Opinion Statement
The incidence of mesothelioma has gone from almost none to the current 2500–3000 cases per year in the USA. This estimate is an extrapolation based on information available from the Surveillance, Epidemiology and End Results (SEER) Program that collects information on approximately 12% of the US population. Mesothelioma is a cancer that is linked to exposure to carcinogenic mineral fibers. Asbestos and erionite have a proven causative role; the possible role of other mineral fibers in causing mesothelioma is being investigated. Asbestos is considered the main cause of mesothelioma in the US and in the Western world. The capacity of asbestos to induce mesothelioma has been linked to it ability to cause the release of TNF-α (that promotes mesothelial cells survival), other cytokines and growth factors, and of mutagenic oxygen radicals from exposed mesothelial cells and nearby macrophages. Some investigators proposed that as a consequence of the regulations to prevent exposure and to forbid and or limit the use of asbestos, the incidence of mesothelioma in the US (and in some European countries) should have started to decline before or around the year 2000, and sharply decline thereafter. Unfortunately, there are no data available yet to support this optimistic hypothesis. Simian virus 40 (SV40) infection and radiation exposure are additional causes, although their contribution to the overall incidence of mesothelioma is unknown. Recent data from several laboratories indicate that asbestos exposure and SV40 infection are co-carcinogens in causing mesothelioma in rodents and in causing malignant transformation of human mesothelial cells in tissue culture. An exciting new development comes from the discovery that genetic susceptibility to mineral fiber carcinogenesis plays a critical role in the incidence of this cancer in certain families. It is hoped that the identification of this putative mesothelioma gene will lead to novel mechanistically driven preventive and therapeutic approaches.
Introduction
Malignant mesothelioma (MM) is a rare but very aggressive tumor that arises from mesothelial cells lining the pleural, peritoneal and pericardial cavities. Pleural mesothelioma is the most common type, accounting for about 70% of all MM cases [1]. MM is sub-typed into three forms according to the histological morphology: epithelial, sarcomatoid, and biphasic. The prognosis of MM is poor, and the median survival time for these three types is 18, 8 and 11 months, respectively [2, 3].
Epidemiology
The first case report of MM was in 1947. In 1960, Wagner et al. reported a MM epidemic among asbestos miners and first demonstrated a relationship between asbestos exposure and MM.
MM was extremely rare until the second half of the 20th century. The incidence of MM has increased significantly, and currently there are about 2,000 to 3,000 cases per year in the United States [4]. The continuing increase in MM incidence has been associated with widespread use of asbestos in the past century. Asbestos was widely used in the shipbuilding and construction industries, especially between the 1940s and 1979 in the United States and Europe, due to its fire-resistant properties [5]. The latency period between the time of initial exposure and diagnosis is about 30 years and ranges from 20 to 50 years [6]. Two-thirds of MM patients are between 50 to 70 years of age. Males are at a much higher risk for MM than females, likely due to occupational exposure. The expected peak incidence of MM varies among countries. Price et al. proposed that MM has already reached its peak incidence in the United State [7]. This hypothesis remains to be confirmed. However, about 70,000 new MM cases are expected in the United States over the course of the next 20 years. In Europe, the anticipated peak year of MM incidence is in the period of 2015–2020, with a predicted incidence of 250,000 cases over the next 40 years [8].
The incidence of MM shows marked variations from one country to another. In some countries, MM incidence is low even though there is high asbestos [9, 10]. The reasons that account for these differences are unclear. Epidemiological studies found that about 5% of individuals with heavy prolonged asbestos exposure develop MM, although it has been proposed that approximately 80% of patients with MM in the United States have been exposed to asbestos. However, there is a significant difference in the percent of MM linked to asbestos exposure by different studies: from zero in some studies to 100% in others [11]. Geographical differences as well as different technical approaches used to attribute exposure are the likely cause of many of these very discrepant results. For example, some investigators will use lung content analysis studies. (i.e. The amount of asbestos found in 1cc or less of lung tissue). To attribute exposure, others will use patients interviews. It should not be unexpected that such different approaches will generate different results. What may appear as a surprise is that even within each of these 2 categories (i.e. Lung content analysis and interviews), the results vary significantly. This is also caused by different approaches in each category. For example, some investigators establish a background level of asbestos in the general population and count only fibers with the length of 5 µm or longer. Others will measure if any type of asbestos fiber is present in the lung, and if it is, they will attribute MM to asbestos (i.e. in other words, in these studies, there is no threshold set for background accounts nor for fiber size). Obviously, such studies will produce very different results. The interview system is even more unreliable when it comes to compare different studies. Some studies rely on work histories and on patients interviews; others are much less stringent and include interviews of friends and relatives when patients are no longer alive. Now it is hard to imagine how accurate friends and relatives could be in reporting whether a given patient was or was not exposed to asbestos 30–50 years before. Conflict of interest related to litigation further contribute to make the whole issue even more difficult to interpret.
Besides asbestos, some other etiologies or cofactors have been linked to MM development, e.g., Simian virus 40 (SV40), genetic predisposition, and certain other mineral fibers such as erionite. Such factors may also contribute to the significant variations in MM incidence in different geographic areas. There have been several reports of clustering of MM in certain areas; for example, in three small villages in Cappadocia, Turkey, an unprecedented mesothelioma epidemic causes 50% of all deaths [12]. The incidence of MM in those villages is about 1:100/year, compared to 1–20/million in the western world. Recently, Luo and colleagues reported that in a county called Da-yao in southwestern China, the annual mortality rate of mesothelioma is up to 365/million, compared to 2–3 per million in the general population in China [14, 2].
Etiology and Pathogenesis
Asbestos
The word asbestos comes from the Greek language, meaning “inextinguishable”. Asbestos refers to a group of naturally occurring hydrated mineral silicate fibers including two major forms: serpentine, represented by chrysotile (white asbestos) and amphibole, which includes crocidolite (blue asbestos), amosite (brown asbestos), anthophyllite, actinolite and tremolite.
The association between amphibole asbestos exposure and MM development is well accepted. In particular, crocidolite is generally considered to be the most oncogenic type of asbestos. The long and thin fibers (especially ≥ 8 µm in length ≤ 0.25 µm in width) are thought to be more dangerous, because they have longer biopersistance in the pleura. These fibers are able to penetrate the lung and cause repeated damage, tissue repair and local inflammation.
Chrysotile is the most common type of asbestos and accounts for about 90% of the world’s asbestos production. Whether chrysotile causes MM is still controversial. Some scientists suggested that chrysotile plays an important role in the pathogenesis of MM, because chrysotile fibers induce DNA damage and chromosome abnormalities in human and rat mesothelial cells in vitro, and cause MM in animals. Some authors suggested that chrysotile may cause MM but at a lower rate compared to amphibole asbestos. Hodgson and Darnton suggested that the exposure specific risk of MM is broadly in the ratio of 1:100:500 for chrysotile, amosite and crocidolite, respectively [14]. Instead, Suzuki et al. proposed that chrysotile is the main contributor to the causation of MM based on their analysis of lung and mesothelial tissues taken from 168 cases of MM, in which they found that chrysotile was the most common asbestos type [15]. Other authors have instead proposed that chrysotile does not cause MM and that it is the amphibole that often contaminates chrysotile that causes MM [16]. Some studies show that chrysotile asbestos is considerably less biopersistent than amphibole asbestos once inhaled in the lungs, and chrysotile fibers do not cause a pronounced inflammatory response compared to the amphibole tremolite [17]. Some of us conducted an extensive review of the literature and found that the data were so radically contradictory and, at times, flawed by conflict of interest that it was not possible to conclude whether chrysotile does or does not cause MM [18].
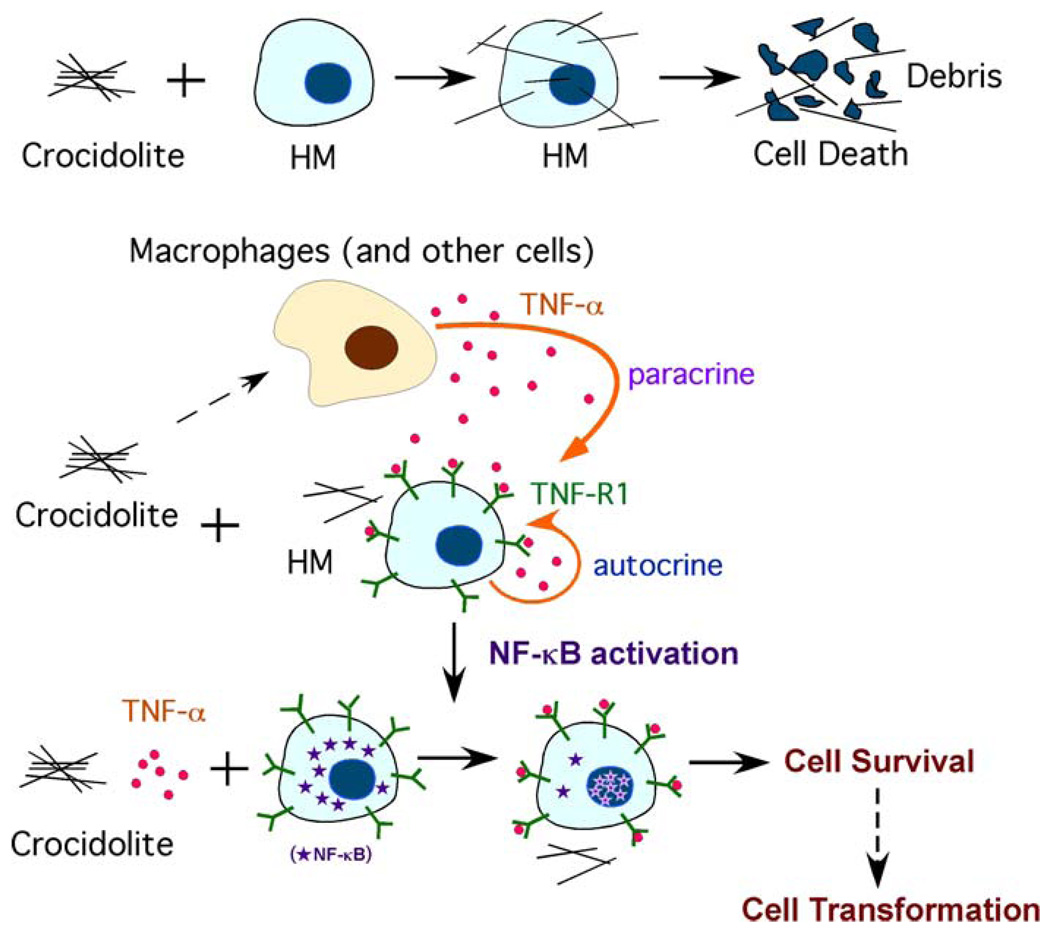
The mechanisms of asbestos carcinogenicity are not fully understood. During the long latency period of MM, many pathogenentic events may occur that can contribute to MM. Compared to other cell types tested, human mesothelial cells are very susceptible to asbestos cytotoxicity. For example, when exposed to amosite asbestos, mesothelial cells were 10 and 100 times more sensitive to the cytotoxic effects of asbestos than normal human bronchial epithelial or fibroblastic cells. Asbestos fibers induce toxicity in a dose-dependent manner. In tissue culture, doses equal to or higher than 5 µg/cm2 of crocidolite fibers induce 100% cell death in less than a week [19]. This observation raises the issue of how can asbestos cause MM if human mesothelial cells exposed to asbestos die. Recent work addressed this paradox and demonstrated a critical role for tumor necrosis factor-alpha (TNF-α) and NF-κB signaling in mediating responses of human mesothelial cells to asbestos [20]. In vivo studies have revealed that, following asbestos exposure there is an inflammatory reaction with a large component of mononuclear phagocytes [21]. Upon differentiation into macrophages, these cells phagocytize asbestos and, in response, release TNF-α. At the same time, asbestos induces human mesothelial cells to express TNF-α receptor TNF-R1 and also stimulates the secretion of TNF-α (both paracrine and autocrine effects). TNF-α binds to its receptor and activates the NF-κB pathway, which increases the percentage of human mesothelial cells that survive asbestos exposure [20]. Human mesothelial cells exposed to asbestos can accumulate DNA damages. Asbestos causes DNA strand breaks mediated by iron-catalyzed free radicals. In addition, by causing the release of reactive oxygen species (ROS) and reactive nitrogen species (RNS), asbestos fibers can indirectly induce genotoxicity including base substitutions, deletions, rearrangements, insertions, sister chromatid exchanges, and chromosomal aberrations which may lead to a broad spectrum of mutations in mammalian cells [22, 23]. The activation of the NF-κB pathway stimulated by TNF-α allows mesothelial cells with asbestos-induced DNA damage to divide rather than die, and if sufficient specific genetic damage accumulates to eventually develop into a MM [20] (Figure 1).
Figure 1.
TNF-α inhibits asbestos induced cytotoxicity via a NF-κB dependent pathway: a possible mechanism for asbestos induced oncogenesis. In tissue culture, crocidolite is very cytotoxic to human mesothelial cells (HM), causing extensive cell death. Crocidolite causes the accumulation of macrophages in the pleura and lung. These macrophages or some other cells release TNF-α when they encounter asbestos. At the same time, asbestos induces HM to secrete TNF-α, with paracrine and autocrine effects. TNF-α induces translocation of the NF-κB subunit, and activation of NF-κB increases HM survival. This allows HM with asbestos-induced DNA damage to divide rather than die and, if key genetic alterations accumulate, to eventually develop into a MM.
In addition to TNF-α, other growth factors and cytokines have been implicated in asbestos carcinogenesis and their role in MM pathogenesis is being investigated. These include: transforming growth factor beta (TGF-β), which might have a role in stimulating tumor growth; platelet-derived growth factor (PDGF), which may act as a regulatory factor in MM cell proliferation, insulin-like growth factor (IGF), which promotes tumor proliferation and cell migration [24]; interleukins such as IL-6 and IL-8, which may promote tumor growth and the development of new capillaries [25]; vascular endothelial growth factor (VEGF), which also promotes tumor angiogenesis [26], and hepatocyte growth factor (HGF), which stimulates mesothelioma cell migration and tumor invasiveness [27].
After interaction with mesothelial cells, asbestos triggers multiple cell-signaling pathways. Crocidolite fibers can induce autophosphorylation of the epidermal growth factor receptor, which stimulates the extracellular signal regulated kinase (ERK1/2) signaling pathway. This effect in turn increases activator protein (AP)-1 activity and mitosis of mesothelial cells [28]. Asbestos also activates the NF-κB pathway, which leads to the activation of multiple pro-survival genes that promote tumor development [20].
Cytogenetic and loss of heterozygosity analyses of MMs have detected frequent deletions of specific regions within chromosome arms 1p, 3p, 4p, 4q, 6q, 9p, 13q, 14q, 15q and 22q [29, 6]. Certain tumor suppressor genes located in these chromosomal regions have also been implicated, including CDKN2A/ARF at chromosome band 9p21 and NF2 at 22q12. Mutations of the p53 gene (TP53) are occasionally observed in MMs [30]. Loss and/or inactivation of these tumor suppressor genes may play a role in the development and progression of MM. For example, CDKN2A/ARF encodes the tumor suppressors p16(INK4a), a cyclin-dependent kinase inhibitor, and p14(ARF), a component of the p53 cell cycle checkpoint; and homozygous deletions of the CDKN2A/ARF locus in MM might simultaneously impair both the retinoblastoma (Rb) and p53 pathways [31]. The NF2 product, Merlin, represses cyclin D1 expression, and loss/inactivation of NF2 in MM leads to cell cycle progression in connection with up regulation of cyclin D1 [32]. Merlin also inhibits Rac/Pak and focal adhesion kinase (FAK) signaling, which play a role in cell migration and spreading, and inactivation of NF2 in MM promotes cell invasiveness annd spreading [33, 34].
SV40
Simian virus 40 (SV40) is a DNA monkey virus that has been associated with MM [35, 36].. The most likely route of SV40 transmission from monkey to human was through the SV40 contaminated polio vaccines produced between 1955 and 1978 [37].
Although over 50 different laboratories have reported a positive association of SV40 with MM, some have not, and this has caused a controversy [38]. For example, Lopez-Rios et al. reported that initially they detected SV40 in about 60% of MM specimens, and then they determined that most of the positive results were caused by plasmid PCR contamination, and that only 6% of the initially positive samples were confirmed to contain SV40 DNA [39]. It appears possible that some studies that lacked proper controls may have reported false positive results. However, many carefully designed and controlled studies have showed the presence of SV40 in human specimens by using several other techniques besides PCR, including Southern blotting, immunostaining, RNA in situ hybridization, microdissection, and electron microscopy [40]. Analysis of human MMs revealed that SV40 sequences are present in tumor cells but not in the normal adjacent tissue [41, 42]. Animal experiments demonstrated a clear association between SV40 and MM. For instance, 100% of hamsters injected intrapleurally with SV40 and 60% of those injected intracardially developed MM within 6 months.
SV40 produces two proteins that are oncogenic: Large T and small t antigens. In human MM biopsies, the large T antigen (Tag) was found to bind and inhibit p53 and pRb tumor suppressor proteins, thus contributing to MM carcinogenesis. A recent study demonstrated that Tag-p53 complex also has growth stimulatory activities that are required for malignant cell growth [43]. This investigation revealed that a multi-protein complex “Tag-p53-pRb-p300” binds to the promoter of the gene encoding insulin-like growth factor I (IGF-I), thereby stimulating IGF-I expression and IGF-I signaling and leading to enhanced cell growth. In other words, the binding of Tag to p53, p300, pRb, on one hand, inactivates the tumor suppressor activities of these proteins, on the other, the Tag-p53-pRb-p300 multi-protein complex acquire its own oncogenic activity by activating the IGF-1/IGF1R pathway. The small t antigen (tag) inhibits the cellular phosphatase 2A (PP2A), a protein involved in the dephosphorylation of many protein substrates, including components of the MAP-kinase (MAPK) pathway. Through inhibition of PP2A, tag may activate MAPK signaling and induce AP-1 activity. In addition, SV40 induces HGF/Met receptor activation [27], telomerase activity [44], and Notch-1 activation in human mesothelial cells and MM biopsies [45].
That SV40 and asbestos might be co-carcinogens was first demonstrated by Bocchetta et al. during in vitro studies of human mesothelial cells [19]. These observations were confirmed by Kroczynska et al., who demonstrated a strong co-carcinogenic effect between asbestos and SV40 [46]. Asbestos and SV40 dl883 (SV40 dl883 does not express tag and it does not cause MM in animals) together caused MM in 90% of hamsters, whereas SV40 dl883 alone did not cause MM in any animal, and asbestos alone caused MM in only 20% of hamsters. Importantly, significantly lower amounts of asbestos were sufficient to cause MM in animals infected with SV40. Molecular studies showed that asbestos and SV40 in combination had a co-stimulatory effect in inducing ERK1/2 phosphorylation and AP-1 activity in both Syrian hamsters and human primary mesothelial cells. AP-1 activation stimulated the expression and activation of matrix metalloproteinases MMP-1 and MMP-9, which in turn led to cell invasion [46]. These findings indicate that mineral fibers and viruses can act as co-carcinogens. Moreover, these data suggest that lower amounts of asbestos may be sufficient to cause MM in individuals infected with SV40. These results are important for determining levels of asbestos exposure that are supposedly “safe”. Such supposedly safe levels may not be truly safe for the millions of individuals who were exposed to SV40 contaminated polio vaccines. Co-carcinogenesis between SV40 and asbestos was later confirmed by Robinson et al [47] and Pietruska et al [48] in different animal models.
Erionite
Among different types of mineral fibers, erionite is the most potent induces of MM. Erionite has been detected in the lungs of villagers in several towns in Cappadocia, Turkey, where 50% or more of deaths are caused by MM [12]. Animal experiments showed that erionite is the most potent fiber in causing MM. Pleural MM was observed in 40 of 40 rats injected with erionite compared to 19 of 40 rats injected with asbestos. Inhalation of the erionite fibers induced a similar effect: 27/28 rats developed MM compared to only 4/124 rats exposed to crocidolite [49]
Genetics
Genetic susceptibility to MM was observed in the Cappadocian villages of Tuzkoy, Karain, and “Old” Sarihidir. Although mineralogical studies showed that all the houses appear to contain similar amounts of erionite, MM was prevalent in certain families but not in others. Pedigree studies of the three MM villages showed that MM seemed to be inherited in an autosomal dominant pattern (Figure 2). When high-risk MM family members married into families with no history of the disease, MM developed in the descendants (Figure 2). Taken together, the results of mineralogical studies and pedigree analysis indicate that the MM epidemic in Cappadocia is caused by erionite exposure in genetically predisposed individuals. Genetically predisposed family members born and raised outside the MM villages did not seem to develop MM, supporting the observation that the combination of genetics and erionite exposure (gene and environment) is involved in causing MM in these villages. Several US families have incidences of MM similar to those found in the Cappadocian families. It is possible that in the US MM families, genetic predisposition and asbestos exposure (or SV40) cause MM. However, the largest deposits of erionite are in the US, therefore, there is the possibility that erionite also played a role in these or other US MM [12].
Figure 2.
Family 1: Pedigree from the now abandoned village of “Old” Sarihidir showing a family of 30 in which 17 died of MM (black symbols), 4 died of other cancers [osteosarcomas (B), leukemia (D), prostate cancer (F), and pancreatic cancer (G)], 4 died of reasons other than cancer [2 traffic accidents (A), 1 intestinal occlusion (C), 1 congestive heart failure (E), and 1 unknown reason (first generation, female; F)], and 4 are alive (white symbols). Five MM developed in individuals who married into the family. They were also from MM families. Bottom, representative examples. Family 9: Pedigree of the family of origin of 65-year-old male (+) who married into family 1. Seven of the 17 people of this two-generation pedigree died of MM, 1 of liver cancer (H), and 5 unknown. The deaths from MM include a 46-year-old female (^) who married into family 9. The family of origin of this woman (family 10) has a very high incidence of MM: 5 of the 7 family members died of MM and 1 of lung cancer (K); the remaining cause of death is unknown. Family 3: When members of family 1 (*) marry into a non–MM family, the cancer appeared in the descendents. A, traffic accident; C, intestinal occlusion; the other causes of death were not cancer-related, but they could not be established with certainty.
Radiation
Several case reports have documented MM in patients who received radiation to the thorax or abdomen [50, 51]. Cancer patients who are treated with radiotherapy have shown increased risks for MM and the average interval between radiotherapy and MM was 21 years [52–54]. Animal studies using rats also support the role of radiation as a causative factor of MM.
Other Carcinogens and MM
There is no evidence showing an association between MM and smoking. There are some anecdotal case reports suggesting that chronic inflammation and scaring of the pleura, intrapleural thorium dioxide, and some chemicals may cause MM [55, 56].
Conclusion
MM is an aggressive malignancy caused by multiple factors that may work alone or in combination. It is hoped that the recent advances in understanding the mechanisms of MM pathogenesis will eventually lead to novel preventive and therapeutic strategies for MM patients. Importantly, some drugs that specifically target the molecular pathways that lead to MM are already available and can be tested in high-risk cohorts [57, 58].
Acknowledgement
The work presented here was supported by the following grants: NCI RO1-CA106567 and PO1-CA114047 to M.C., PO1-CA114047 project 3, P30-CA06927 and the Commonwealth of Pennsylvania to J.R.T., and a grant from the Riviera Country Club of Illinois to H.Y.
References
- 1.Bridda A, Padoan I, Mencarelli R, et al. Peritoneal mesothelioma: a review. MedGenMed. 2007;9:32. [PMC free article] [PubMed] [Google Scholar]
- 2.Becklake MR, Bagatin E, Neder JA. Asbestos-related diseases of the lungs and pleura: uses, trends and management over the last century. Int J Tuberc Lung Dis. 2007;11:356–369. [PubMed] [Google Scholar]
- 3.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 4.Grondin SC, Sugarbaker DJ. Malignant mesothelioma of the pleural space. Oncology (Williston Park) 1999;13:919–926. [PubMed] [Google Scholar]
- 5.Ismail-Khan R, Robinson LA, Williams CC, Jr, et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13:255–263. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]
- 6.Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29:2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- 7.Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol. 2004;159:107–112. doi: 10.1093/aje/kwh025. [DOI] [PubMed] [Google Scholar]
- 8.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379–387. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- 10.Becklake MR, Bagatin E, Neder JA. Asbestos-related diseases of the lungs and pleura: uses, trends and management over the last century. Int J Tuberc Lung Dis. 2007;11:356–369. [PubMed] [Google Scholar]
- 11.Carbone M, Rdzanek MA. Pathogenesis of malignant mesothelioma. Clin Lung Cancer. 2004;5 Suppl 2:S46–S50. doi: 10.3816/clc.2004.s.002. [DOI] [PubMed] [Google Scholar]
- 12.Carbone M, Emri S, Dogan AU, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 13.Luo S, Liu X, Mu S, et al. Asbestos related diseases from environmental exposure to crocidolite in Da-yao, China. I. Review of exposure and epidemiological data. Occup Environ Med. 2003;60:35–41. doi: 10.1136/oem.60.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg. 2000;44:565–601. [PubMed] [Google Scholar]
- 15.Suzuki Y, Yuen SR, Ashley R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: pathological evidence. Int J Hyg Environ Health. 2005;208:201–210. doi: 10.1016/j.ijheh.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Yarborough CM. Chrysotile as a cause of mesothelioma: an assessment based on epidemiology. Crit Rev Toxicol. 2006;36:165–187. doi: 10.1080/10408440500534248. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein DM, Chevalier J, Smith P. Comparison of Calidria chrysotile asbestos to pure tremolite: final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal Toxicol. 2005;17:427–449. doi: 10.1080/08958370591002012. [DOI] [PubMed] [Google Scholar]
- 18.Powers A, Carbone M. The role of environmental carcinogens, viruses and genetic predisposition in the pathogenesis of mesothelioma. Cancer Biol Ther. 2002;1:348–353. [PubMed] [Google Scholar]
- 19.Bocchetta M, Di Resta I, Powers A, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci U S A. 2000;97:10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Bocchetta M, Kroczynska B, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe N, Tanaka S, Xia W, et al. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ Health Perspect. 1997;105 Suppl 5:1257–1260. doi: 10.1289/ehp.97105s51257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu A, Zhou H, Yu DZ, et al. Mechanisms of the genotoxicity of crocidolite asbestos in mammalian cells: implication from mutation patterns induced by reactive oxygen species. Environ Health Perspect. 2002;110:1003–1008. doi: 10.1289/ehp.021101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla A, Gulumian M, Hei TK, et al. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Klominek J. Chemotaxis and chemokinesis of malignant mesothelioma cells to multiple growth factors. Anticancer Res. 2004;24:1625–1630. [PubMed] [Google Scholar]
- 25.Galffy G, Mohammed KA, Dowling PA, et al. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res. 1999;59:367–371. [PubMed] [Google Scholar]
- 26.Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 27.Cacciotti P, Libener R, Betta P, et al. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci U S A. 2001;98:12032–12037. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res. 2002;62:6065–6069. [PubMed] [Google Scholar]
- 29.Murthy SS, Testa JR. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell. Physiol. 1999;180:150–157. doi: 10.1002/(SICI)1097-4652(199908)180:2<150::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 30.Altomare DA, Vaslet CA, Skele KL, et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–8095. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- 31.Testa JR, Giordano A. SV40 and cell cycle perturbations in malignant mesothelioma. Semin. Cancer Biol. 2001;11:31–38. doi: 10.1006/scbi.2000.0344. [DOI] [PubMed] [Google Scholar]
- 32.Xiao GH, Gallagher R, Shetler J, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol. Cell. Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 34.Poulikakos PI, Xiao GH, Gallagher R, et al. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 35.Carbone M, Pass HI, Miele L, et al. New developments about the association of SV40 with human mesothelioma. Oncogene. 2003;22:5173–5180. doi: 10.1038/sj.onc.1206552. [DOI] [PubMed] [Google Scholar]
- 36.Pass HI, Bocchetta M, Carbone M. Evidence of an important role for SV40 in mesothelioma. Thorac Surg Clin. 2004;14:489–495. doi: 10.1016/j.thorsurg.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Cutrone R, Lednicky J, Dunn G, et al. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;6:10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- 38.Wong M, Pagano JS, Schiller JT, et al. New associations of human papillomavirus, Simian virus 40, and Epstein-Barr virus with human cancer. J Natl Cancer Inst. 2002;94(24):1832–1836. doi: 10.1093/jnci/94.24.1832. [DOI] [PubMed] [Google Scholar]
- 39.López-Ríos F, Illei PB, Rusch V, et al. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364:1157–1166. doi: 10.1016/S0140-6736(04)17102-X. [DOI] [PubMed] [Google Scholar]
- 40.Gazdar AF, Butel JS, Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- 41.Shivapurkar N, Wiethege T, Wistuba II, et al. Presence of simian virus 40 sequences in malignant mesotheliomas and mesothelial cell proliferations. J Cell Biochem. 1999;76:181–188. doi: 10.1002/(sici)1097-4644(20000201)76:2<181::aid-jcb2>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Carbone M, Rizzo P, Pass H. Simian virus 40: the link with human malignant mesothelioma is well established. Anticancer Res. 2000;20:875–877. [PubMed] [Google Scholar]
- 43.Bocchetta M, Eliasz S, De Marco MA, et al. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68:1022–1029. doi: 10.1158/0008-5472.CAN-07-5203. [DOI] [PubMed] [Google Scholar]
- 44.Foddis R, De Rienzo A, Broccoli D, et al. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene. 2002;21:1434–1442. doi: 10.1038/sj.onc.1205203. [DOI] [PubMed] [Google Scholar]
- 45.Bocchetta M, Miele L, Pass HI, Carbone M. Notch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cells. Oncogene. 2003;22:81–89. doi: 10.1038/sj.onc.1206097. [DOI] [PubMed] [Google Scholar]
- 46.Kroczynska B, Cutrone R, Bocchetta M, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci U S A. 2006;103:14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson C, van Bruggen I, Segal A, et al. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: malignant transformation is dose dependent. Cancer Res. 2006;66:10786–10794. doi: 10.1158/0008-5472.CAN-05-4668. [DOI] [PubMed] [Google Scholar]
- 48.Pietruska JR, Kane AB. SV40 oncoproteins enhance asbestos-induced DNA double-strand breaks and abrogate senescence in murine mesothelial cells. Cancer Res. 2007;67:3637–3645. doi: 10.1158/0008-5472.CAN-05-3727. [DOI] [PubMed] [Google Scholar]
- 49.Saffiotti U. mesothelioma carcinogenesis:in vivo models. In: Pass HI, Vogelzang NJ, Carbone M, editors. malignant Mesothelioma. New York, NY: Springer; 2005. p. 60. [Google Scholar]
- 50.Amin AM, Mason C, Rowe P. Diffuse malignant mesothelioma of the peritoneum following abdominal radiotherapy. Eur J Surg Oncol. 2001;27:214–215. doi: 10.1053/ejso.2000.1024. [DOI] [PubMed] [Google Scholar]
- 51.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 52.Brown LM, Howard RA, Travis LB. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:2741–2742. doi: 10.1002/cncr.22309. [DOI] [PubMed] [Google Scholar]
- 53.Teta MJ, Lau E, Sceurman BK, et al. Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer. 2007;109:1432–1438. doi: 10.1002/cncr.22526. [DOI] [PubMed] [Google Scholar]
- 54.Cavazza A, Travis LB, Travis WD, et al. Post-irradiation malignant mesothelioma. Cancer. 1996;77:1379–1385. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1379::AID-CNCR24>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 55.Andersson M, Wallin H, Jönsson M, et al. Lung carcinoma and malignant mesothelioma in patients exposed to Thorotrast: incidence, histology and p53 status. Int J Cancer. 1995;63:330–336. doi: 10.1002/ijc.2910630304. [DOI] [PubMed] [Google Scholar]
- 56.Comin CE, de Klerk NH, Henderson DW. Malignant mesothelioma: current conundrums over risk estimates and whither electron microscopy for diagnosis? Ultrastruct Pathol. 1997;21:315–320. doi: 10.3109/01913129709021929. [DOI] [PubMed] [Google Scholar]
- 57.Beck AK, Pass HI, Carbone M, Yang H. Ranpirnase as a potential antitumor ribonuclease treatment for mesothelioma and other malignancies. Future Oncol. 2008;4:341–349. doi: 10.2217/14796694.4.3.341. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman AJ, Pass HI. Current concepts in malignant pleural mesothelioma. Expert Rev Anticancer Ther. 2008;8:293–303. doi: 10.1586/14737140.8.2.293. [DOI] [PubMed] [Google Scholar]