Abstract
Background
Electrocardiographic Imaging (ECGI) is a novel electrophysiologic imaging modality which may help guide patient selection and lead placement for cardiac resynchronization therapy (CRT).
Objective
The aim of this study is to apply noninvasive electrocardiographic imaging (ECGI) to pediatric heart failure patients with congenital heart disease (CHD) undergoing evaluation for cardiac resynchronization therapy (CRT).
Methods
ECGI was applied in 8 CHD patients, who were either being evaluated for CRT or undergoing CRT. An electrical dyssynchrony index (ED) was computed from the ECGI epicardial activation maps as the standard deviation of activation times at 500 epicardial sites of the systemic ventricle. A normal ED of 20±4 ms was calculated from a control group of normal pediatric patients.
Results
Four patients had an ECGI assessment for ED but did not undergo CRT implant. Two other patients had ECGI assessment pre-CRT that demonstrated abnormal ED and went on to CRT implant. In both cases, the resynchronization lead was placed at the site of latest electrical activation (as determined by ECGI) in pre-CRT baseline rhythm. A total of four patients (2 responders, 2 non-responders) were studied with post-CRT in multiple rhythms. Responders had an average ED of 22msec in optimal CRT conditions. The non-responder had very elevated ED (37 ms) in all rhythms including optimal CRT settings. ED and ECG QRS duration showed weak correlation (r=0.58).
Conclusions
ECGI can be used in pediatric heart failure patients to evaluate ventricular ED and identify suitable candidates for CRT. Also, ECGI can guide resynchronization lead placement to the area of latest electrical activation. It could also be used in noninvasive follow ups for assessing synchrony and the electrophysiological substrate over time.
Keywords: Cardiac resynchronization therapy, congenital heart disease, electrocardiographic imaging, pediatrics
Introduction
Cardiac resynchronization therapy (CRT) has been extensively studied in adult heart failure patients with two ventricles and a systemic left ventricle (LV). There has been less investigation of CRT in patients with congenital heart disease (CHD) who comprise a substantially different population characterized by unusual anatomy including univentricular hearts, systemic right ventricles (RV) and other anomalies. CRT has been demonstrated to benefit certain CHD patients but significantly varies by substrate (1).
Electrocardiographic Imaging (ECGI) is a novel noninvasive functional imaging modality for cardiac electrophysiology (EP). It images epicardial potentials (voltage maps), electrograms, activation (isochrones) and repolarization patterns (2-7). ECGI is based on 250-channel body surface electrocardiograms and an accurate heart-torso anatomy derived from ECG-gated thoracic CT. Previously, it has been applied to image cardiac EP in adult heart failure patients undergoing CRT (5). ECGI has been also applied successfully to guide intracardiac mapping and ablation of accessory pathways in pediatric patients with complex congenital heart disease and structurally abnormal hearts (6,7). We hypothesize that ECGI can: 1) be used to identify CHD patients who have substantial ventricular electrical dyssynchrony (ED) in the baseline rhythm and thus may benefit from CRT; 2) help guide resynchronization lead placement by identifying both the electrophysiologic substrate and the area of latest electrical activation; 3) evaluate intraventricular electrical dyssynchrony post-CRT.
Methods
The study population included pediatric patients who met the following criteria: 1) age ≤21 years, 2) presence of congenital heart disease, 3) heart failure symptoms, and 4) undergoing evaluation for CRT or having a CRT device in place. Patients were chosen irrespective of their QRS duration. All patients were maximized on oral therapy prior to CRT.
For patients undergoing evaluation for CRT, an ECGI was performed in baseline rhythm. If the patient had a pacemaker in place and was not pacemaker dependent, ECGI was performed in both paced and underlying rhythm. Post-implant CRT patients were tested during various pacing conditions including: 1) CRT-optimal (CRT-OPT, which refers to the optimized CRT settings including an optimal intraventricular (V-V) delay), 2) CRT-nominal (CRT-NOM, which refers to the baseline factory settings with LV early by 4 ms) 3) single ventricular site pacing (either RV/LV, or anterior/posterior), and 4) underlying rhythm (when applicable). From the ECGI activation isochrones, an electrical dyssynchrony (ED) index (measured in msec) was computed as the standard deviation of activation times at 500 discrete epicardial sites on the systemic ventricle. ED is a measure of the degree of dyssynchrony of electrical activation of the systemic ventricle. Normal values for the ED index were determined to be 20±4msec from a control group of 22 patients (age 5-41). These patients all had structurally normal hearts with normal ventricular function and QRS duration of 81±9 msec. An ED of 24-28msec (the “two-sigma limit”) was considered mildly dyssynchronous, and 28-32msec moderately dyssynchronous. An ED index > 32 msec (above the ‘three-sigma limit’) was considered severely dyssynchronous. While ECGI images of normal ventricular activation have been described before (2,3), the intraventricular electrical dyssynchrony (ED) index in the normal population was specifically evaluated for this study. No dependence of ED on age (r=0.19) was observed in the control group. ED was abnormal (above the three-sigma limit) only when there were substantial isolated areas of late activation on the ventricle. All patients in this study with abnormal ED had symptoms of heart failure. We have not encountered a case with abnormal ED but with normal function and no eveidence of mechanical dyssynchrony.
If a patient was found to have ED greater than the normal range, the area of latest activation was identified as a potential site for resynchronization lead placement (8) and shared with the medical and surgical team. For patients who had a CRT device in place, ECGI was used to determine the ED index under various pacing conditions. Duration of follow-up for post-CRT studies was 4-6 months. All protocols were reviewed and fully approved by Human Research Protection Office at Washington University. Informed written consent was obtained from all patients and/or legal guardians prior to the study.
Statistical analysis
Data are expressed as the mean ± standard deviation for continuous variables or as percentages for categorical variables. Correlation testing was performed using Pearson product-moment correlation coefficient (r).
Results
Eight pediatric patients (age 12±6 years) were enrolled in the study with a total of 4 pre-CRT, 2 pre and post-CRT and 2 post-CRT studies. There was a wide variety of congenital heart lesions (Table 1) including: 1) systemic left ventricle, 2) systemic right ventricle, or 3) univentricular heart.
Table 1.
Patient data, including anatomy, QRS duration, ED index and interpretation, and clinical status
| Patient# Age/Gender |
Diagnosis | Pre/Post evaluation | Conditions | QRS duration (msec) | Electrical Dyssynchrony Index (msec) | Recommendation from ECGI | Outcome |
|---|---|---|---|---|---|---|---|
| 1 13/M |
Congenital Marfan's, s/p MVR and AVR s/p Ao Root replacement | PRE | SR | 120 | 22 | ED normal | Patient stabilized on optimal oral medical therapy |
| 2 17/M |
Tricuspid Atresia, L-TGA, s/p Fontan | PRE | Paced (DDD) | 125 | 20 | ED normal | Patient stabilized following AV optimizations and medical therapy |
| 3 18/M |
Tricuspid Atresia, L-TGA, s/p Fontan | PRE | SR | 137 | 28 | ED moderately abnormal | Optimized on medical therapy with improvement in symptoms |
| 4 3/M |
HLHS, s/p Glenn shunt | PRE | SR | 100 | 37 | ED severely abnormal | Required IV inotropic support and listed for OHT; patient expired awaiting OHT |
| 5 5/M |
L-TGA, s/p DDD epicardial pacemaker | PRE POST |
Paced (DDD) CRT-OPT CRT-NOM Pulm ventricle Syst ventricle SR |
100 68 70 100 85 70 |
36 21 29 36 32 23 |
ED severely abnormal ED normal ED moderately abnormal ED severely abnormal ED severely abnormal ED normal |
Recommended site for lead; left basal posterolateral Clinical improvement on device and medical therapy |
| 6 8/M |
HLHS, Mitral Atresia, DORV s/p Fontan and DDD epicardial pacemaker | PRE POST |
Paced (DDD) CRT-OPT CRT-NOM Anterior Lead Posterior Lead |
172 126 136 175 172 |
50 27 29 31 50 |
ED severely abnormal ED mildly abnormal ED moderately abnormal ED severely abnormal ED severely abnormal |
Recommended site for lead; left mid-posterolateral Clinical improvement on device and medical therapy |
| 7 17/F |
DILV, s/p Fontan and DDD epicardial pacemaker | POST | CRT-OPT CRT-NOM Anterior Lead Posterior Lead |
98 95 145 167 |
18 21 32 34 |
ED normal ED normal ED severely abnormal ED severely abnormal |
Clinical improvement on device and medical therapy |
| 8 21/F |
DILV, D-TGA, s/p Fontan and DDD epicardial pacemaker | POST | CRT-OPT Anterior Lead Posterior Lead |
170 138 175 |
37 36 38 |
ED severely abnormal ED severely abnormal ED severely abnormal |
Clinical nonresponder; went onto OHT |
Pre-CRT Evaluation
A total of 6 patients had ECGI as part of their pre-CRT assessment. Figure 1 shows the pre-CRT ECGI activation maps in Patients #1-4. The left panel in each row shows the anterior 4 chamber projection of the heart and the right panel shows the posterior view. Patients #1 and 2 were found to have normal ED indices, with Patient #1 having an ED=22.4msec in sinus rhythm [SR], Patient #2 had ED=20.5msec in dual chamber (DDD) paced rhythm; both patients had CRT therapy deferred (Table 1) and were optimized on medical therapy. Patient #1 (Figure 1, first row) has synchronous systemic LV activation with the only delayed spot (dark blue, right panel) near the RV base, consistent with a peripheral right bundle branch block (RBBB) activation pattern. For Patient #2 (Figure 1, second row), the ECGI activation map was obtained during paced rhythm (lead in a posterior apical location, see asterisk). Patient #3 (Figure 1, third row) had a higher ED (28.42ms) during SR with moderate dyssynchrony. The clinical care team decided to first optimize his medical therapy with angiotensin-converting enzyme (ACE) inhibitors and beta-blockers and the patient had significant symptomatic improvement. To date, the patient has not been implanted with CRT.
Figure 1.
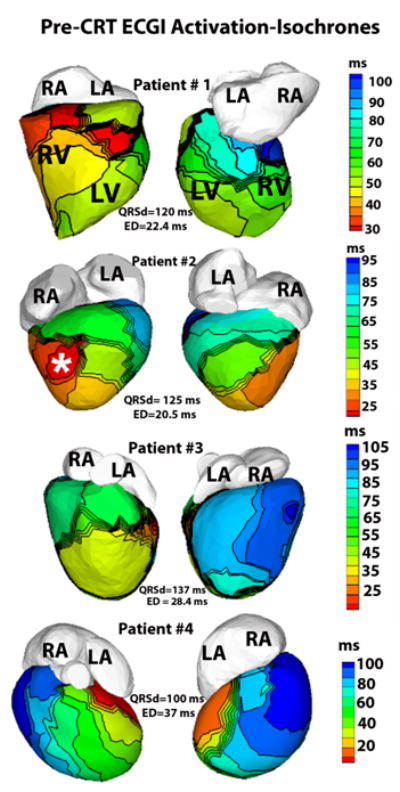
ECGI activation-isochrones in Patients #1-4 for pre- implant evaluation. Left panel of each row shows the anterior view and the right panel shows inferior view. The isochronal lines on the epicardial surface are depicted in black. Activation maps are constructed during sinus rhythm in patients #1, 3 and 4 and during epicardial dual chamber pacing in Patient #2. White asterisk (second row) shows the location of the tip of the epicardial pacing lead, digitized from CT images. LA-left atrium, RA-right atrium, LV-left ventricle, RV-right ventricle.
Three more patients had ECGI pre-implant which revealed severely abnormal ED indices: Patient #4 (ED=37msec) during sinus rhythm (ECGI activation map Figure 1, last row), Patient #5 (ED=36.4msec) during dual chamber [DDD] pacing (pre-CRT ECGI activation map Figure 2, top panel), and Patient #6 (ED=50msec) during DDD pacing (pre-CRT activation map Figure 3, top panel). All patients were first optimized on oral heart failure medical therapy. Patient #4 was optimized on medical therapy and had a brief period of improvement; he then decompensated quickly requiring intravenous inotropic support and was upgraded to status IA (highest status) for a heart transplant. Unfortunately, this patient expired awaiting cardiac transplant. Patients #5 and 6 went on to receive CRT (see next section).
Figure 2.
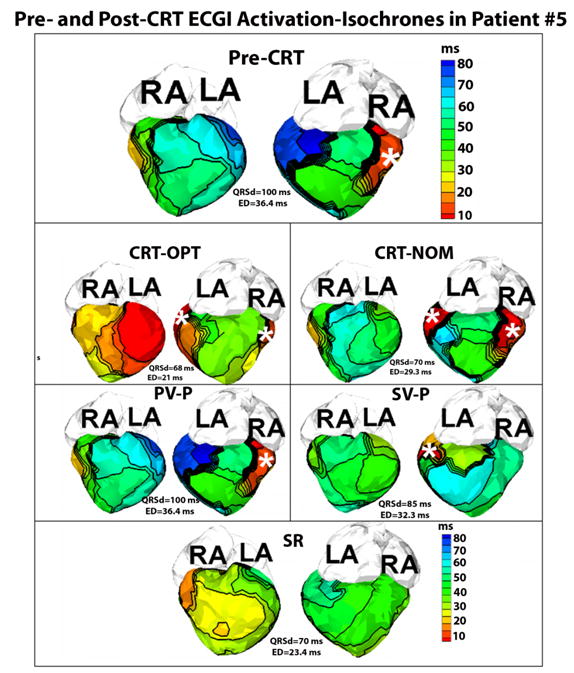
Pre- and Post-CRT ECGI activation-isochrones in Patient #5. Each panel shows the anterior view (left) and the inferior view (right) of the epicardial surface activation map. The top panel shows the pre-CRT activation map during epicardial DDD pacing (pacing site marked by asterisk). Latest activation (dark blue, right, top panel) is observed in the basal posterolateral area of the systemic right ventricle and this area was chosen as the site for implanting the resynchronization lead. Panels CRT-OPT,CRT-NOM, PV-P, SV-P and SR show the ECGI activation-isochrones of the same patient, six months after the implant during optimized CRT pacing, nominal CRT pacing, pacing from the pulmonary LV lead only, pacing from the systemic RV resynchronization lead only, and sinus rhythm (SR), respectively. The epicardial pacing sites are marked by white asterisks.
Figure 3.
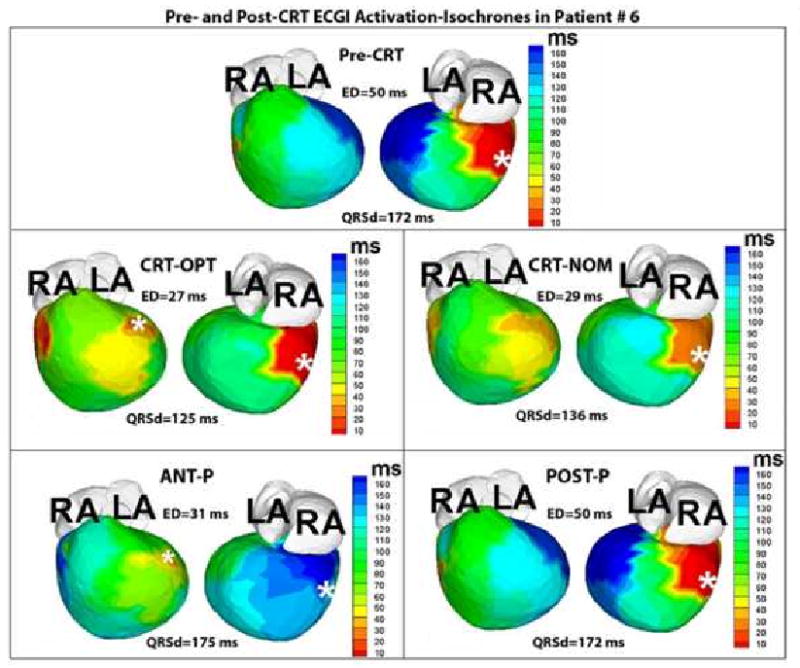
ECGI activation maps from Patient #6 pre- and post-CRT. The map in the top panel is obtained during dual chamber paced rhythm (pacing site indicated by the white asterisk). The area of latest activation (dark blue, right, top panel) was localized to the mid lateral segment. The following panels are obtained in CRT-OPT, CRT-NOM, ANT-P, and POST-P conditions, with the white asterisks denoting sites of pacing leads.
Pre and Post CRT
Patient #5 is a 6 year old male with congenitally corrected Transposition of the Great Arteries (ccTGA) status post 16mm homograft from the left ventricle to pulmonary arteries with high grade 2nd degree A-V conduction block. He was implanted with a dual chamber epicardial pacemaker at 15 months of age. His pre-CRT ECGI activation map, obtained in paced rhythm, showed severely abnormal ED (ED=36.4ms, Figure 2) and he was implanted with CRT. Using the initial (pre-implant) ECGI activation isochrones (Figure 2, top panel), an area of late electrical activation was identified on the posterolateral basal area of the systemic ventricle (dark blue, right panel, top panel Figure 2). This information was shared with the cardiothoracic surgeon implanting the resynchronization lead as the preferred site for lead placement. The patient had a successful epicardial lead placed in the operating room in the designated location. He had subsequent improvement in symptoms and returned to baseline activity. Six months post-implant (and post-CRT optimization), ECGI was repeated under 5 conditions: 1) CRT-OPT; 2) CRT-NOM; 3) pacing from the pulmonary ventricle lead alone (PV-P); 4) pacing from the systemic ventricle lead alone (SV-P); 5) sinus rhythm (SR). Figure 2 shows the anterior and posterior views of the epicardial activation maps in each of these five conditions. His CRT-OPT setting restored a normal ED index of 21msec with other pacing conditions having higher ED indices (Table 1). Also during follow up, the patient was found to have a slow underlying sinus rhythm; ECGI demonstrated a normal ED of 23.4ms. It showed that his sinus rhythm produces synchronized ventricular activation; however due to the intermittent nature of his A-V node conduction, he was mostly in a paced rhythm. The activation sequence generated by the pacemaker was dysynchronous (ED=36.4 ms) which may have contributed to his heart failure symptoms.
Patient #6 is an 8 year old male with Hypoplastic Left Heart Syndrome, Mitral Atresia, and Double Outlet Right Ventricle who had a DDD epicardial pacemaker implanted at the age of 3 months for post-operative complete heart block. The pacing lead was placed in a right posterior area (white asterisk, pre-CRT panel, Figure 3). At 4 years of age, he had a fenestrated extracardiac Fontan. Over the next several years, he developed worsening symptoms of heart failure and was maximized on medical therapy. His pre-CRT epicardial activation map (Figure 3) showed a severely abnormal ED at baseline paced rhythm (ED=50msec). The left anterior basal and inferior basal areas of the ventricle (in dark blue, Figure 3, Pre-CRT) were the areas of latest activation in the pre-CRT baseline rhythm and were designated as suitable sites for the resynchronization lead. The patient underwent surgical implant of an epicardial lead at the left anterior basal area of the ventricle as it is easier to access surgically compared to the inferior area. Three months following his implant and optimization, the patient had repeat ECGI under the following conditions (Figure 3): 1) CRT-OPT, 2) CRT-NOM, 3) Anterior Lead pacing only (ANT-P), and 4) posterior lead pacing only (POST-P). Under the CRT-OPT condition, the ED reduced to 27msec, or mildly abnormal ED, though this was much improved from his baseline paced rhythm. The ED was also elevated (ED=29msec, mildly abnormal) in CRT-NOM settings. For the ANT-P (ED=31msec) and POST-P (ED=50msec) pacing conditions, there was moderate and severely abnormal ED respectively. Clinically, the patient has substantially improved and remains on both medical and device therapy. In this case, the severe dyssynchrony seen in his baseline paced rhythm may have contributed to the patient's poor clinical status pre-CRT.
Post-CRT
A total of 4 patients were evaluated post-CRT implant with ECGI (see above section for patient #5 and #6). Patient #7, a 17 year old female with Double Inlet Left Ventricle (DILV) and L-Transposition of the Great Arteries (L-TGA) s/p fenestrated Fontan and dual chamber epicardial pacemaker for second degree heart block and sinus node dysfunction (1991), had an upgrade to CRT in 2006 for heart failure (New York Heart Association Class [NYHA] III). The second ventricular lead was empirically placed on the mid-lateral aspect of the left border of the heart, approximately 180° apart from the first ventricular lead. Since the upgrade to CRT and optimization of medical therapy (including ACE inhibitor and beta-blocker), she has done well clinically with improvement in NYHA to Class II. ECGI activation maps (Figure 4) were constructed during 4 different pacing conditions: 1) CRT-OPT; the ED index was restored to a normal value of 18.4ms; 2) CRT-NOM settings which resulted in a slightly higher but normal dyssynchrony index (ED=21.2ms); 3) pacing from anterior lead alone (ANT-P); 4) pacing from the posterior lead alone (POST-P). Single ventricular site pacing from either the anterior or posterior lead resulted in abnormal ED indices (ED=32.8ms with anterior lead pacing, ED=34.7ms with posterior lead pacing). The patient had no junctional or ventricular escape rate during which intrinsic activation could be evaluated with the ECGI technique.
Figure 4.
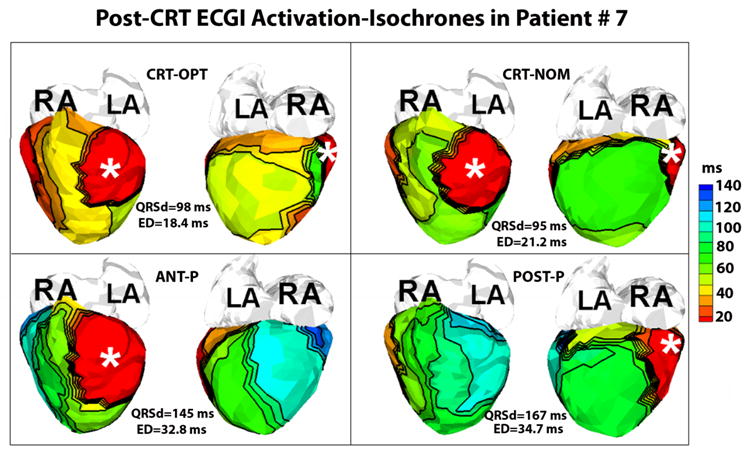
Post-CRT ECGI activation isochrones in Patient #7 with two epicardial leads (white asterisks). Each panel shows the anterior (left) and inferior (right) views of the epicardial surface activation-map. Panels CRT-OPT, CRT-NOM, ANT-P and POST-P show the epicardial activation-isochrones during optimized CRT pacing, nominal CRT pacing, pacing from the anterior lead alone and pacing alone from the posterior lead alone, respectively. The optimized CRT settings yield the lowest electrical dyssynchrony (ED) index (or maximal synchrony of ventricular activation).
Patient #8, a 21 year old white female born with DILV and L-TGA, status post fenestrated Fontan procedure had an epicardial dual chamber pacemaker (DDD) implanted for heart block 3 years prior to presentation with the ventricular lead placed on the right-sided inferoapical region of the ventricle. She had an upgrade to a CRT device with the second ventricular epicardial lead implanted anteriorly approximately 180 degrees opposite to the first ventricular lead. ECGI was performed under 3 conditions: 1) CRT-OPT (ED=37.3ms, severely abnormal, Figure 5, top panel); 2) Anterior lead pacing (ED=36.5ms, severely abnormal, Figure 5, second row); and 3) Inferior-apical lead pacing (ED=38.8ms, severely abnormal, Figure 5, third row). Activation isochrones (Figure 5) showed regions of slow conduction (illustrated by crowded isochrones) and lines of conduction block (illustrated by thick black lines) located on both the anterior and inferior surfaces, encompassing the pacing leads (white asterisks). Activation patterns and lines of slow conduction were similar in all three pacing conditions, demonstrating delayed electrical activation (in blue and green) of the left sided lateral wall and the posterior region (right panels); there was persistent electrical dyssynchrony that did not improve with CRT. Clinically, the patient had progression of heart failure symptoms despite maximal medical and device therapy; she was listed for cardiac transplant. A suitable donor was found and the patient was successfully transplanted.
Figure 5.
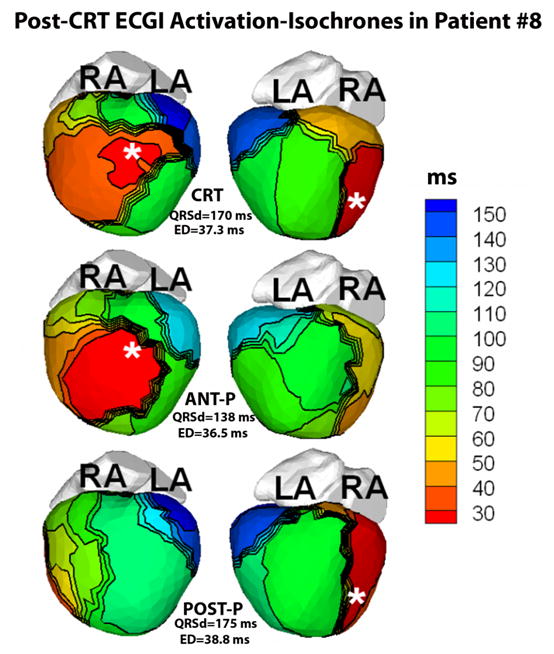
ECGI imaged activation isochrones in Patient #8 during CRT pacing (top row, CRT), pacing from the anterior lead only (second row, ANT-P) and pacing from the inferior lead only (third row, POST-P). Each row shows the anterior (left) and inferior (right) views of the epicardial surface activation-map. White asterisks mark the location of the epicardial pacing leads. Crowded isochrones indicate slow conduction and thick black lines indicate conduction block. The identical pattern of delayed activation (in blue, green) of the left lateral wall and the inferior region (right) in all three pacing modes indicates that a major target area of the ventricle is non-responsive to pacing. Three months later, the patient underwent a heart transplant and was reported to be in a stable and satisfactory condition.
QRS duration and ED
Data from a total of 22 conditions were obtained during the course of the study (Table 1). Correlation analysis between QRS duration and ED index demonstrated an r-value of 0.58.
Discussion
Pre-CRT echocardiographic testing used to determine dyssynchrony is fraught with limitations, mostly inter-observer variability. Unusual anatomy of CHD patients further imposes technical limitations on the utility of standard echocardiographic methods for objective evaluation. ECGI provides an objective rather than a subjective method in the assessment of dyssynchrony. The use of ECGI in selecting patients likely to benefit from CRT presupposes that mechanical dyssynchrony is preceded by ED, and identification of patients with substantial ED inherently selects patients with mechanical dyssynchrony. For patients with heart failure in absence of appreciable systemic ventricle dyssynchrony (ED in the normal range), CRT may not be the appropriate therapy and may account for part of the high non-responder rate (9). ED index is expected to be higher for cases with isolated late areas of ventricular activation (the dysynchronous pattern thought to be commonly associated with heart failure) rather than generalized slow conduction. For example, in patient #2, a wide QRS (125 ms) reflected generalized slow conduction associated with pacing but ED index was still low (20 ms). In spite of a long QRS duration, a very small area of the ventricle activated later than 80th percentile of QRS duration (deep blue, Patient #2, Figure 1), preserving the general synchrony of ventricular activation as reflected by a lower ED index. In comparison, patient # 4 had substantially large isolated areas of late activation (deep blue, Patient #4, Figure 1) and had an elevated ED index.
It has been reported before that RV pacing has deleterious effects on LV function in adults and one of the causes may be the pacing induced dyssynchrony arising from the the ‘left bundle branch block’ pattern of activation associated with RV pacing. There has been a lack of similar studies in the congenital heart disease population. In fact, the present study is one of the first to show that single site pacing in congenital heart block patients (# 5,6,7) produces dysynchronous intraventricular activation and may be one of the causes of subsequent heart failure observed in these patients.
Resynchronization of ventricular myocardium, the goal of CRT, has been difficult to reliably achieve in the pediatric population with non-responder rates of 12-13% (1, 10). Currently there are no objective criteria for placement of the resynchronization lead with the CRT resynchronization leads usually placed 180 degrees apart on the epicardial surface (1). Placement of the resynchronization lead at a site of late electrical activation is more likely to achieve resynchronization, as demonstrated in patient #5 and 6. No objective registration was performed between the epicardial area of latest activation indicated on the ECGI-CT images and the location of the lead placed by the surgeons. ECGI images showed the surgeons the general anatomic area for placement of the lead. For patient #5, the empirical area for the resynchronization lead would be left basal anterior which is 180 degrees apart from the previously implanted right-sided posterior lead. But ECGI maps showed a more inferior location as the area of the latest activation in the baseline rhythm; hence the resynchronization lead was placed inferiorly rather than anteriorly. Though this area is still close to the empirical area that would be chosen, ECGI provides an objective basis for the selection.
Previous ECGI studies (5) have demonstrated that defining the electrophysiologic substrate may be important for patients before CRT implant. One of the vital considerations pre-implant should be electrical viability of the cardiac muscle. If the underlying myocardial substrate has altered electrical characteristics (i.e., regions of slow conduction, inexcitable tissue, or lines of conduction block) which inhibit the pacing wavefront from activating a substantial target area of the myocardium as in Patient #8, CRT will not yield the desired response. This is demonstrated by the identical activation patterns and similar ED indices during the different conditions of pacing in Patient #8. For this patient, the resynchronization lead was placed adjacent to an area of slow conduction. Pacing from this area resulted in a functional line of block, leaving the half of the ventricular mass devoid of CRT. As such, only half the ventricular mass was activated early resulting in persistent dyssynchrony and insufficient resynchronization. Ideally, CRT leads should be placed at sites within the EP substrate conducive to rapid activation of a substantial target area of the myocardium. Performing ECGI in patients with complex congenial lesions prior to CRT implant may identify regions of slow conduction and block and help circumvent these areas while selecting a site for lead placement. ECGI may be used to noninvasively assess the electrical viability of the ventricular muscle pre-implant and thus avoid inappropriate placement of CRT devices.
ECGI images obtained in a CRT clinical responder with single ventricle physiology (Patient #7, Figure 4) imply that electrical synchrony may correlate with clinical response. While this has been demonstrated in adult patients (5), this is the first case of a clinical responder and improvement in ED in a pediatric patient with complex congenital heart disease.
QRS duration on the body-surface ECG is often used as a surrogate marker for electrical dyssynchrony with the assumption that longer QRS durations correlate with ED. We had the ability to correlate QRS duration with ED index in 21 conditions and demonstrated lack of strong positive correlation (r=0.58) between them. Data from clinical CRT responders in the study (Patients #5, 6, and 7) suggest that improvement in clinical response is accompanied by more synchronized ventricular activation by CRT (lower ED).
Study Limitations
ECGI was not used in this study to optimize CRT devices or study the relation between mechanical and electrical synchrony. The present study showed that this novel imaging modality can be applied to the congenital heart disease (CHD) patients to study the ventricular activation patterns and underlying EP substrate. It may be used to decide whether a prospective CHD patient may benefit from CRT based on the electrical intraventricular dyssynchrony and substrate in the baseline rhythm. It may be also used to follow-up CRT patients to evaluate their electrical dyssynchrony post-implant. An important aspect of CRT is optimization of the device including A-V delay, V-V delay, etc which may lead to better response in suitable patients. ECGI may be used in conjunction with the existing echo techniques for device optimization in prospective patients in future. This preliminary study shows that ECGI may be an important tool to add to the clinical armamentarium available for diagnosis and treatment of CHD patients, tailored to each patient.
The normal range for ED was calculated from patients with 2 ventricles and a systemic LV. This normal range may not apply to patients with systemic right ventricles and single ventricles as discussed in this manuscript. This study was performed at a single center in a unique patient population. The relatively small number of patients (n=8) precludes statistical conclusions to be drawn from the study. Unlike routine surface 12-lead ECG or echocardiography, ECGI is not yet an established routine clinical procedure, and requires multi-disciplinary expertise and time for careful analysis. With ECGI evolving as a clinical tool, large-scale multi-center studies will become possible, permitting statistical analysis of CRT response in large groups of CHD patients.
Conclusions
This study reports the first experience with application of a novel noninvasive cardiac EP imaging modality for evaluation of CRT in a small group of pediatric CHD patients. It shows that ECGI can be used objectively to measure electrical dyssynchrony, which does not correlate with QRS duration. ECGI may help to correctly identify patients (with substantially large ED compared to control) who would likely benefit from CRT. ECGI activation maps can help guide resynchronization lead placement by defining the EP substrate and identifying the area of latest electrical activation. ECGI data also show the incremental improvement in electrical synchronization achieved by CRT optimization compared to the nominal settings. In the future, ECGI may be used to optimize electrical synchrony which may correlate with good clinical response in patients selected for CRT. It could also be used in noninvasive follow ups for assessing synchrony and the electrophysiological substrate over time.
Acknowledgments
The study was supported by Merit Award R37-HL-033343 and Grant R01-HL-49054 from the National Heart, Lung, and Blood Institute to Y. Rudy. Dr. Rudy is the Fred Saigh Distinguished Professor at Washington University in St. Louis.
Footnotes
Disclosures: Y. R. chairs the scientific advisory board and holds equity in CardioInsight Technologies (CIT). CIT does not support any research conducted by Y.R., including that presented here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dubin AM, Janousek J, Rhee E, Strieper MJ, Cecchin F, Law IH, Shannon KM, Temple J, Rosenthal E, Zimmerman FJ, Davis A, Karpawich PP, Al Ahmad A, Vetter VL, Kertesz NJ, Shah M, Snyder C, Stephenson E, Emmel M, Sanatani S, Kanter R, Batra A, Collins KK. Resynchronization Therapy in Pediatric and Congenital Heart Disease Patients: An International MultiCenter Study. J Am Coll Cardiol. 2005;46:2277–2283. doi: 10.1016/j.jacc.2005.05.096. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci U S A. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghanem RN, Jia P, Ramanathan C, Ryu K, Markowitz A, Rudy Y. Noninvasive electrocardiographic imaging (ECGI): comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2:339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: observation of variable electrophysiologic responses. Heart Rhythm. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh S, Avari JN, Rhee EK, Woodard PK, Rudy Y. Noninvasive Electrocardiographic imaging (ECGI) of a univentricular heart with Wolff-Parkinson-White syndrome. Heart Rhythm. 2008;5:605–608. doi: 10.1016/j.hrthm.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S, Avari JN, Rhee EK, Woodard PK, Rudy Y. Noninvasive electrocardiographic imaging of epicardial activation before and after catheter ablation of the accessory pathway in a patient with Ebstein anomaly. Heart Rhythm. 2008;5:857–860. doi: 10.1016/j.hrthm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker M, Franke A, Breithardt OA, Ocklenburg C, Kaminski T, Kramann R, Knackstedt C, Stellbrink C, Hanrath P, Schauerte P, Hoffmann R. Impact of left ventricular lead position on the efficacy of cardiac resynchronization therapy: a two-dimensional strain echocardiography study. Heart. 2007;93:1197–1203. doi: 10.1136/hrt.2006.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and Left Ventricular Dyssynchrony in Patients with End-Stage Heart Failure. J Cardiovasc Electrophysiol. 2004;15:544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 10.Cecchin F, Frangini PA, Brown DW, Fynn-Thompson F, Alexander ME, Triedman JK, Gauvreau K, Walsh EP, Berul CI. Cardiac Resynchronization Therapy (and Multisite Pacing) in Pediatrics and Congenital Heart Disease: Five Years Experience in a Single Institution. J Cardiovasc Electrophysiol. 2009;20:58–65. doi: 10.1111/j.1540-8167.2008.01274.x. [DOI] [PubMed] [Google Scholar]


