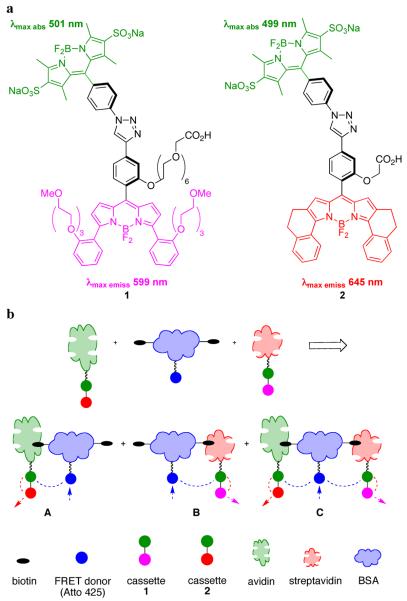
Fluorescence resonance energy transfer (FRET) is a key technique for monitoring protein-protein interactions.1,2 However, a restriction of FRET is that the fluorescence emission of the donor must overlap with the absorption of the acceptor. This limits the spectral output dispersion that can be achieved using a single donor and a set of acceptors. To address this issue, our group has been involved in the design and syntheses of “through-bond energy transfer (TBET) cassettes” in which a donor relays energy to an acceptor predominantly via twisted π-electron systems.3,4 A major obstacle, however, has been syntheses of water-soluble TBET-cassettes that efficiently relay energy to the acceptor, and emit it as fluorescence in aqueous environments. This communication describes cassettes, 1 and 2 (Figure 1a), that have good spectral properties in this regard, and their application to observe protein-protein interactions in vitro and in live cells.
Figure 1.
a Featured TBET-cassettes; b FRET from one protein can be used to excite the donors of TBET-cassettes on another protein to reveal interactions.
Three innovations led to the synthesis of cassettes 1 and 2. First, functionalized, water-soluble BODIPY donor fragments were prepared via sulfonation of lipophilic precursors.5 Second, constrained and functionalized BODIPY acceptor synthons were made from aryl-substituted pyrroles.6 Third, copper-mediated alkyne/azide cycloadditions7 were used to join the donor and acceptor fragments giving 1,2,3-triazole-based linkers. Cassettes 1 and 2 are soluble in aqueous media. We define the “overall quantum yield” for the cassettes when excited at the donor absorption maximum (500 nm) to be the number of photons emitted from the acceptor part divided by the number absorbed for excitation. These are 0.30 and 0.24 for 1 and 2, respectively, in pH 7.4 phosphate buffer. The efficiency of energy transfer from the donor to the acceptor is 0.94 and 0.92 in phosphate buffer. Finally, cassettes 1 and 2 have absorption maxima at around 500 nm, and fluoresce maximally at 599 nm and 645 nm in pH 7.4 phosphate buffer. Thus, they have huge “apparent Stokes shifts”: 98 and 146 nm.
A well-behaved system for studying protein-protein interactions was needed to test cassettes 1 and 2. This was generated via the interaction of biotin-functionalized bovine serum albumin (BSA) with avidin or streptavidin.8 Thus, BSA was reacted with N-hydroxysuccinimide (NHS) activated forms of: (i) the FRET donor Atto425 (a coumarin derivative; Sigma); then, (ii) biotin; the dye:biotin:BSA ratio was 5.2:6.6:1.0. Cassette 1 was activated (di-isopropylcarbodiimide, NHS) then coupled to streptavidin (dye:protein 2.5:1.0). Similarly, cassette 2 was coupled to avidin (dye:protein 1.6:1.0). The strategy followed was to mix these proteins, excite the Atto425 donor, and observe emissions from cassettes 1 and 2. Strong enhancements mean that binary complexes like A and B, three-component tertiary complexes like C, and/or higher order assemblies were formed. This experiment would not be possible using Atto-425 as a FRET donor and single dyes based on the acceptor components of 1 and 2 because the fluorescence emission from Atto-425 (centered on 480 nm) has minimal overlap with the absorbance spectrum of the TBET-acceptor from 1 and almost none with that in 2 (λmax abs 556 and 631 nm, respectively, in pH 7.4 phosphate buffer).
Several precautions were necessary before the key energy transfer experiment could be performed and interpreted. First, 1-streptavidin and 2-avidin were pretreated with BSA to monitor the impact of BSA-(strept)avidin interactions with no biotin involved. Second, to correct for variations of dye fluorescence properties in different protein environments, each probe was examined in each of the relevant protein-protein interactions, one dye at a time to give baseline “before FRET” values.
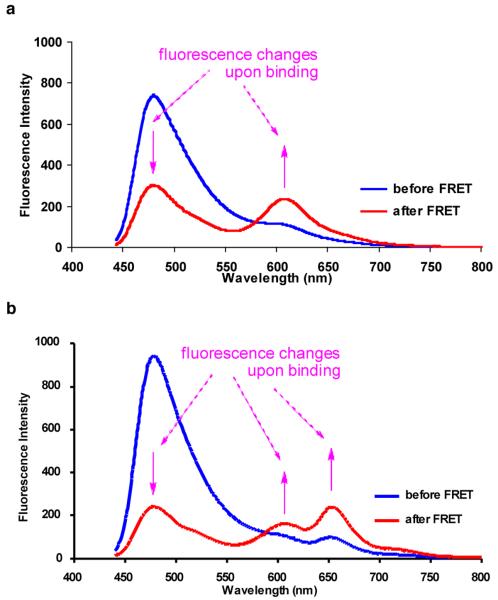
Figure 2a shows illustrative data for cassette 1 involved in a two-protein interaction; fluorescence from the FRET donor diminishes while that from the cassette is enhanced when the proteins are mixed (FRET efficiency from Atto425 to 1 was measured as 59%). Similar data was obtained for cassette 2 in the same experiments (see supporting Figure S16). Data in Figure 2b is for the corresponding three protein interactions (Atto425-BSA-biotin and 1-streptavidin and 2-avidin). Here the FRET donor fluorescence is diminished and that from the acceptor parts of both cassettes is enhanced. The overall FRET efficiency from Atto425 to cassettes 1 and 2 was measured as 74%.
Figure 2.
a Two protein interactions: Atto425-BSA-biotin + 1-streptavidin; b three protein interactions: Atto425-BSA-biotin + 1-streptavidin + 2-avidin. Throughout, the mixtures were excited at 430 nm. The concentrations of the proteins used were (x 10-7 M, in pH 7.4 phosphate buffer): Atto425-BSA-biotin, 2; 1-streptavidin, 3; 2-avidin, 9.
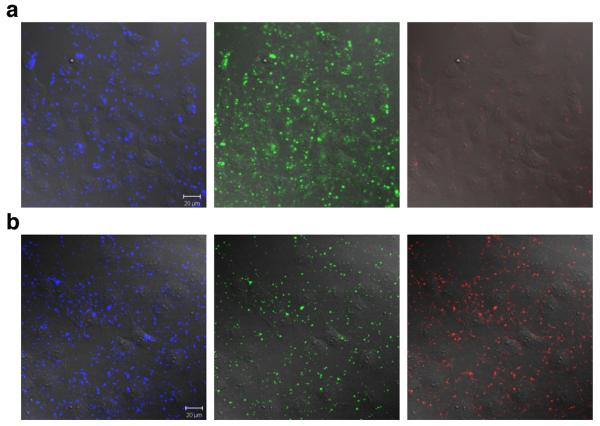
The protein-protein interactions were then investigated in COS-7 cells using Pep-19 to import the labeled proteins inside the cells. For each experiment, the FRET donor was first imported (10 min incubation at 37°C), the cells were thoroughly washed, and incubated for another 10 min at 37°C with the acceptors (1-streptavidin and/or 2-avidin as appropriate). No significant fluorescence upon excitation at 458 nm was observed in the acceptors channels (565-615 nm and 640-704 nm), when the FRET donor, 1-streptavidin, and 2-avidin were imported into cells individually (see supporting Figure S22-24). When the FRET donor and the acceptor were imported inside the cells, significant signal from the acceptor proteins (1-streptavidin or 2-avidin) was observed upon excitation at 458 nm indicating interactions between them (Figure 3a; similar data is given for cassette 2 in the supporting Figure S20). Similarly, when the FRET donor then a mixture of 1-streptavidin and 2-avidin were imported, interactions between all the labeled proteins were evident (Figure 3b).
Figure 3.
Cellular studies corresponding to the in vitro ones in Figure 2. a Atto425-BSA-biotin + 1-Streptavidin; b Atto425-BSA-biotin + 1-Streptavidin + 2-avidin. Overlay of fluorescence and DIC images of cells. Blue channel 480-520 nm, green channel 565-615 nm, and red channel 640-704 nm; all excited at 458 nm. Images were collected after 10 min incubation at 37 °C with the FRET donor, washing, and another 10 min incubation at 37 °C with the acceptors. Scale bar is 20 μm.
In conclusion, the through-bond energy transfer cassettes 1 and 2 are water-soluble probes that can be conjugated to proteins. These cassettes allow increased dispersion of fluorescence emissions from a single excitation source. A protein-FRET-donor system can be used to excite these cassettes on other proteins to test for protein-protein interactions in vitro and in living cells.
Supplementary Material
Acknowledgement
The TAMU/LBMS-Applications Laboratory directed by Dr Shane Tichy is thanked for assistance with mass spectrometry. Support for this work was provided by The National Institutes of Health (GM72041) and by The Robert A. Welch Foundation.
Footnotes
Supporting Information Available: Characterization data for cassettes 1 and 2. Data for electronic spectra of 1, 2, 1-streptavidin, 2-avidin, Atto425-BSA-biotin and Atto425-BSA. Full details of the control, and key energy transfer experiments. Procedures for determining dye:biotin:protein ratios. This material is available free of charge via Internet at http://pubs.acs.org.
References
- (1).Yan Y, Marriott G. Curr. Opin. Chem. Biol. 2003;7:635–640. doi: 10.1016/j.cbpa.2003.08.017. [DOI] [PubMed] [Google Scholar]
- (2).Miyawaki A, Tsien RY. Methods Enzymol. 2000;327:472–500. doi: 10.1016/s0076-6879(00)27297-2. [DOI] [PubMed] [Google Scholar]
- (3).Jiao G-S, Thoresen LH, Burgess K. J. Am. Chem. Soc. 2003;125:14668–14669. doi: 10.1021/ja037193l. [DOI] [PubMed] [Google Scholar]
- (4).Bandichhor R, Petrescu AD, Vespa A, Kier AB, Schroeder F, Burgess K. J. Am. Chem. Soc. 2006;128:10688–89. doi: 10.1021/ja063784a. [DOI] [PubMed] [Google Scholar]
- (5).Li L, Han J, Nguyen B, Burgess K. J. Org. Chem. 2008;73:1963–1970. doi: 10.1021/jo702463f. [DOI] [PubMed] [Google Scholar]
- (6).Chen J, Burghart A, Derecskei-Kovacs A, Burgess K. J. Org. Chem. 2000;65:2900–2906. doi: 10.1021/jo991927o. [DOI] [PubMed] [Google Scholar]
- (7).Meldal M, Tornoe CW. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- (8).Dong D, Zheng D, Wang F-Q, Yang X-Q, Wang N, Li Y-G, Guo L-H, Cheng J. Anal. Chem. 2004;76:499–501. doi: 10.1021/ac035184p. [DOI] [PubMed] [Google Scholar]
- (9).Morris MC, Depollier J, Mery J, Heitz F, Divita G. Nature Biotech. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.