Abstract
An epidemiologic study of the autoimmune diseases taken together has not been done heretofore. The National Patient Register of Denmark is used to estimate population prevalence of 31 possible or probable autoimmune diseases. Record linkage is used to estimate 465 pairwise comorbidities in individuals among the 31 diseases, and familial aggregation among sibs, parents and offspring. The prevalence of any of the 31 diseases in the population is more than 5%. Within individuals, there is extensive comorbidity across the 31 diseases. Within families, aggregation is strongest for individual diseases, and weak across diseases. These data confirm the importance of the autoimmune diseases as a group, and suggest that common etiopathologies exist among them.
Keywords: Prevalence, comorbidity, autoimmune diseases, population register, sex ratio, familial aggregation
In 2002 the National Institutes of Health Autoimmune Diseases Coordinating Committee concluded that “the existing data do not allow for reliable estimates of the prevalence of autoimmune diseases on a national scale”(1). In a review in 1997, Jacobson et al searched for prevalence studies in the published literature from 1965 to 1997(2). There was heterogeneity as to the strength of the research literature, according to the disease category. For example, in that review there were 80 studies of Multiple Sclerosis, 38 of Rheumatoid Arthritis, 23 of Systemic Lupus Erythematosus, 9 of Myasthenia Gravis, and 15 of Type 1 Diabetes. Most of the other disease categories in the 24 studied had only a handful of studies, and there were 14 diseases with zero, one, or two studies of prevalence. An update in 2003 added a handful more of studies to this literature(3).
It has been clear for a long time that most autoimmune diseases are more common in females. One prior review included 24 diseases (4), and another, 16 diseases(5).
There are a limited number of studies of comorbidity of autoimmune diseases, that is, more than one disease occurring in the same individual(6), in spite of the growth in interest in their commonalities(7). A review of studies of comorbidity, which included case reports on single individuals, located about two dozen studies in total(8). A systematic review of 52 studies of comorbidity of Thyroiditis, Type 1 Diabetes, Rheumatoid Arthritis, and Multiple Sclerosis appeared recently (9). Gershwin et al(10) found prevalence of Systemic Lupus Erythematosis, Autoimmune Thyroid Disease, Sjogren’s Syndrome, and Polymyositis to be 6-20 times higher in cases of Primary Biliary Cirrhosis than in controls.
There are a limited number of studies of familial aggregation of autoimmune diseases, and there is substantial heterogeneity among those that do exist. The majority are uncontrolled studies on small clinic-based samples, with almost no consideration of confounding factors. However, a few tentative findings have emerged. Results of a cohort study in Sardinia, Italy reported a six-fold increased risk of Diabetes in healthy siblings of multiple sclerosis patients, and greater than a three-fold increased risk of Diabetes in healthy siblings of individuals with Multiple Sclerosis in the presence of other relatives with Multiple Sclerosis (11). A recent Danish population-based cohort study confirmed this association; they reported that first-degree relatives of patients with Multiple Sclerosis had 63% increased risk for the development of Type I Diabetes (12). Controlled studies also suggest an increased prevalence of Type I Diabetes within family members of Rheumatoid Arthritis (13, 14, 15), an increased prevalence of autoimmune Thyroiditis within families with Type I Diabetes(16), and an increased prevalence of autoimmune Thyroiditis and Rheumatoid Arthritis within families with Idiopathic Inflammatory Myopathy (17). An increased risk of autoimmune Thyroiditis, Rheumatoid Arthritis and Systemic Lupus Erythematosus among other autoimmune diseases was also reported in families with primary Sjogren ’s Syndrome (18). In another study, relatives of cases of Primary Biliary Cirrhosis were ten times as likely to have Primary Biliary Cirrhosis as controls, and prevalence of Systemic Lupus Erythematosis, Autoimmune Thyroid Disease, and Sjogren’s Syndrome were also significantly elevated in that study(10).
Several studies have also reported an increased risk of multiple sclerosis in first-degree relatives of Multiple Sclerosis patients(19). A hospital-based case-control study in Norway showed greater than a 12-fold increased risk of Multiple Sclerosis in first degree relatives of Multiple Sclerosis patients. This was confirmed in a nationwide cohort study in Denmark, which showed that first-degree relatives of Multiple Sclerosis patients had a seven-fold increased risk of Multiple Sclerosis (20). A similar risk was reported in a study using data from the Swedish Hospital Discharge Register (21).
A focus on one disease at a time may make it difficult to discover clues to etiology(22) underlying more than one disease, or leading from one to another, in spite of considerable logic for the presence of a general predisposition to several or many diseases, such as is the case for autoimmune processes and etiologies. Recognizing these problems, the Autoimmune Diseases Coordinating Committee recommended population-based disease registries to support “.... comprehensive, multidisciplinary approaches to analyze relationships among autoimmune diseases....(1).” In this paper we report prevalence, sex ratios, individual comorbidity, and familial aggregation of 31 autoimmune diseases using population registries of Denmark.
METHODS
Study population
Data from the Danish Civil Registration System(23) was used to identify all persons alive and living in Denmark on December 31, 2001 (5,472,032 persons). This study population and their parents and siblings were linked with the National Hospital Register using the unique personal identification number (CPR number) which is assigned to all persons alive and living in Denmark in April, 1968 or later. The National Hospital Register has collected data on all admissions to Danish Hospitals since 1977. With the exception of very few private clinics performing a small proportion of elective surgical procedures, it includes information from all general hospitals in Denmark. Since 1995 it has included all contacts in emergency rooms and outpatient clinics. All treatment is free of charge for residents of Denmark. From 1977-1993, people were diagnosed according to the Danish version of the World Health Organization International Classification of Diseases, eighth revision (ICD8)(24, 25) and from 1994 onwards according to the International Classification of Diseases 10th edition (ICD10)(26). The period of the case definition includes an era in which ICD8 was in use and a period where ICD10 was in use. The classification of diseases changed somewhat over this period. The general tendency of the change was to specify more precisely the potential autoimmune aspects of diseases.
This study focused on 31 probable or possible autoimmune diseases (Table 1). People in the study population were classified with a history of an autoimmune disease if they have been admitted to hospital or been in outpatient care in a hospital-based clinic with a diagnosis of the disease in question before January 1, 2002. The names given here for the disease are as stated in the ICD10, with the codes chosen to isolate as closely as possible the form of the disease with the autoimmune basis. This manner of analysis provides for each person to have a history of more than one autoimmune disease.
TABLE 1.
Lifetime Prevalence of 31 Autoimmune Diseases From two Reviews and among 5,472,032 persons in Denmark in 2001 per 1000 population
| Disease | ICD8 | ICD10 | Number of “High” Comorbidities$ | Prior Studies* | Number of Cases† | Prevalence |
|---|---|---|---|---|---|---|
| Thyrotoxicosis | 242.0 | E05 | 1 | 11.51 | 34,434 | 6.29 |
| Autoimmune Thyroiditis | 245 | E06.3 | 5 | 7.92# | 3,388 | 0.62 |
| Insulin Dependent Diabetes | 250.0 | E10 | 6 | 1.92£ | 51,783 | 9.46 |
| Primary Adrenocortical Insufficiency | 255.1 | E27.1 | 7 | 0.14 | 983 | 0.18 |
| Celiac Disease | 269.0 | K90.0 | 9 | 9.9a | 2,722 | 0.50 |
| Pernicious Anemia | 281.0 | D51.0 | 8 | 1.51 | 2,947 | 0.54 |
| Autoimmune Haemolytic Anemia | 283.9 | D59.1 | 11 | -- | 912 | 0.17 |
| Idiopathic Thrombocytopenic Purpura | 287.19 | D69.3 | 3 | -- | 3,940 | 0.72 |
| Multiple Sclerosis | 340.0 | G35 | 4 | 0.58 | 9,961 | 1.82 |
| Guillain Barre Syndrome | 354.0 | G61.0 | 6 | ∼0.38b | 3,926 | 0.72 |
| Iridocyclitis | 366.0 | H20 | 5 | 0.02 | 8,126 | 1.49 |
| Wegener’s Granulomatosis | 446.29 | M31.3 | 6 | 0.03 | 568 | 0.10 |
| Crohn’s Disease | 563.0 | K50 | 2 | 0.54c | 12,309 | 2.25 |
| Ulcerative Colitis | 563.19 | K51 | 5 | 1.61d- | 20,669 | 3.78 |
| Primary Biliary Cirrhosis | 571.90 | K74.3 | 10 | 0.04 | 666 | 0.12 |
| Chronic Hepatitis, n.e.c. | 571.93 | K73 | 11 | 0.004 | 2,472 | 0.45 |
| Interstitial Cystitis | 595.0 | N30.1 | 3 | 3.0e | 39,496 | 7.22 |
| Endometriosis | 625.3 | N80 | 0 | -- | 26,882 | 4.91 |
| Pemphigoid | 693 | L12.0 | 5 | -- | 895 | 0.16 |
| Pemphigus | 694 | 2 | -- | 195 | 0.04 | |
| Psoriasis vulgaris | 696 | L40.0 | 6 | 15.0f | 10,788 | 1.97 |
| Alopecia Areata | 704.00 | L63 | 5 | 17.0g-- | 1,172 | 0.21 |
| Vitiligo | 709.0-19 | L80 | 10 | 4.0 | 1,576 | 0.29 |
| Seropositive Rheumatoid Arthritis | 712 | M05 | 10 | 8.6¤ | 20,831 | 3.81 |
| Dermatopolymyositis | 716 | M33 | 12 | 0.05 | 949 | 0.17 |
| Myositis | 717.90 | M60 | 4 | -- | 45,051 | 8.23 |
| Polymyalgia Rheumatica | 717.99 | M31.5 | 3 | 6.1h | 6,136 | 1.12 |
| Myasthenia Gravis | 733.09 | G70.0& | 6 | 0.05 | 977 | 0.18 |
| Systemic Sclerosis | 734.0 | M34 | 9 | 0.04 | 1,257 | 0.23 |
| Systemic Lupus Erythematosis | 734.19 | M32.1@ | 14 | 0.24 | 1,732 | 0.32 |
| Sjogren’s Sydrome | 734.90 | M35.0 | 14 | 0.14 | 2,615 | 0.48 |
Notes for Table 1
Including G70.0, G70.2, G70.8 and G70.9
Including M32.1, M32.8 and M32.9.
A “high” comorbidity is defined in the text.
Prior studies from review of Jacobson et al, 1997, as updated by Cooper et al, 2003.
Ages > 19.
Ages < 20.
Adult
The specific autoimmune diseases are not mutually exclusive: that is, a person may have more than one autoimmune disease.
(39)
(40). Incidence was estimated in that study as 1.26/100,000/year. Assuming little mortality and 30 years of risk, the prevalence was estimated to be 37.8.
(41)
(42)
(43)
(44)
(45)
(46)
The National Hospital Register has advantages not found in other epidemiologic research studies. For example, it is unlikely another type of research than register-based would generate data across such a broad range of autoimmune diseases as considered here. In the National Hospital Register cases not treated in hospitals or hospital based clinics will not be recorded, as well as cases not in the medical system at all. Some diseases, such as hypothyroidism and Type 1 diabetes, may be effectively treated in primary care and thus not enter the registers at each visit, but we expect that many of the persons with autoimmune diseases are likely to eventually come to the attention of specialists even if care is initially provided by a primary care practitioner.
Estimation of Prevalence
The focus here is on the lifetime prevalence of disease, which we define as the proportion in the population in 2001 which has the disease currently or has a history of the disease. The life time prevalence for each disease was estimated as the proportion of the persons alive and living in Denmark on December 31, 2001 who were diagnosed with the disease in the period from 1977 to 2001. Individuals with more than one disease were counted in the numerators of each disease. Since the register began operation in 1977, there is a full record on all diagnoses given at Danish hospitals only for persons younger than 25 years of age on December 31, 2001. Persons older than that will enter the data-set only if a visit is recorded after 1977. This implies an underestimation of the prevalence.
Estimation of Comorbidity
For each of the 465 pairwise combinations of the 31 diseases, logistic regression (LOGISTIC procedure in SAS version 9.1) was used to estimate the ratio of the odds of having a history of a disease among people with and without a history of the other disease. To account for age and sex specific variation in the occurrence of the disease, all estimates of odds ratios were adjusted for sex and its interaction with age (on December 31, 2001). For most diseases age was categorized in 5 year intervals up to 80 years, while for the more rare diseases 10 year intervals were used. The youngest age group was sometimes grouped in larger intervals (0-9, 0-19 or 0-29 years). The lower 95% confidence limit was calculated by Wald’s test.
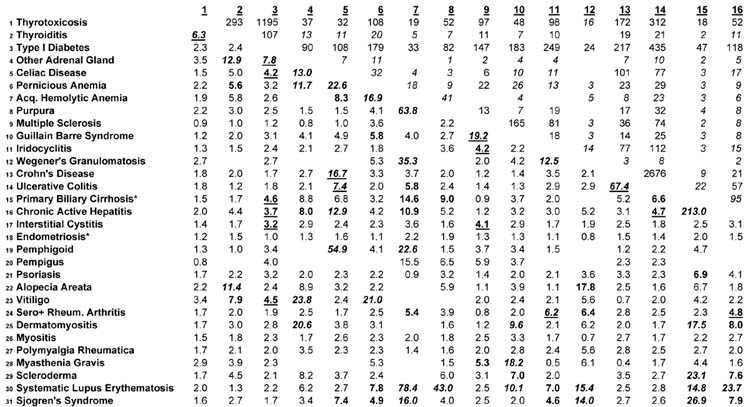
For each pairwise combination of diseases the expected number of people with both diseases was calculated as the sum of expected cases for each sex on the assumption of no association between the two diseases. The rarity of the autoimmune diseases makes estimation of confidence intervals problematic when studying comorbidity, because there are so few co-occurring cases, even in the entire population. The estimation of the confidence interval is called into question when the number of cases expected on the hypothesis of no association is below 5, as it is for many of the co-occurrences in Table 2. Table 2 shows observed, not expected cases, above the diagonal. To ensure that the reader is appropriately circumspect about this issue, we have italicized entries above the diagonal in Table 2 when the expected numbers of cases is lower than 5, and the presentation includes explicit designation of low frequency situations.
Table 2.
Comorbidity of 31 Autoimmune Diseases in Denmark Numbers of Co-occurring Cases above the diagonal Pairwise Odds Ratios Below the Diagonal
 |
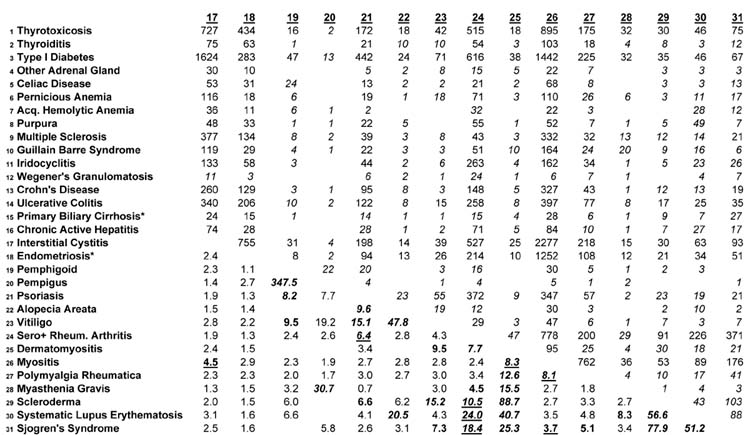 |
Numbers of Co-occurring Cases are italicized if the number of expected cases with both diseases is below 5 on the assumption of no association
Odds Ratios with Lower 95% Confidence Intervals > 5.0 are boldface and italicized
Odds Ratios with Lower 95% Confidence Intervals > 3.0 and < 5.0 are boldface
Boldfaced Odds Ratios are underlined if the number of expected cases with both diseases exceeds 5 on the assumption of no association
Familial aggregation for diseases was studied in similar fashion. We also adjusted for the three variables indicating whether the identity of the mother and father are known and whether we know the identity of any siblings. The situation in which the expected number of co-occurring cases, on the hypothesis of no association, was less than 5, is noted in the presentation.
The study was approved by the Danish Data Protection Agency.
RESULTS
The 31 individual autoimmune diseases are rare, in general, but vary in lifetime prevalence by about two orders of magnitude, from Pemphigus (0.04/1000) and Primary Biliary Cirrhosis (0.12/1000), with less than than 20 per 100,000, to nearly 10 per 1000 (that is, one percent, as with Diabetes and Myositis: Table 1). The column entitled “prior studies” shows data from the two prior reviews (2, 3). Eight studies appearing after those reviews or for diseases not included in those reviews are added to Table 1. For six of the 31 diseases there are no prior estimates of prevalence.
The total number of persons with one or more autoimmune disease is 289,228. The lifetime prevalence proportion for any autoimmune disease among the population of 5,472,032 is thus 5.29%. The portion of Table 2 above the diagonal may be used to estimate the lifetime prevalence of any pair of co-occurring diseases, using the figure in the table as the numerator and the total population of Denmark in 2001 as the denominator. Thus, for example, the prevalence of the co-occurrence of type 1 diabetes and rheumatoid arthritis is 616/5,472,032, or about 1 in 10,000. It is also possible to estimate the number with either disease, by adding the two prevalence frequencies in Table 1 and subtracting the number of jointly occurring cases.
Most autoimmune diseases are more common in females (Figure 1). Sjogren’s Syndrome, the thyroid diseases, SLE, and scleroderma (systemic sclerosis) are dominated by females, in close agreement with data presented on 10 diseases by Whitacre(4). There is good agreement on four further diseases presented here and in that earlier work (rheumatoid arthritis, multiple sclerosis, ulcerative colitis, and Type 1 diabetes: our presentation does not include sarcoidosis).
Figure 1.
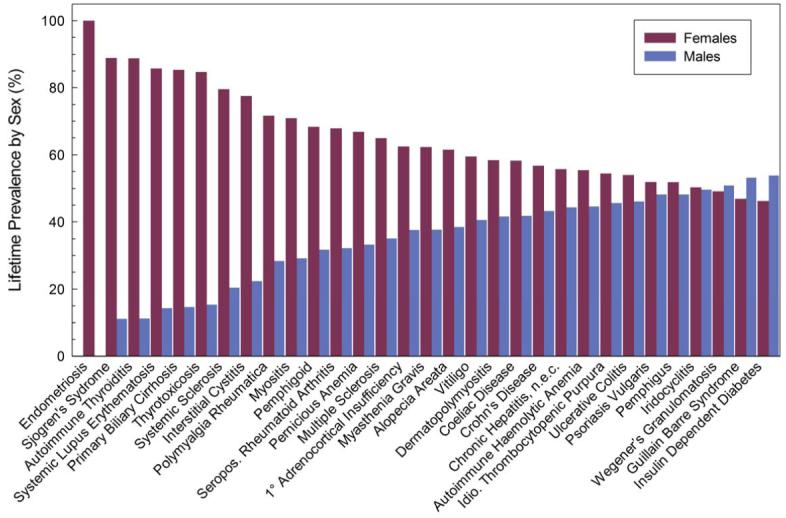
Prevalence of 31 autoimmune diseases by sex
There is considerable comorbidity in the autoimmune diseases, shown in the numbers of co-occurring cases above the diagonal, and the Odds Ratios below the diagonal, in Table 2. In 31 diseases, there are 465 combinations of two diseases, as in the Table. The Odds Ratios are estimated from the logistic regression predicting one disease from another, with the sample size being the population of Denmark on December 31, 2001 (5,472,032) and the numbers of cases as in Table 1: for example, 34,434 cases of Thyrotoxicosis; 3,388 cases of Thyroiditis, and so forth. The strength and statistical stability of the Odds Ratios are distinguished using italicized and boldface fonts. Odds Ratios whose lower 95% confidence limit is greater than 5.0 are in boldface and italicized; Odds Ratios whose lower 95% confidence limit is between 3.0 and 5.0 are boldface; other Odds Ratios are in normal font. Boldfaced Odds Ratios are underlined when the expected number of cases with both diseases is above 5 on the hypothesis of no association between the two diseases—these represent the strongest evidence of comorbidity. Blanks in the table indicate no co-occurring cases in the population at all (36 pairwise combinations with not a single co-occurring case in the entire population of Denmark). For efficiency of presentation, below, the boldface Odds Ratios are collectively denoted as “high.” We have examined the 465 Odds Ratios for each sex and not been struck by the differences in comorbidity, and for efficiency present the sexes combined in Table 2. The middle column in Table 1 helps assess the degree of comorbidity, reporting, for each disease, the number of other diseases with “high” Odds Ratios. The connective tissue diseases have higher comorbidities, in general, than other diseases: for example, there are 14 other diseases which are highly comorbid with Systemic Lupus Erythematosus; and likewise 14 with Sjogren’s Syndrome. This compares with relatively limited comorbidity of the thyroid diseases, for example, and no diseases comorbid with Endometriosis (females only). The presence of comorbidities reported to be “high” is influenced by the prevalence of the diseases, because the confidence intervals for rare diseases are wider.
The pattern of comorbidities is diverse. The general pattern is for the presence of any one disease to be associated with higher prevalence of any other. Of the 429 Odds ratios estimated, only 12 were less than 1.0, indicating a negative association (possibly “protective” effect) of one disease against another. There are 101 out of 465 “high” Odds Ratios in Table 2. The Odds Ratios for the two sexes (not shown) have roughly similar patterns: 50 of the “high” Odds Ratios reported by males are reported also for that pair of diseases for females. Endometriosis, for which the evidence of autoimmune cause is relatively weak (27), has the lowest comorbidity, with no “high” odds ratios.
The pattern of co-occurrence in families differs distinctly from that in individuals, in that it is concentrated in single diseases. Three separate tables of 465 pairwise odds ratios for familial aggregation in sibs, parents, and for sibs and parents combined were estimated, but they are not shown because there was not a single odds ratio that would be designated as “high” according to the threshold discussed above with reference to Table 2. Therefore, the presentation focuses on the familial aggregation of individual diseases (Table 3). Two of the diseases had no co-occurring cases in families (Wegener’s Granulomatosis and Pemphigus), and 16 others (denoted with asterisks) had expected numbers of co-occurring cases less than five, leading us to be cautious about the interpretation of the results. For four other diseases (Acquired Hemolytic Anemia, Primary Biliary Cirrhosis, Pemphigoid, and Dermatomyositis) there were no cases among either the parents or sibs. For these diseases the overall parent-or-sib odds ratio will necessarily be lower than the respective sib odds ratio or parent odds ratio, because the number of people with both a personal history and a family history of the disease is the same in the two situations but the number of people with a family history and without a personal history of the disease is larger when both parents and siblings are considered.
Table 3.
Familial Aggregation of 31 Autoimmune Diseases† in Denmark
| Disease in cohort member | Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| History of the given disease in: | |||
| Parents or Sibs | Parents | Sibs | |
| Thyrotoxicosis | 3.4 (3.1-3.6) | 2.9 (2.6-3.1) | 5.2 (4.6-6.0) |
| Thyroiditis* | 13.9 (9.8-19.6) | 9.2 (5.7-14.6) | 30.1 (18.2-49.7) |
| Type I Diabetes | 4.0 (3.8-4.2) | 3.0 (2.9-3.2) | 8.8 (8.3-9.4) |
| Other Adrenal Gland* | 68.4 (43.9-106.7) | 29.3 (13.9-62.1) | 205.4 (123.1-342.7) |
| Intestinal Malabsorption* | 53.0 (44.0-63.8) | 41.8 (32.5-53.9) | 83.2 (66.0-104.9) |
| Pernicious Anemia* | 16.2 (9.9-26.6) | 12.5 (7.0-22.4) | 57.4 (23.3-141.3) |
| Acquired Hemolytic Anemia* | 3.6 (0.5-25.8) | 15.2 (2.1-108.4) | |
| Purpura | 22.9 (19.4-27.0) | 22.8 (18.2-28.6) | 32.0 (26.4-38.8) |
| Multiple Sclerosis | 6.0 (5.2-6.7) | 5.3 (4.5-6.3) | 7.1 (5.8-8.8) |
| Guillain Barre Syndrome | 2.3 (1.3-4.1) | 1.7 (0.8-3.5) | 5.0 (2.1-12.1) |
| Uveitis | 3.3 (2.6-4.2) | 2.9 (2.1-4.0) | 4.1 (3.0-5.8) |
| Wegener’s Granulomatosis | |||
| Crohn’s Disease | 5.4 (4.8-6.0) | 5.1 (4.3-5.9) | 5.7 (4.9-6.6) |
| Ulcerative Colitis | 4.4 (4.1-4.8) | 4.1 (3.7-4.6) | 5.0 (4.5-5.6) |
| Primary Biliary Cirrhosis (90%)* | 31.5 (9.9-100.2) | 35.0 (11.0-111.4) | |
| Chronic Active Hepatitis* | 8.3 (5.1-13.4) | 5.3 (2.6-10.6) | 16.8 (8.7-32.6) |
| Interstitial Cystitis | 1.7 (1.6-1.9) | 1.6 (1.4-1.7) | 2.2 (2.0-2.5) |
| Endometriosis* | 2.3 (2.1-2.5) | 1.9 (1.7-2.2) | 2.8 (2.5-3.2) |
| Pemphigoid* | 23.1 (9.5-56.1) | 32.6 (13.4-79.4) | |
| Pemphigus | |||
| Psoriasis | 7.5 (6.7-8.5) | 7.3 (6.3-8.4) | 8.6 (7.1-10.5) |
| Alopecia Areata* | 22.7 (13.1-39.4) | 10.7 (3.4-33.4) | 33.6 (18.0-63.0) |
| Vitiligo* | 7.9 (4.1-15.2) | 5.1 (1.7-15.9) | 10.8 (4.8-24.3) |
| Seropositive Rheumatoid Arthritis | 3.4 (3.0-3.8) | 3.0 (2.6-3.4) | 5.9 (4.7-7.4) |
| Dermatomyositis* | 3.9 (0.6-27.7) | 4.9 (0.7-35.2) | |
| Myositis | 2.2 (2.1-2.4) | 2.1 (1.9-2.2) | 2.6 (2.3-2.9) |
| Polymyalgia Rheumatica | 3.6 (2.2-5.7) | 3.6 (2.2-6.0) | 3.2 (0.8-12.8) |
| Myasthenia Gravis* | 78.5 (49.7-124.1) | 92.7 (57.3-149.8) | 91.3 (40.4-206.4) |
| Scleroderma* | 7.2 (2.3-22.6) | 2.9 (0.4-20.9) | 26.2 (6.5-105.5) |
| Systematic Lupus Erythematosis* | 18.1 (11.7-28.0) | 5.8 (2.4-14.0) | 48.9 (29.5-81.1) |
| Sjogren’s Syndrome* | 12.8 (7.0-23.3) | 14.8 (8.1-27.0) | 16.6 (4.1-67.1) |
ICD definitions of Diseases as categorized as Table 1.
Less than five co-occurring cases in the family expected on the hypothesis of no association
There is considerable familial aggregation in almost all 31 diseases. The possible exceptions, with overall parent and sib odds ratios below 3.0, are Guillain Barre Syndrome (2.3; 95% CI 1.3-4.1), Intersitital Cystitis (1.7; 95% CI 1.6-1.9), and Myositis (2.2; 95% CI 2.1-2.4). Endometriosis, with a limited number of expected cases, also had low familial aggregation. There are several diseases with odds ratios above 20.0 (Other Adrenal Gland, Intestinal Malabsorption, Primary Biliary Cirrhosis, Alopecia Areat, and Myasthenia Gravis, and Purpura, but with the exception of Purpura (22.0, 95% CI 19.4-27.0) these all have small numbers of cases. Purpura, Psoriasis, Multiple Sclerosis, and Crohn’s disease have the strongest and most credible evidence for familial aggregation.
The odds ratios for sibs are higher than the odds ratios for parent to child aggregation, which suggests common environmental influences over and above genetic transmission. For more than ten diseases the sib odds ratio is more than double the parent to offspring odds ratio. The only exceptions to this trend are Polymyalgia Rheumatica and Myasthenia Gravis.
DISCUSSION
Many autoimmune diseases are rare enough to suspect that estimates from earlier smaller studies are unstable. Comparison of the prevalence in Denmark with data from prior studies is fraught with difficulties since there are ethnic and national differences in the prevalence of the diseases. For some diseases there are discrepancies that suggest under-reporting in the context of the health system in Denmark. For example, the estimate of the lifetime prevalence of Autoimmune Thyroiditis is less than one tenth the estimate from prior research, and it seems possible that this condition is frequently treated without recourse to specialty care. Likewise, it may be that Vitiligo is less frequently noticed and treated in Denmark than in countries with darker-skinned populations. For some diseases, such as Type 1 Diabetes, the prevalence in this study is higher than prior research would suggest. This may have resulted from diagnosing and reporting in the register Type 2 diabetics who were taking insulin as Type 1.
The analysis with register data is inevitably tied to the International Classification of Diseases, with its strengths and limitations. Some potentially important autoimmune diseases are not included (e.g., Anti-Phospholipid Syndrome); and for others, autoimmune etiologies may not be totally isolated (e.g., Diabetes in ICD-8); for yet others, interesting subcategories of disease are not available for analysis (e.g., subcategories of Autoimmune Thyroiditis, listed under ICD-9 E06.3 but not with separate designations). The diagnoses are those recorded by treating physicians in the routine practice of care.
The results on comorbidity are consistent with a prior systematic analysis in finding positive association between Autoimmune Thyroiditis and Type 1 Diabetes, and Autoimmune Thyroiditis and Rheumatoid Arthritis, and a negative association between Multiple Sclerosis and Rheumatoid Arthritis(9). The results on Primary Biliary Cirrhosis and Systemic Lupus Erythematosis, and Sjogren’s Syndrome, are consistent with an earlier case control study, but the low comorbidity found in Denmark for Primary Biliary Cirrhosis and Autoimmune Thyroid Disease is not consistent with the relatively high comorbidity found in that earlier study(10). Prior data are from uncontrolled studies, for the most part, and the results presented here have broad confidence intervals, so that the confirmation is only suggestive. The results presented here are to be considered exploratory, awaiting further confirmation. In some cases, such as the Odds Ratio of 213.0 for Primary Biliary Cirrhosis and Chronic Active Hepatitis, there may be misdiagnosis, shifting from one to the other on successive specialist visits; or the diseases themselves may employ related pathogenetic processes (e..g, Multiple Sclerosis and Guillain-Barré syndrome both involving demyelinization: OR of 19.2). Some comorbidities may represent clinical overlap (e.g., Pemphigus and Pemphigoid: OR of 347.5). The disease with the lowest degree of comorbidity was Endometriosis, which suggests it belongs to another family of diseases than Autoimmune.
Some diseases, such as Celiac Disease or Rheumatoid Arthritis, have comorbidities extending across the entire range of the autoimmune diseases. There may be a common cause linked to a consequence of the disease. For example, the comorbidity associated with Celiac Disease may be related to increased permeability of the intestine, allowing passage of a wide range of antigen-like substances which may stimulate autoimmune processes in various organ systems(28, 29, 30, 31, 32). Prior research on comorbidity of Celiac Disease and Primary Biliary Cirrhosis estimated incidence rate ratios (IRR’s) based on two co-occurring cases in Denmark (IRR of 27.6) and 22 co-occurring cases in Sweden (IRR of 25.1) (33). These estimates are higher than our odds ratio of 6.8 in Table 2, based on three co-occurring cases. However these authors failed to adjust for age and sex, which may have heightened the degree of association, since sex is strongly associated with Primary Biliary Cirrhosis. Even with these differences all three estimates show what might be termed high comorbidity. For many diseases the high comorbidities are for diseases within the same organ system, such as the odds ratios within the connective tissue diseases or the endocrine diseases—for these diseases the common process may be local to that system. Some of the high comorbidities reported here would not have been predicted from the literature or from what is known about the diseases to date, such as the comorbidity of Pemphigoid and Celiac Disease (OR of 54.9).
Many pitfalls exist in the data. Due to the potential incomplete registration of both diseases in question, estimates of odds ratios may be biased if admission with an autoimmune disease changes the likelihood of being registered with another previously undiagnosed autoimmune disease. This bias would exist also for occurrence in families, and perhaps most strongly in sibs. This bias is likely to be high (34). Furthermore, it could be the situation that the diagnoses themselves generate positive biases, in that clinicians examining a medical record may be more likely to diagnose a disease which they perceive to be likely. Possibly these comorbidity biases are stronger within individuals than within families.
The comorbidity may represent common etiopathologic processes. One possibility is a shared genetic basis(35, 36), generating a system-wide lower threshold for recognition of self antigens as foreign. But the high individual comorbidity, compared to relatively lower familial aggregation, suggests that genes common to the group of autoimmune diseases as a whole are only a part of the explanation. Prior results on familial aggregation across different diseases is scant (reviewed above), but these data from Denmark do not confirm some prior results. For example, the odds ratio for Diabetes for those with Multiple Sclerosis in the family, versus those with no Multiple Sclerosis in the family, is about 1.0 in these data; the analogous odds ratio for Diabetes with Rheumatoid Arthritis in the family is about 1.5; for Diabetes and Autoimmune Thyroiditis about 1.5; for Autoimmune Thyroiditis and Rheumatoid Arthritis about 1.3 (data not shown in tables). The high familial aggregation for individual diseases suggests that the genetic origins of the diseases are specific, not general. The tendency for familial aggregation to be stronger in sibs than from parents to offspring suggests a common environmental cause, not a genetic cause. These results are consistent with a gene by environment interaction which is one feature of many autoimmune diseases(37, 38)
The prevalence of most autoimmune diseases is low, in general. But when considered collectively, as here, they represent a sizable prevalence of more than 5%-- the first available systematic data on this particular issue. These results suggest more conclusively than has been known before the extensive comorbidity between autoimmune diseases. These comorbidities deserve further exploration for possible etiologic clues that may emanate from linking one set of findings on pathogenesis to another.
Acknowledgements
Supported by NIMH Grant MH53188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Autoimmune Diseases Coordinating Committee . Bethesda, Maryland, National Institute of Allergy and Infectious Diseases. National Institutes of Health; 2002. Autoimmune Diseases Research Plan. [Google Scholar]
- 2.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical Immunology and Immunopathology. 1997;84:223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 4.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 5.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein A. Clinical Judgement. Williams & Wilkins Company; Baltimore: 1967. [Google Scholar]
- 7.Rodriguez-Reyna TS, Alarcon-Segovia D. The different faces of shared autoimmunity. Autoimmun Rev. 2006;5:86–8. doi: 10.1016/j.autrev.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Sloka S. Observations on recent studies showing increased co-occurrence of autoimmune diseases. J Autoimmun. 2002;18:251–7. doi: 10.1006/jaut.2002.0588. [DOI] [PubMed] [Google Scholar]
- 9.Somers EC, Thomas SL, Smeeth L, Hall AJ. Autoimmune diseases co-occurring within individuals and within families: a systematic review. Epidemiology. 2006;17:202–17. doi: 10.1097/01.ede.0000193605.93416.df. [DOI] [PubMed] [Google Scholar]
- 10.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrosu MG, Cocco E, Lai M, Spinicci G, Pischedda MP, Contu P. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet. 2002;359:1461–5. doi: 10.1016/S0140-6736(02)08431-3. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen NM, Westergaard T, Frisch M, Rostgaard K, Wohlfahrt J, Koch-Henriksen N, Melbye M, Hjalgrim H. Type 1 diabetes and multiple sclerosis: A Danish population-based cohort study. Arch Neurol. 2006;63:1001–4. doi: 10.1001/archneur.63.7.1001. [DOI] [PubMed] [Google Scholar]
- 13.Panczel P, Falus A, Meretey K, Romics L, Gyodi E, Vertes P, Bohm U, Petranyi G. [Association between cumulative familial incidence of type I diabetes and rheumatoid arthritis] Orv Hetil. 1985;126:1281–4. 1287–9. [PubMed] [Google Scholar]
- 14.Thomas DJ, Young A, Gorsuch AN, Bottazzo GF, Cudworth AG. Evidence for an association between rheumatoid arthritis and autoimmune endocrine disease. Ann Rheum Dis. 1983;42:297–300. doi: 10.1136/ard.42.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JP, Cash JM, Doyle SZ, Peden S, Kanik K, Amos CI, Bale SJ, Wilder RL. Familial clustering of rheumatoid arthritis with other autoimmune diseases. Hum Genet. 1998;103:475–82. doi: 10.1007/s004390050853. [DOI] [PubMed] [Google Scholar]
- 16.Anaya JM, Castiblanco J, Tobon GJ, Garcia J, Abad V, Cuervo H, Velasquez A, Angel ID, Vega P, Arango A. Familial clustering of autoimmune diseases in patients with type 1 diabetes mellitus. J Autoimmun. 2006;26:208–14. doi: 10.1016/j.jaut.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Ginn LR, Lin JP, Plotz PH, Bale SJ, Wilder RL, Mbauya A, Miller FW. Familial autoimmunity in pedigrees of idiopathic inflammatory myopathy patients suggests common genetic risk factors for many autoimmune diseases. Arthritis Rheum. 1998;41:400–5. doi: 10.1002/1529-0131(199803)41:3<400::AID-ART4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Anaya JM, Tobon GJ, Vega P, Castiblanco J. Autoimmune disease aggregation in families with primary Sjogren’s syndrome. J Rheumatol. 2006;33:2227–34. [PubMed] [Google Scholar]
- 19.Midgard R, Gronning M, Riise T, Kvale G, Nyland H. Multiple sclerosis and chronic inflammatory diseases. A case-control study. Acta Neurol Scand. 1996;93:322–8. doi: 10.1111/j.1600-0404.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen NM, Westergaard T, Rostgaard K, Frisch M, Hjalgrim H, Wohlfahrt J, Koch-Henriksen N, Melbye M. Familial risk of multiple sclerosis: a nationwide cohort study. Am J Epidemiol. 2005;162:774–8. doi: 10.1093/aje/kwi280. [DOI] [PubMed] [Google Scholar]
- 21.Hemminki K, Li X, Johansson SE, Sundquist K, Sundquist J. Re: “Familial risk of multiple sclerosis: a nationwide cohort study”. Am J Epidemiol. 2006;163:873–4. doi: 10.1093/aje/kwj130. [DOI] [PubMed] [Google Scholar]
- 22.Morris JN. Uses of Epidemiology. Third Edition Churchill Livingstone; Edinburgh: 1975. [Google Scholar]
- 23.Pedersen Carsten B., Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Danish Medical Bulletin. 2006;53:441–9. [PubMed] [Google Scholar]
- 24.World Health Organization . Manual of the international statistical classification of diseases, injuries, and causes of death (ICD-8) World Health Organization; Geneva: 1967. [PMC free article] [PubMed] [Google Scholar]
- 25.Sundhedsstyrelsen . Klassifikation af sygdomme-systematisk del, 8 revision. Aarhus Stiftsbogtrykkerie; Aarhus: 1976. [Google Scholar]
- 26.World Health Organization . International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. World Health Organization; Geneva: 1992. [PubMed] [Google Scholar]
- 27.Odukoya OA, Wheatcroft N, Weetman AP, Cooke ID. The prevalence of endometrial immunoglobulin G antibodies in patients with endometriosis. Hum Reprod. 1995;10:1214–9. doi: 10.1093/oxfordjournals.humrep.a136121. [DOI] [PubMed] [Google Scholar]
- 28.Collin P, Kaukinen K, Valimaki M, Salmi J. Endocrinological Disorders and Celiac Disease. Endocrine Reviews. 2002;23:464–83. doi: 10.1210/er.2001-0035. [DOI] [PubMed] [Google Scholar]
- 29.Elliott DE, Murray JA, Weinstock JV. Inflammatory Bowel and Celiac Disease. In: Rose NR, Mackay IR, editors. The Autoimmune Diseases. Academic Press; New York: 1998. pp. 477–509. [Google Scholar]
- 30.Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, Maseri A, Gasbarrini G. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–8. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]
- 31.James M, Scott B. Coeliac disease: the cause of the various associated disorders? European Journal of Gastroenterology and Hepatology. 2001;13:1119–21. doi: 10.1097/00042737-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Petaros P, Martelossi S, Tommasini A, Torre G, Caradonna M, Ventura A. Prevalence of autoimmune disorders in relatives of patients with celiac disease. Digestive Disease Science. 2002;47:1427–31. doi: 10.1023/a:1015830110836. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen HT, Thulstrup AM, Blomqvist P, Norgaard B, Fonager K, Ekbom A. Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut. 1999;44:736–8. doi: 10.1136/gut.44.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2:47–53. [PubMed] [Google Scholar]
- 35.Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF, Trent JM. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc Natl Acad Sci U S A. 1998;95:9979–84. doi: 10.1073/pnas.95.17.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wandstrat A, Wakeland E. The genetics of complex autoimmune diseases: non-MHC susceptibility genes. Nat Immunol. 2001;2:802–9. doi: 10.1038/ni0901-802. [DOI] [PubMed] [Google Scholar]
- 37.Mackay IR. The etiopathogenesis of autoimmunity. Semin Liver Dis. 2005;25:239–50. doi: 10.1055/s-2005-916330. [DOI] [PubMed] [Google Scholar]
- 38.Alper CA, Husain Z, Larsen CE, Dubey DP, Stein R, Day C, Baker A, Beyan H, Hawa M, Ola TO, Leslie RD. Incomplete penetrance of susceptibility genes for MHC-determined immunoglobulin deficiencies in monozygotic twins discordant for type 1 diabetes. J Autoimmun. 2006;27:89–95. doi: 10.1016/j.jaut.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maki M, Mustalahti K, Kokkonen J, Kilmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, Hopfl P, Knip M. Prevalence of celiac disease among children in Finland. The New England Journal of Medicine. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 40.Cuadrado J, Pedro-Cuesta J, Ara J, Cemillan C, Diaz M, Duarte J, Fernandez F, Garcia-Lopez F, Carcia-Merino A, Velasquez J, Martinez-Matos J, Pardo P, Tobias A. Public health surveillance and incidence of adulthood Guillain-Barre syndrome in Spain, 1998-1999: the view from a sentinel network of neurologists. Neurol Sci. 2004;25:57–65. doi: 10.1007/s10072-004-0231-6. [DOI] [PubMed] [Google Scholar]
- 41.Munkholm P, Langholz E, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of Crohn’s disease in the county of Copenhagen, 1962-87: a sixfold increase in incidence. Scand J Gastroenterol. 1992;27:609–14. doi: 10.3109/00365529209000127. [DOI] [PubMed] [Google Scholar]
- 42.Langholz E, Munkholm P, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of ulcerative colitis in Copenhagen county from 1962 to 1987. Scand J Gastroenterol. 1991;26:1247–56. doi: 10.3109/00365529108998621. [DOI] [PubMed] [Google Scholar]
- 43.Leppilahti M, Sairanen J, Tammela T, Aaltomaa S, Lehtoranta K, Auvinen A. Prevalence of clinically confirmed interstitial cystitis in women: a population based study in Finland. The Journal of Urology. 2005;174:581–3. doi: 10.1097/01.ju.0000165452.39125.98. [DOI] [PubMed] [Google Scholar]
- 44.Gelfand J, Weinstein R, Porter S, Neimann A, Berlin J, Margolis D. Prevalence and treatment of Psoriasis in the United Kingdom - a population based study. Archives of Dermatology. 2005;141:1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 45.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–33. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 46.Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. Epidemiology of polymyalgia rheumatica in Olmsted County, Minnesota, 1970-1991. Arthritis Rheum. 1995;38:369–73. doi: 10.1002/art.1780380311. [DOI] [PubMed] [Google Scholar]


