11 This data is in contrast to that derived from this study where we noted some activity in vivo (skeletal muscle) and in vitro (up to 20% of human umbilical vein endothelial cell and vascular smooth muscle cells transduced with the NCX1-promoter showed weak GFP expression by flow cytometry). We hypothesized that extra-cardiac expression might be caused by residual promoter activity arising from the 3′-self-inactivating long-terminal repeat (3′-SIN-LTR). Therefore, we created a lentiviral construct without any promoter other than the intrinsic 3′-SIN-LTR driving expression of enhanced GFP (eGFP). As evident in Figure 4, the 3′-SIN-LTR retained significant promoter activity in several cell lines; however, the mean fluorescence intensity was ~150 times lower than in CMV-transduced cells (Figure 4b). This is consistent with the SIN design of lentiviral vectors in which the LTR promoter activity has been greatly reduced.12 However, our results suggest that this residual promoter activity is still sufficient to reduce cardiac specificity, especially of short promoter sequences. This is supported by the fact that increasing the intramolecular distance between the 3′-SIN-LTR and the reporter gene, e.g., by introducing an internal ribosomal entry site element which, by itself, is devoid of any promoter activity, lead to a marked attenuation of the residual promoter activity of the 3′-SIN-LTR. Likewise, the MLC-2v 2.7-kilobase promoter showed less expression in extra-cardiac cell lines than the short 250-base pair proximal promoter fragment (Figure 2a and b).
Figure 4. Residual promoter activity of 3′-self-inactivating long-terminal repeat (3′-SIN-LTR).
(a) Representative fluorescent images of neonatal rat ventricular myocytes (NRVMs), skeletal myoblasts (sk. myoblasts), HEK293T, and cardiac fibroblasts transduced with “promoterless” 3′-SIN-LTR-eGFP and 3′-SIN-LTR-IRES-eGFP constructs as well as CMV-eGFP. Exposure time was 2 seconds except for cells transduced with CMV (200 ms). Cell confluency >90%, ×10 magnification for all images. (b) Corresponding analysis by flow cytometry. It becomes evident that the 3′-SIN-LTR still retained significant promoter activity in all cell lines tested; however, the mean fluorescence intensity was ~150 times less than in CMV-transduced cells. This is consistent with the SIN design of third generation lentiviral vectors in which the LTR promoter activity has been greatly reduced. Yet, it is not completely abrogated and increasing the distance between the 3′-SIN-LTR and the reporter gene, e.g., by introducing an IRES element, leads to a further attenuation of the residual promoter activity of the 3′-SIN-LTR. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; IRES, internal ribosomal entry site.
The cardiac NCX1-promoter reports cardiogenic differentiation in embryonic and adult CDCs
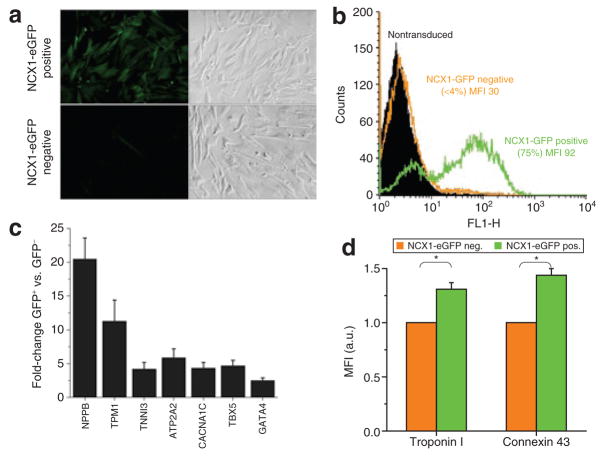
To test the ability to report cardiogenic differentiation in stem cells, embryoid bodies of a mouse embryonic stem cell line were transduced with NCX1-eGFP. Selective labeling of beating areas in embryoid bodies supported the specificity of the NCX1-eGFP construct (Supplementary Figure S1, Supplementary Video S1). Likewise, when CDCs were transduced with NCX1-eGFP lentivirus and subsequently separated in GFP-positive and GFP-negative cell populations by fluorescence-activated cell sorting (n = 5), NCX1-eGFP-positive CDC subpopulations could be expanded (Figure 1c) and retained their specific properties in vitro, as evident by the high percentage of eGFP-positive CDCs after 4 weeks of culture (Figure 5a and b). Quantitative real-time polymerase chain reaction (quantitative RT-PCR) showed that transcripts of several cardiac marker genes, including cardiac troponin I, α-tropomyosin, pro-brain natriuretic peptide, T-box transcription factor 5, cardiac ion channels and transporters, including the cardiac sarcoplasmic reticulum CA2+-ATPase (SERCA2) and the L-type calcium channel, were more abundantly expressed in GFP-positive cell populations (Figure 5c). Likewise, cardiac troponin I and connexin 43 proteins were more abundant in NCX1-GFP-positive versus eGFP-negative cells, as determined by flow cytometry (Figure 5d).
Figure 5. NCX1-eGFP-labeled cardiosphere-derived cells (CDCs) express cardiac markers.
(a) Fluorescent and corresponding bright light image of human CDCs 4 weeks after fluorescence-activated cell sorting (FACS) into NXC1-eGFP-positive and -negative cell populations. (b) Corresponding flow cytometric analysis 4 weeks after FACS. (c) Expression of several cardiac marker genes in human CDCs as measured by semiquantitative real-time polymerase chain reaction (n = 5). Fold-changes indicate relative expression between GFP-positive and GFP-negative cell populations. Expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase. Transcripts encoding for cardiac genes are upregulated in GFP-positive cells (NPPB = pro-brain natriuretic peptide. TPM1 = α-tropomyosin. TNNI3 = cardiac troponin I. ATP2A2 = sarcoplasmic Ca2+-ATPase (SERCA2). CACNA1C = α-subunit of the L-type calcium channel. TBX5 and GATA4 are cardiac transcription factors; P < 0.05 for all transcripts). (d) Troponin I and connexin 43 protein were more abundant in NCX1-GFP-positive versus NCX1-GFP-negative cells as determined by flow cytometry. * denotes P < 0.05 (n= 5). a.u., arbitrary units; eGFP, enhanced green fluorescent protein; MFI, mean fluorescence intensity.
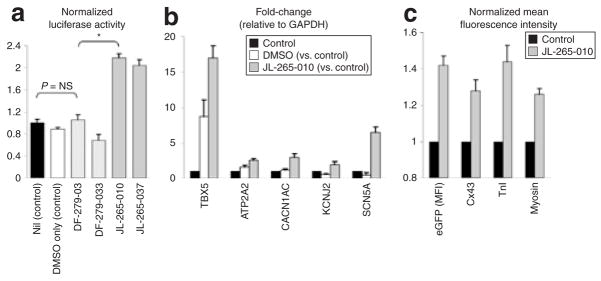
The NCX1 gene is an important signature gene of cardiac fate in stem cells. In this regard, our NCX1-luciferase reporter lentivirus could serve as an ideal biosensor of cardiac fate and differentiation in high throughput chemical library screens or assays for cardiogenic factors. To test this possibility, we used sulfonyl hydrazones, a family of cardiogenic small molecules identified in a chemical library screen for activators of Nkx2.5 in P19CL6 embryonic carcinoma cells (H. Sadek, unpublished results), to promote cardiac differentiation in CDCs. As control, we compared sulfonyl hydrazones to isoxazoles, a second family of compounds that activated Nkx2.5 in the primary screen but were found to be noncardiogenic (H. Sadek, unpublished results). Indeed, the NCX1-luciferase reporter gene was activated in lentivirus-transduced human CDCs by the cardiogenic sulfonyl hydrazone compounds but the isoxazoles had no effect on expression of this reporter gene (Figure 6a). Correlating with this result, sulfonyl hydrazones strongly induced several endogenous cardiac messenger RNAs, including TBX5 and SCN5A, as well as cardiac proteins in human CDCs (Figure 6b and c). These results demonstrate that, as a biosensor of cardiac differentiation in human CDCs, NCX1-luciferase can accurately discriminate between cardiogenic and noncardiogenic small molecules, validating the specificity of this reporter system.
Figure 6. Cardiogenic small molecules.
(a) Two sulfonyl hydrazone compounds (JL-265-010 and JL-265-037) shown to induce cardiac differentiation in a cardiogenic subline of P19 embryonic carcinoma cell lines (H. Sadek, unpublished results), also potently activated the NCX1-luciferase reporter system in human cardiosphere-derived cells, while the isoxazole compounds DF-279-03 and DF-279-033 were confirmed to be noncardiogenic (n = 5; * denotes P < 0.05). Cells were incubated for 7 days with 10 μmol/l of each compound [dissolved in dimethyl sulfoxide (DMSO)]. (b) The sulfonyl hydrazone compound JL-265-010 lead to marked induction of cardiac gene expression (n = 4; P < 0.05 for all transcripts; JL-265-010 versus control). TBX5 = T-box 5 cardiac transcription factor; ATP2A2 = sarcoplasmic Ca2+-ATPase (SERCA2); CACNA1C = α-subunit of the L-type calcium channel; KCNJ2 = subunit of the inward rectifier potassium channel; SCN5A = α-subunit of the cardiac sodium channel. (c) The cardiogenic small molecule JL-265-010 lead to a significant increase in mean green fluorescent protein (GFP) intensity in NCX1-GFP positive cells, accompanied by a significant increase in troponin I, myosin, and connexin 43 protein as determined by flow cytometry. All comparisons between JL-265-010 and control cells reached statistical significance (n = 4; P < 0.05). MFI, mean fluorescence intensity.
DISCUSSION
This study describes a powerful new tool for the in vitro selection and enrichment of cardiomyocyte progenitors out of a heterogeneous cell population using a lentiviral vector–based approach. While lentiviral transduction of human CDCs consistently yielded efficiencies of >90%, AAV labeling efficiencies ranged between 15 and 50% for AAV serotypes 2, 2.5, 3, and 5. Thus, these findings are comparable to viral transduction of human mesenchymal stem cells, with clear superiority of lentiviral vectors over AAV2 and the remaining AAV serotypes.13,14
Showing a quantitative assessment of promoter activity, we demonstrate that the cardiac NCX1 promoter is the most cardiac-specific in the context of lentiviral vectors in vitro and in vivo. Even though the other cardiac-specific promoters, including αMHC, MLC-2v, and TnI have been shown to confer cardiac specificity in transgenic animal models,11,15,16 several caveats need to be considered in the context of viral vectors. Most importantly, the interaction between promoter sequences and the selected vector can dramatically affect tissue-specific expression. In this respect, we noted that the viral SIN-LTR 3′-region still retains important promoter activity despite extensive deletions. Thereby, the 3′-SIN-LTR influences expression levels of the reporter gene in addition to the heterologous cardiac-specific promoter, leading to significant expression in noncardiac cell lines. This was of particular concern for short promoters including the TnI and MLC2v–250-base pair fragment. In accordance with this notion, increasing the distance between the 3′-SIN-LTR and the reporter gene by including a transcriptionally silent internal ribosomal entry site abrogated the effect of the 3′-SIN-LTR on reporter gene expression (Figure 4).
The feline cardiac NCX1 promoter has been shown to be sufficient for driving the normal spatiotemporal pattern of NCX1 expression in cardiac development. In transgenic mice, the luciferase reporter gene was expressed in a heart-restricted pattern in both early and late embryos.10 In addition to being one of the earliest markers of cardiac differentiation (~E8.5 in embryonic stem cells17), expression from the cardiac NCX1-promoter is also maintained after birth.17 Herein, we were able to show that the cardiac-specificity of the NCX1-promoter is preserved at later stages of cardiac differentiation in the context of lentiviral vectors, as NCX1-eGFP vectors selectively labeled beating areas of embryoid bodies (Supplementary Video S1 and Supplementary Figure S1), NRVMs (Figure 2a and b) and adult myocardium (Figure 3).
Using expression of a reporter gene under the transcriptional control of the cardiac NCX1-promoter, we were able to select cardiomyocyte precursors from human CDCs. We demonstrated that NCX1-eGFP-positive cell populations express cardiac messenger RNAs and proteins such as cardiac troponin I, α-tropomyosin, pro-brain natriuretic peptide, T-box transcription factor 5 as well as several transcripts encoding for cardiac ion channels (Figure 5c and d). Of note, transduction with lentiviral constructs at an MOI ~35 did not increase cell death and the rate of apoptosis in adult CDCs (Figure 1e). Likewise, it did not slow the proliferation of transduced cells, as NCX1-eGFP positive subpopulations of CDCs could be isolated and expanded in vitro (Figure 1c). Thus, our study shows that lentiviral vectors containing the cardiac NCX1-promoter can be used to purify cardiomyocyte precursors out of a heterogeneous population of cells. The ability to selectively enrich for cardiomyocytes and their precursors has potential relevance for the development of cell-based therapies.
In addition, long-term follow up of NCX1 lentiviral–injected animals by bioluminescence imaging showed robust transduction of the myocardium for periods >6 months (Figure 3c). This is in contrast to a previous study where a profound reduction (more than sevenfold, depending on the promoter construct used) was noted for copy numbers of integrated lentivirus DNA in cardiac extracts by RT-PCR.18 As lentiviral constructs bearing the cardiac NCX1-promoter confer robust and prolonged cardiac gene expression, they might have important potential applications in gene therapy approaches as well, as they enable the targeted expression of genes in myocardium, thereby minimizing the unwanted side effects of extra-cardiac gene expression. Our chemical biology studies also demonstrate the potential usefulness of the NCX1 viral reporter system for chemical library screens in search of small molecules that can selectively activate the cardiac gene program in stem cells. Therefore, lentiviral constructs bearing the cardiac NCX1 promoter represent a promising tool in both stem cell biology applications and gene therapy approaches.
MATERIALS AND METHODS
AAV
AAV vectors expressing eGFP (serotypes 1, 2, 2.5, 3, 4, 5, 6, 8, and 9) or luciferase (serotype 2) were obtained from the Vector Core of the University of North Carolina (Dr. R. Jude Samulski). Adult CDCs (cardiosphere-derived cells, CDCs) were isolated as previously described (see Supplementary Materials and Methods).1,2 2.5 × 104 cells were transduced with the different AAV serotypes in a total volume of 1 ml in a 24-well plate with an MOI of 1 × 105 and 2.5 × 105.
Construction and production of lentiviral vectors
The lentiviral vector backbone was pRRLsin18.cPPT.CMV.eGFP.WPRE (Inder Verma, Salk Institute, La Jolla, CA), a SIN vector that expresses eGFP under the control of the human CMV promoter. The SIN design of the human immunodeficiency virus-1 vector had been achieved by introducing a 400-nucleotide deletion in the 3′-long terminal repeat which also included the TATA-box, thereby greatly diminishing the internal promoter activity of the LTR.12
The CMV promoter was replaced by one of the following cardiac promoters: (i) sodium-calcium exchanger [NCX1 (ref. 10)], (ii) L-type calcium channel α-subunit (α1C19), (iii) α-myosin heavy chain (αMHC11), (iv) myosin ventricular light chain 2 (MLC-2v15,20), and (v) troponin I (TnI16) (Table 1). Correct orientation of the insert was confirmed by sequencing in all cases. Table 1 describes the subcloning strategies selected to construct the vectors used in this study. The cardiac promoters were a kind gift of the following investigators: NCX1—Drs. Donald R. Mennick and Terrence X. O’Brien, Medical University of South Carolina, Charleston, South Carolina; α1C—Dr. Stanley Nattel, University of Montreal, Canada; MLC-2v—Dr. Peter Gruber, University of Pennsylvania; αMHC—Dr. Jeffrey Robbins, University of Cincinnati; troponin I—Dr. Steffano Schiaffino, University of Padova, Italy.
Table 1.
Summary of the cardiac-specific promoters and the subcloning strategies used to generate the lentiviral vectors for this study. All promoters were subcloned into pRRLsin18.cPPT.CMV. eGFP.WPRE (Inder Verma, Salk Institute, La Jolla, CA).
| Promoter | Species | Fragment/length | Original vector | Reference(s) |
|---|---|---|---|---|
| α-Myosin heavy chain (αMHC3) | Mouse | SphI–SalI/2,783 kilobase (kb) | αMHC promoter in BluescriptII SK+ (Stratagene); clone 26 | 11 |
| Myosin light chain 2, ventricular (long) (MLC-2v-2.7 kb) | Rat | MluI–XhoI/2.7 kb | 2.7-kb EcoRI fragment of MLC-2v5′-flanking region with promoter and TSS inserted into multiple cloning site of pGL3-basic vector (Promega) | 15,20 |
| Myosin light chain 2, ventricular (short) (MLC-2v-250 bp) | Rat | HindIII–EcoRI/250 base pair (bp) | 250-bp MLC-2v promoter was contained in the MLCSVOA vector | 15,20 |
| Cardiac troponin I (cTNI) | Rat | SalI fragment/246 bp | Cardiac troponin I cloned into pCAT-basic (Promega) | 16 |
| Cardiac sodium-calcium exchanger (NCX1) | Cat | SpeI–NheI/2,040 bp | 2-kb NCX1 promoter containing 1,850-bp 5′-untranslated region (5′-UTR), 110-bp promoter HI, 68-bp 3′-UTR cloned in pGL2 basic vector (Promega) | 10 |
| Cardiac L-type calcium channel (α-1c) | Human | XhoI–HindIII/1,576 bp | α-1C Promoter in pGL3-F7R1 vector (Promega) | 19 |
“Promoterless” 3′-SIN-LTR-eGFP and 3′-SIN-LTR-IRES-eGFP constructs were generated by excising the CMV promoter with ClaI and XbaI from the pRRLsin18.cPPT.CMV.eGFP.WPRE vector, blunting the ends with Klenow enzyme, and directly ligating the vector ends or inserting an internal ribosomal entry site from the encephalomyocarditis virus, respectively. NCX1-luciferase constructs were made by exchanging eGFP in NCX1-eGFP vectors (Table 1) with the firefly luciferase reporter gene (luc) from the PGL2-basic vector (Promega) by blunt ligation.
For viral vector production, HEK293T cells were seeded in T175-cm2 flasks to obtain a 80–90% confluency on the day of transfection, and co-transfected with a four plasmid vector system using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described previously.2,21 The crude viral suspension was harvested from 293T cell cultures 48 and 72 hours after transfection, filtered (0.2 mm), and concentrated using either Centricon Plus-70 filter columns (100,000 molecular weight cut-off; Millipore, Billerica, MA) for in vitro use or ultracentrifuged at 70,000g and 8 °C for 2 hours and the pellet resuspended in phosphate-buffered saline for in vivo injections. Vector aliquots were subsequently stored at −80 °C until use. Vector titers were determined using an enzyme-linked immunosorbent–based assay (PerkinElmer Life Sciences, Boston, MA), measuring the p24 Gag protein content according to the manufacturer’s instructions. Based on parallel assessment of p24 levels and serial viral dilutions in 293T cells with CMV-eGFP vectors from six independent viral preparations, we calculated the conversion to be 15.4 transducing units per pg p24 (data not shown).
Cell culture and flow cytometry
In AAV- or lentiviral-transduced cells, expression of eGFP was assayed using a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ) 6–7 days after transduction when expression was determined to have reached a plateau, based on measurement of mean fluorescence intensity and percentage of eGFP positive cells. Likewise, luciferase activity of 105 cells was assessed 7 days after transduction, as reported elsewhere.22
Myocardial specificity of transgene expression was determined in a total of seven cell lines. Culture conditions are summarized in Table 2. Low passage number cells (<6) were plated at 2 × 104 cells per well in a 24-well plate and transduced with lentivirus (4.5 × 104 pg p24, corresponding to an MOI of 35) in a total volume of 2 ml in the presence of 8 μg/ml polybrene. NRVMs, with low proliferative potential and high purity (the contamination of fibroblast was reduced to <5–10% with preplating steps23) were transduced at 1 × 105 cells per well in a 24-well plate and transduced with 2.27 × 105 pg p24 lentivirus (MOI 35) in the presence of 8 μg/ml polybrene. Sixteen hours later, viral vector–containing medium was replaced with fresh medium.
Table 2.
Cell lines and culture conditions
| Cell line | Species | Cell culture medium | Reference |
|---|---|---|---|
| Human embryonic kidney cells 293T (HEK293T) | Human | DMEM + 10% FBS | ATCC CRL-11268 |
| NRVMs | Rat | Medium 199 + 2% FBS | 23 |
| A7r5 (vascular smooth muscle) | Rat | DMEM + 10% FBS | CRL-1444 (ATCC) |
| Ventricular CFs | Guinea pig | DMEM + 10% FBS | 23 |
| Skeletal myoblasts (Sk-Myo) | Human | Skeletal muscle cell basal medium (SkBM-2) + SkGM-2 SingleQuots (Cambrex, Walkersville, MD) | SkMC 2561 (Cambrex; Walkersville, MD) |
| Umbilical vein endothelial cells (HUVEC) | Human | Endothelial cell basal medium (EBM-2) + EGM-2 SingleQuots (Cambrex, Walkersville, MD) | CRL-1730 (ATCC) |
| DF | Human | DMEM + 10% FBS | C-12300 (PromoCell, Heidelberg, Germany) |
| Embryonic stem cells | Mouse | GMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (Equitech-Bio, Kerrville, TX), 1% nonessential amino acids (Invitrogen), 0.1 mmol/l 2-mercaptoethanol, 1mmol/l sodium pyruvate, 0.1 g/ml penicillin, 0.1 mg/ml streptomycin, 100 μg/ml hygromycin (Gibco-BRB), and 1,000 U/ml leukemia inhibitory factor (ESGRO; Chemicon International, Temecula, CA) | 26,27 |
Abbreviations: ATCC, American Type Culture Collection; CFs, cardiac fibroblasts; DF, dermal fibroblasts; DMEM, Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum; GMEM, Glasgow minimum essential medium; HUVEC, human umbilical vein endothelial cell; NRVMs, Neonatal rat ventricular myocytes;
CDCs (2.5 × 105 cells) were transduced with NCX1-eGFP lentivirus (5.7 × 105 pg p24; MOI ~35) in a total volume of 3 ml in a T25 flask in the presence of 8 μg/ml polybrene. The percentage of dead and apoptotic NCX1-eGFP-transduced CDCs was determined by flow cytometry using 7-amino-actinomycin and Annexin V-APC (BD Biosciences, San Jose, CA), respectively. Proliferation of NCX1-eGFP-transduced CDCs was assessed using the colorimetric WST-8 assay (Cell counting kit-8; Dojindo Molecular Technologies, Gaithersburg, MD).
Flow cytometric detection of intracellular antigens for immuno-phenotyping was performed using the BD Cytofix/Cytoperm Kit (Beckton Dickinson, Franklin Lakes, NJ) following the manufacturer’s instructions with myosin light chain (Abcam, Cambridge, MA), cardiac troponin I and connexin 43 rabbit antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), followed by staining with a anti-rabbit Alexa-647 secondary antibody.
RT SYBR-Green PCR (quantitative RT-PCR)
One million NCX1-transduced CDCs were sorted using FACSVAntage SE cell sorter (Becton-Dickinson, Franklin Lakes, NJ) and total RNA extracted from the top 10% GFP-positive- and GFP-negative-sorted cell populations using the RNeasy Micro RNA extraction kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase Set (Qiagen, Valencia, CA) to eliminate genomic DNA contamination, and complementary DNA was synthesized from 1 μg total RNA using AffinityScript multiple temperature reverse transcriptase (Stratagene, La Jolla, CA) and Oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Gene-specific primers were designed based on the qPrimerDepot database24 in order to amplify fragments of ~100–150 base pairs in length close to the 3′-end of the transcript (Table 3). Gel electrophoresis and the configuration of the dissociation curves were used to assess the specificity of the amplicon. RT-PCR was performed in duplicate for each sample with 25 ng of complementary DNA and 300 nmol/l primer in the Applied Biosystems 7900HT RT-PCR system (Applied Biosystems, Foster City, CA) using the QuantiTect SYBR Green PCR Kit according to the recommendations of the manufacturer (Qiagen, Valencia, CA). PCR-amplification of complementary DNA started with a “hot start”–activation of the Taq polymerase at 95 °C for 15 minutes, followed by 40 cycles of 15-second denaturation at 95 °C, annealing for 30 seconds at 58 °C, and 30-second elongation at 72 °C. All experimental results for the samples with a coefficient of variation >10% were retested. Relative quantification of the signals was done by normalizing the signals of the different transcripts to glyceraldehyde-3-phosphate dehydrogenase.25
Table 3.
Oligonucleotides used for qRT-PCR
| Name | Sequence of primer |
|---|---|
| GAPDH | Forward: 5′-TTA AAA GCA GCC CTG GTG AC-3′ |
| Reverse: 5′-CTC TGC TCC TCC TGT TCG AC-3′ | |
| NPPB | Forward: 5′-TGT GGA ATC AGA AGC AGG TG-3′ |
| Reverse: 5′-TTT GGG AGG AAG ATG GAC C–3′ | |
| TPM1 | Forward: 5′-CCA ACT CTT CCT CAA CCA GC-3′ |
| Reverse: 5′-CTC AAA GAT GCC CAG GAG AA-3′ | |
| TNNI3 | Forward: 5′-CAG TAG GCA GGA AGG CTC AG–3′ |
| Reverse: 5′-GTG AAG AAG GAG GAC ACC GA-3′ | |
| ATP2A2 | Forward: 5′-TCA GCA GGA ACT TTG TCA CC-3′ |
| Reverse: 5′-GGG CAA AGT GTA TCG ACA GG-3′ | |
| CACNA1C | Forward: 5′-TAG GCA TTG GGG TGA AAG AG-3′ |
| Reverse: 5′-GAA GAT GAT TCC AAC GCC AC-3′ | |
| TBX5 | Forward: 5′-AGA CTC GCT GCT GAA AGG AC-3′ |
| Reverse: 5′-GGA GCT GCA CAG AAT GTC AA-3′ | |
| GATA4 | Forward: 5′-TGC CGT TCA TCT TGT GGT AG-3′ |
| Reverse: 5′-CCG ACA CCC CAA TCT CG-3′ |
Abbreviations: ATP2A2, sarcoplasmic Ca2+-ATPase (SERCA); CACNA1C,α-subunit of the L-type calcium channel; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NPPB, pro-brain natriuretic peptide; TPM1, α-tropomyosin; qRT-PCR, quantitative real-time polymerase chain reaction; TNNI3, cardiac troponin I. TBX5 and GATA4 are cardiac transcription factors.
In vitro luciferase assay and in vivo bioluminescence imaging
Eight- to ten-week-old male Wistar–Kyoto rats underwent left thoracotomy in the fourth or fifth intercostal space under general anesthesia (isoflurane inhalation, 4% for induction and 2.5% for maintenance; n = 7). The heart was exposed and the NCX1-luciferase lentivirus (~4×107 transducing units in 200 μl volume) injected at two sites in the anterolateral wall of the left ventricle using a 30G needle. Subsequently, the chest was closed and the animals were allowed to recover. In three separate animals, the NCX1-luciferase vector was injected into the right pectoral muscle. All procedures were approved by the local animal care committee.
For serial long-term follow-up of myocardial luciferase levels, bioluminescence imaging was performed under general anesthesia starting from day 7 after injection of the virus. D-Luciferin (sodium salt; Gold Biotechnology, St. Louis, MO) was dissolved in phosphate-buffered saline and given via an intraperitoneal injection at a dose of 150 mg/kg body weight before imaging. The rats were then placed in the IVIS 200 Xenogen in vivo imaging system chamber (Xenogen, Alameda, CA). For visualization purposes, the luminescent image (exposure time 1 minute) was overlaid on a photographic image. The signal intensity is represented by radiance (p/s/cm2/sr) and encoded by pseudocolors on the luminescent image.
Contrary to the luminescent signal from the pectoral muscle injections, the signal from the myocardium is attenuated by the chest wall. Therefore, comparison of luciferase activity from both injection sites was carried out by in vitro luciferase assays rather than in vivo imaging. After killing the animals 7 days after surgery, the area around the injection site was dissected, and five samples, each weighing 200 mg, homogenized in 1-ml lysis buffer (Promega, Madison, WI) supplemented with 0.5 ml of 10% bovine serum albumin (Sigma). After centrifugation at 24,000g for 45 minutes at 4 °C, the supernatant was used to perform luciferase assays as reported elsewhere.22
Statistical analysis
All results are presented as mean ± SEM. Significance of differences between any two groups was determined by the Student’s t-test. A final value of P < 0.05 was considered significant for all analyses. All probability values reported are two-sided.
Supplementary Material
Figure S1. Lenti NCX1-eGFP labeled beating area of embryoid body (overlay with Supplementary Video 1).
Video S1. Lenti NCX1-eGFP labeled beating area of embryoid body (overlay with Supplementary Figure 1).
Acknowledgments
We thank Mark Pittenger (Johns Hopkins University, Baltimore, MD) and Elisa Messina (University “La Sapienza”, Rome, Italy) for manuscript review and Mohammed Zauher for isolation of NRVMs and help with cell culture. This study was supported by a grant from the German Research Foundation (DFG, grant BA 3341/1-1; A.S.B.) and by the Donald W. Reynolds Foundation and National Institutes of Health. E.K. is funded by the Michel Mirowski, fellowship of the Heart Rhythm Society.
References
- 1.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 2.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 4.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 6.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 7.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, et al. Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J. 2000;14:2540–2548. doi: 10.1096/fj.00-0002com. [DOI] [PubMed] [Google Scholar]
- 9.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 10.Müller JG, Isomatsu Y, Koushik SV, O’Quinn M, Xu L, Kappler CS, et al. Cardiac-specific expression and hypertrophic upregulation of the feline Na+-Ca2+ exchanger gene H1-promoter in a transgenic mouse model. Circ Res. 2002;90:158–164. doi: 10.1161/hh0202.103231. [DOI] [PubMed] [Google Scholar]
- 11.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 12.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chng K, Larsen SR, Zhou S, Wright JF, Martiniello-Wilks R, Rasko JE. Specific adeno-associated virus serotypes facilitate efficient gene transfer into human and non-human primate mesenchymal stromal cells. J Gene Med. 2007;9:22–32. doi: 10.1002/jgm.990. [DOI] [PubMed] [Google Scholar]
- 14.McMahon JM, Conroy S, Lyons M, Greiser U, O’shea C, Strappe P, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- 15.Henderson SA, Spencer M, Sen A, Kumar C, Siddiqui MA, Chien KR. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem. 1989;264:18142–18148. [PubMed] [Google Scholar]
- 16.Di Lisi R, Millino C, Calabria E, Altruda F, Schiaffino S, Ausoni S. Combinatorial cis-acting elements control tissue-specific activation of the cardiac troponin I gene in vitro and in vivo. J Biol Chem. 1998;273:25371–25380. doi: 10.1074/jbc.273.39.25371. [DOI] [PubMed] [Google Scholar]
- 17.Fijnvandraat AC, van Ginneken AC, Schumacher CA, Boheler KR, Lekanne Deprez RH, Christoffels VM, et al. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J Mol Cell Cardiol. 2003;35:1461–1472. doi: 10.1016/j.yjmcc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Fleury S, Simeoni E, Zuppinger C, Déglon N, von Segesser LK, Kappenberger L, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- 19.Pang L, Koren G, Wang Z, Nattel S. Tissue-specific expression of two human Ca(v)1.2 isoforms under the control of distinct 5′ flanking regulatory elements. FEBS Lett. 2003;546:349–354. doi: 10.1016/s0014-5793(03)00629-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KJ, Ross RS, Rockman HA, Harris AN, O’Brien’ TX, van Bilsen M, et al. Myosin light chain-2 luciferase transgenic mice reveal distinct regulatory programs for cardiac and skeletal muscle-specific expression of a single contractile protein gene. J Biol Chem. 1992;267:15875–15885. [PubMed] [Google Scholar]
- 21.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber PJ, Li Z, Li H, Worrad D, Huang B, Abdullah I, et al. In vivo imaging of MLC2v-luciferase, a cardiac-specific reporter gene expression in mice. Acad Radiol. 2004;11:1022–1028. doi: 10.1016/j.acra.2004.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekar RB, Kizana E, Smith RR, Barth AS, Zhang Y, Marbán E, et al. Lentiviral vector-mediated expression of GFP or Kir2.1 alters the electrophysiology of neonatal rat ventricular myocytes without inducing cytotoxicity. Am J Physiol Heart Circ Physiol. 2007;293:H2757–H2770. doi: 10.1152/ajpheart.00477.2007. [DOI] [PubMed] [Google Scholar]
- 24.Cui W, Taub DD, Gardner K. qPrimerDepot: a primer database for quantitative real time PCR. Nucleic Acids Res. 2007;35:D805–D809. doi: 10.1093/nar/gkl767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuta E, Miake J, Yano S, Furuichi H, Manabe K, Sasaki N, et al. Subtype switching of T-type Ca2+ channels from Cav3.2 to Cav3.1 during differentiation of embryonic stem cells to cardiac cell lineage. Circ J. 2005;69:1284–1289. doi: 10.1253/circj.69.1284. [DOI] [PubMed] [Google Scholar]
- 27.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Lenti NCX1-eGFP labeled beating area of embryoid body (overlay with Supplementary Video 1).
Video S1. Lenti NCX1-eGFP labeled beating area of embryoid body (overlay with Supplementary Figure 1).