Abstract
Ultraviolet B (UVB) radiation causes cutaneous inflammation. One important clinical consequence of UVB-induced inflammation is increased pain or hyperalgesia, which is likely mediated by enhanced sensitivity of cutaneous sensory neurons. Previous studies have demonstrated that UVB radiation generates the lipid mediator, platelet-activating factor (PAF), as well as oxidized phospholipids that act as PAF-mimetics. These substances exert effects through the PAF receptor (PAF-R). This study was designed to assess whether PAF-R is involved in UVB-induced hyperalgesia. Intradermal injection of carbamoyl PAF (CPAF; 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine) resulted in an enhanced response to mechanical stimuli in wild-type mice but not in PAF-R knockout (KO) mice. There was no significant change in paw withdrawal to noxious thermal stimuli in either genotype after intradermal injection of CPAF. Exposure of the hind paw to 1,500 J m−2 UVB radiation caused an increased sensitivity to both mechanical and thermal stimulation in wild-type mice but not in PAF-R KO mice. The thermal hyperalgesia caused by UVB irradiation was inhibited in mice that lacked PAF-R in bone marrow-derived cells. These data demonstrate that the PAF-R is important for UVB-induced hyperalgesia. Further investigation of the role of PAF-R signaling in UVB-induced hyperalgesia could provide better understanding of the pathological processes initiated by UVB-induced skin damage.
INTRODUCTION
Ultraviolet B (UVB, 290–320 nm) irradiation has profound effects on human skin, causing cutaneous inflammation and cell death. Acute short-term UVB irradiation of keratinocytes produces oxidative stress and DNA damage (Norins, 1962; Stewart et al., 1996). UVB irradiation, acting as a potent pro-oxidative stressor (Peus et al., 1998), induces the production of many cytokines, including tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-8, IL-10, and prostaglandins (reviewed by Ullrich 1995; Leverkus et al., 1998). These inflammatory cytokines have been implicated in UVB-induced inflammation and epidermal cell apoptosis.
In addition to its ability to induce the production of protein cytokines, UVB irradiation also induces the production of the lipid mediator, platelet-activating factor (PAF, 1-alkyl-2-acetyl-glycero-3-phosphocholine) and PAF-mimic species, through photooxidization of cellular polyunsaturated phosphatidylcholine, from epidermal cells (Barber et al., 1998; Marathe et al., 2005). PAF is a potent activator of many cell types, including platelets, monocytes, polymorphonuclear leukocytes, mast cells, and vascular endothelium, and exerts its biological effects through a single, highly specific G-protein-coupled receptor, PAF receptor (PAF-R) (reviewed by Ishii et al., 2002; Zimmerman et al., 2002). The PAF-R is linked to numerous signal transduction pathways and a wide range of pathological conditions including oxidative damage and bacterial infection. Human keratinocytes express functional PAF-Rs (Travers et al., 1995), and intradermal injection of PAF induces cutaneous inflammation (Hellewell and Williams, 1989; Travers et al., 1998a). Increased levels of PAF (3 ng ml−1) are found in the fluid of human blisters caused by inflammatory skin diseases such as bullous pemphigoid or pemphigus vulgaris compared with levels (0.5 ng ml−1) in noninflammatory blister caused by suction (Travers et al., 1998b) Activation of PAF-R stimulates the synthesis of various inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-8, IL-10, and eicosanoids (Roth et al., 1996; Poubelle et al., 1991; Ruis et al., 1991; Aepfelbacher et al., 1992), thus playing a key role in inflammatory processes.
There are reports suggesting that PAF may play a role in pain signaling. PAF-R mRNA is present in mouse dorsal root ganglia and dorsal horn of the spinal cord (Morita et al., 2004). Injection of PAF into the rat hind paw increases sensitivity to mechanical stimulation, a phenomenon known as hyperalgesia (Dallob et al., 1987). PAF-R antagonists can block the hyperalgesia induced by Bothrops jararaca venom (Teixeira et al., 1994) or formalin (Teather et al., 2002). Many compounds released during tissue damage and inflammation can induce hyperalgesia to mechanical stimuli or pressure, thermal stimuli, or both by increasing the sensitivity of cutaneous sensory neurons (Treede et al., 1992). Additionally, Tusada et al. (2007) recently demonstrated that mice with a lack of PAF-R have diminished hyperalgesic responses to the injection of the noxious and inflammatory substances, formalin and capsaicin. It is not clear from the few published studies whether PAF-induced hyperalgesia is mediated by direct actions of the agent on sensory neurons that transmit pain signals, that is, nociceptors, or instead, if the hyperalgesia is a result of PAF-induced inflammation.
As one of the important biological effects of UVB-induced pathological responses is hyperalgesia, this study was designed to assess whether the PAF-R and UVB-produced PAF-mimetics are involved in UVB-induced hyperalgesia. As there is a lack of effectively selective PAF-R antagonists, PAF-R knockout (KO) mice generated by Ishii and colleagues (Ishii et al., 1998) were used. Our results demonstrate that administration of the stable PAF agonist, carbamoyl-PAF (CPAF; 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine), causes mechanical hyperalgesia in the wild-type but not PAF-R KO mice. Furthermore, both the mechanical and thermal hyperalgesia induced by UVB irradiation were attenuated by a lack of PAF-R in the KO mice. The mechanical hyperalgesia induced by UVB irradiation was preserved by either the presence of PAF-R in bone marrow-derived cells or non-bone marrow-derived cells, as demonstrated in mice undergoing bone marrow transplant. In contrast, the absence of PAF-R in bone marrow-derived cells inhibited the thermal hyperalgesia caused by UVB irradiation. These data suggest an important role for PAF-R in UVB-induced hyperalgesia.
RESULTS
CPAF induces mechanical hyperalgesia
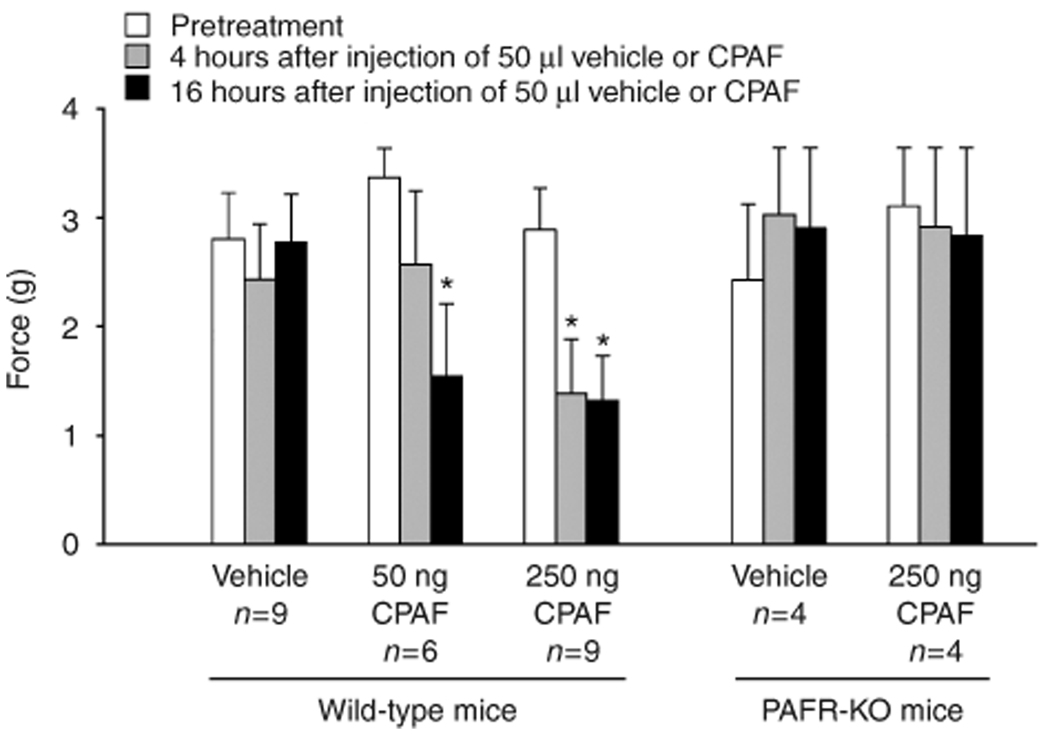
Activation of PAF-R in epidermal cells has been found to stimulate the synthesis of TNF-α and prostaglandins (Ruis et al., 1991; Aepfelbacher et al., 1992), which are known to enhance the sensitivity of cutaneous sensory neurons (Richardson and Vasko, 2002). To assess whether activation of skin PAF-R induces hyperalgesia, the hind paws of PAF-R-expressing wildtype mice and PAF-R KO mice were intradermally injected with the PAF-R agonist CPAF or vehicle control. Concentrations of 50 and 250 ng were chosen on the basis of previous investigations that demonstrated a maximum effect of CPAF to induce calcium influx in human keratinocyte-derived cell lines between 50 and 500 ng (Zhang et al., 2005) and the ability of 100 ng of CPAF to reproducibly cause inflammation when injected into the rodent ear skin (Travers et al., 1998b). Paw withdrawal in response to the force of Von Frey hairs (g), a measure of mechanical nociception, was measured before and 4 and 16 hours after injection. As shown in Figure 1, the force eliciting paw withdrawal before intradermal injection was similar between PAF-R-expressing and KO mice. Injection of 50 ml of 0.25% BSA vehicle did not alter the withdrawal threshold in mice of either genotype. Intradermal injection of 50 ng of CPAF in wild-type mice induced a significant decrease in the force that elicited paw withdrawal (3.4±0.3 g compared with 1.5±0.7 g before and 16 hours after injection, respectively, n = 6, P<0.05, using a repeated-measures analysis of variance (ANOVA) with Dunnet’s post-hoc analysis). Injection of 250ng CPAF in wild-type mice caused a similar decrease in withdrawal threshold 16 hours after injection (2.9±0.4 g compared with 1.3±0.4 g, before and after injection, respectively, n = 9, P<0.05, using a repeated-measures ANOVA with Dunnet’s post-hoc analysis); moreover, a significant decrease in withdrawal threshold was also observed 4 hours after treatment (2.9±0.4 g compared with 1.4±0.5 g, before and after injection, respectively). No paw swelling or abnormal gait was observed in any of the CPAF or vehicle-injected mice, and there was no difference in baseline response to mechanical stimulation between genotypes. These data demonstrate that activation of skin PAF-R in wild-type mice induces mechanical hyperalgesia, and the effect of CPAF is dose- and time-related. In contrast, intradermal injection of 250 ng CPAF into the hind paws of PAF-R KO mice did not alter the paw withdrawal threshold to mechanical stimulation, demonstrating the requirement for PAF-R in the induction of mechanical hyperalgesia caused by the PAF agonist.
Figure 1. Intradermal injection of CPAF induces mechanical hyperalgesia in wild-type but not PAF-R KO mice.
Columns represent the mean ± SEM force of Von Frey hair that caused paw withdrawal before (open columns) and 4 hours (gray columns) and 16hours (black columns) after intradermal injection of BSA or CFAP (50 µl volume) into the hind paw. An asterisk indicates a statistically significant difference from pretreatment levels using a repeated-measures ANOVA with Dunnet’s analysis (*P<0.05).
CPAF alone does not induce thermal hyperalgesia
Different subclasses of nociceptive sensory neurons can mediate mechanical and thermal stimuli (Besson and Chaouch, 1987). For this reason, the induction of thermal hyperalgesia and mechanical hyperalgesia are not always elicited in the same manner. To evaluate whether activation of skin PAF-R with CPAF intradermal injection induces thermal hyperalgesia in mice hind paws, the latency of paw withdrawal in response to heat-producing lamp was measured after CPAF injection in mice hind paws. As shown in Figure 2, there was no difference in baseline response to thermal stimulation between genotypes. Moreover, there was no significant change in paw withdrawal latency observed in either wild-type or PAF-R KO mice 4 and 16 hours after injection with 250 ng of CPAF. These results suggest that activation of skin PAF-R alone is not sufficient to induce thermal hyperalgesia in mice.
Figure 2. Intradermal injection of CPAF does not induce thermal hyperalgesia.
Columns represent the mean ± SEM (n = 4 mice per group) paw withdrawal latency in seconds 4 hours (gray columns) and 16 hours (black columns) after intradermal injection of BSA or CFAP (specific PAF-R agonist, 50 µl volume) into the hind paw. There were no statistically significant differences between groups using a repeated-measures ANOVA.
UVB irradiation induces greater mechanical and thermal hyperalgesia in skin in PAF-R-expressing wild-type mice in comparison with PAF-R KO mice
Increased pain sensitivity of skin is one of the major symptoms of acute UV exposure. It has been found that UVB irradiation of epidermal cells or cellular phospholipid produces PAF-mimetic species that activate epidermal PAF-R (Walterscheid et al. 2002; Marathe et al., 2005). Human sunburn is thought to start occurring at UVB irradiation exposures in the range between 400 and 800 J m−2 (Autier et al., 2000). To determine whether PAF-R is involved in UVB-induced hyperalgesia, responses of wild-type and PAF-R KO mice to thermal and mechanical stimulation were measured before and after exposure to UVB irradiation. The mice were restrained without anesthesia and one hind paw was exposed to 1,500 J m−2 UVB irradiation. At this dose, there was neither paw swelling nor abnormal gait observed in any of the UVB-irradiated mice. The paw withdrawal in response to mechanical or thermal stimulation was measured before and 4 and 28 hours after irradiation as described previously (Chaplan et al., 1994; Evans et al., 2000).
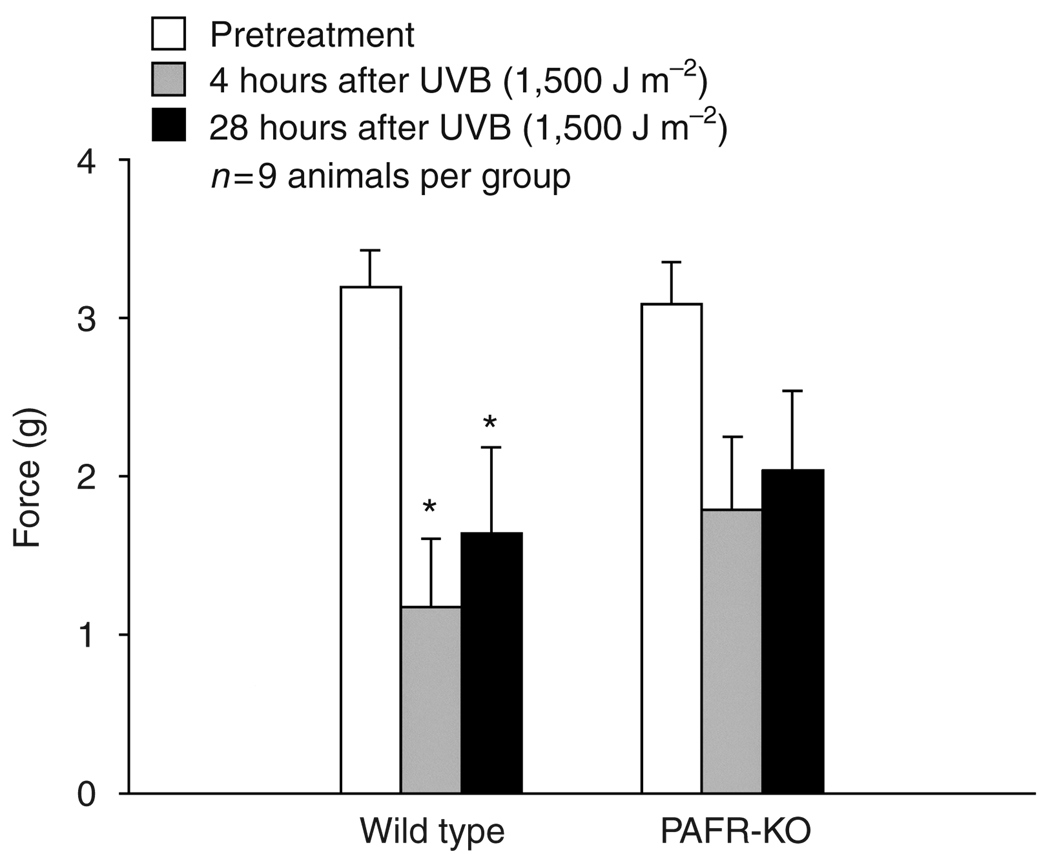
As shown in Figure 3, UVB irradiation in wild-type mice induced a significant decrease in the force that elicited paw withdrawal 4 and 28 hours after UVB irradiation (3.3 ± 0.2, 1.2 ± 0.4, 1.6 ± 0.5 g, before and 4 and 28 hours after UVB irradiation, respectively, n = 9, P<0.05, using a repeated-measures ANOVA with Dunnet’s post-hoc analysis). In contrast, there was a small but not statistically significant decrease observed in PAF-R KO mice either 4 or 28 hours after UVB irradiation (3.1±0.3, 1.9±0.5, and 2.0±0.5 g, before and 4 and 28 hours after UVB irradiation, respectively). These data suggest that PAF-R activation is involved in the optimal induction of UVB-mediated mechanical hyperalgesia in murine skin.
Figure 3. Ultraviolet B irradiation induces mechanical hyperalgesia in wild-type but not PAF-R KO mice.
Columns represent the mean ± SEM (n = 9 mice per group) force of Von Frey hair that caused paw withdrawal before (open columns), 4 hours (gray columns) and 28 hours (black columns) after exposure to 1,500 J m−2 UVB radiation. An asterisk indicates a statistically significant difference from pretreatment levels using a repeated-measures ANOVA with Dunnet’s analysis (*P<0.05).
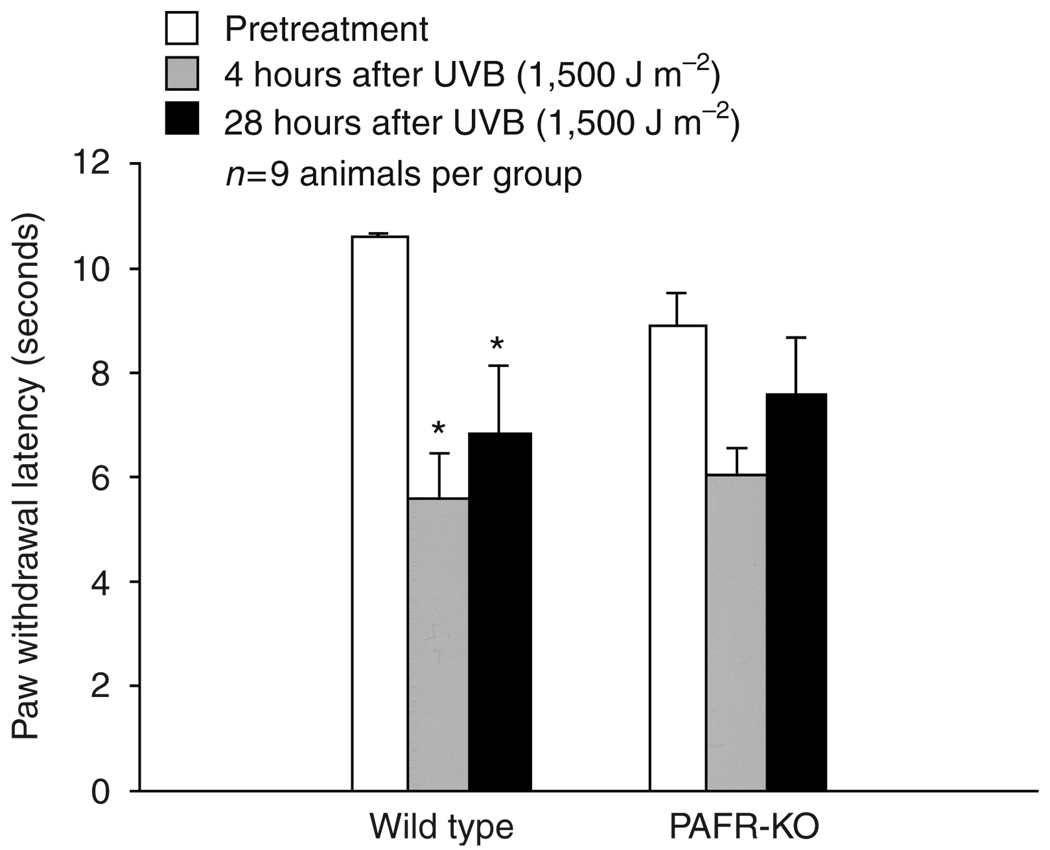
The next studies examined the role of the PAF-R in UVB-mediated thermal hyperalgesia. Although intradermal injection of CPAF did not induce thermal hyperalgesia in wild-type mice, UVB irradiation of PAF-R-expressing wild-type mice significantly shortened the withdraw latency in response to thermal stimulation 4 and 28 hours after treatment (Figure 4). In response to thermal stimulation, the wild-type mice withdrew the paw after 10.6 ±0.6seconds exposure to radiant heat before UVB irradiation and at 5.6 ± 0.9 seconds 4 hours after UVB irradiation and at 6.8 ± 1.3 seconds 28 hours after UVB irradiation (n = 9, P<0.05, a repeated-measures ANOVA with Dunnet’s posthoc analysis). Again, there was a small but not statistically significant change in withdrawal latency observed in PAF-R KO mice after UVB irradiation at 4 or 28 hours (withdrawal latency of 8.9 ±0.6 seconds before and 6.0 ±0.5 and 7.6 ± 1.0 seconds 4 and 28 hours after UVB exposure, respectively, n = 9). These results demonstrate that the PAF-R is also involved in the thermal hyperalgesic response induced by UVB irradiation of mouse skin. High-dose UVB (7,500 J m−2) resulted in identical hyperalgesic responses to thermal and mechanical stimulation in both genotypes and caused significant paw tissue damage and swelling (data not shown). These findings fit with the notion that PAF is but one mediator involved in UVB-mediated hyperalgesia.
Figure 4. Ultraviolet B irradiation induces thermal hyperalgesia in wild-type but not PAF-R KO mice.
Columns represent the mean ± SEM (n = 9 mice per group) paw withdrawal latency 4 hours (gray columns) and 28 hours (black columns) after exposure to 1500 J m−2 UVB radiation. An asterisk indicates a statistically significant difference between genotypes at a particular time point using a repeated-measures ANOVA with Dunnet’s analysis (*P<0.05).
The importance of the presence of PAF-R in bone marrow-derived cells for UVB-mediated hyperalgesia
To determine which types of cells in the skin are needed in UVB-induced hyperalgesia mediated by PAF-R, chimeric mice were produced using bone marrow transplantation. This allowed for the construction of mice with PAF-R on all but bone marrow-derived cells and mice with PAF-R only on bone marrow-derived cells. As a control, bone marrow from wild-type mice was also transplanted into wild-type mice to determine if irradiation and transplantation procedure itself altered thermal or mechanical sensitivities.
Four months after the transplantation, mice were tested for mechanical and thermal sensitivity before and after UVB irradiation as described above. There were no differences in baseline mechanical or thermal sensitivity between wild-type mice receiving wild-type bone marrow, wild-type mice receiving PAF-R-deficient bone marrow or PAF-R KO mice receiving wild-type bone marrow (Figure 5 and Figure 6). In addition, there were no significant differences in baseline behavioral responses of these groups and those of the animals in the previous CPAF or UVB treatments.
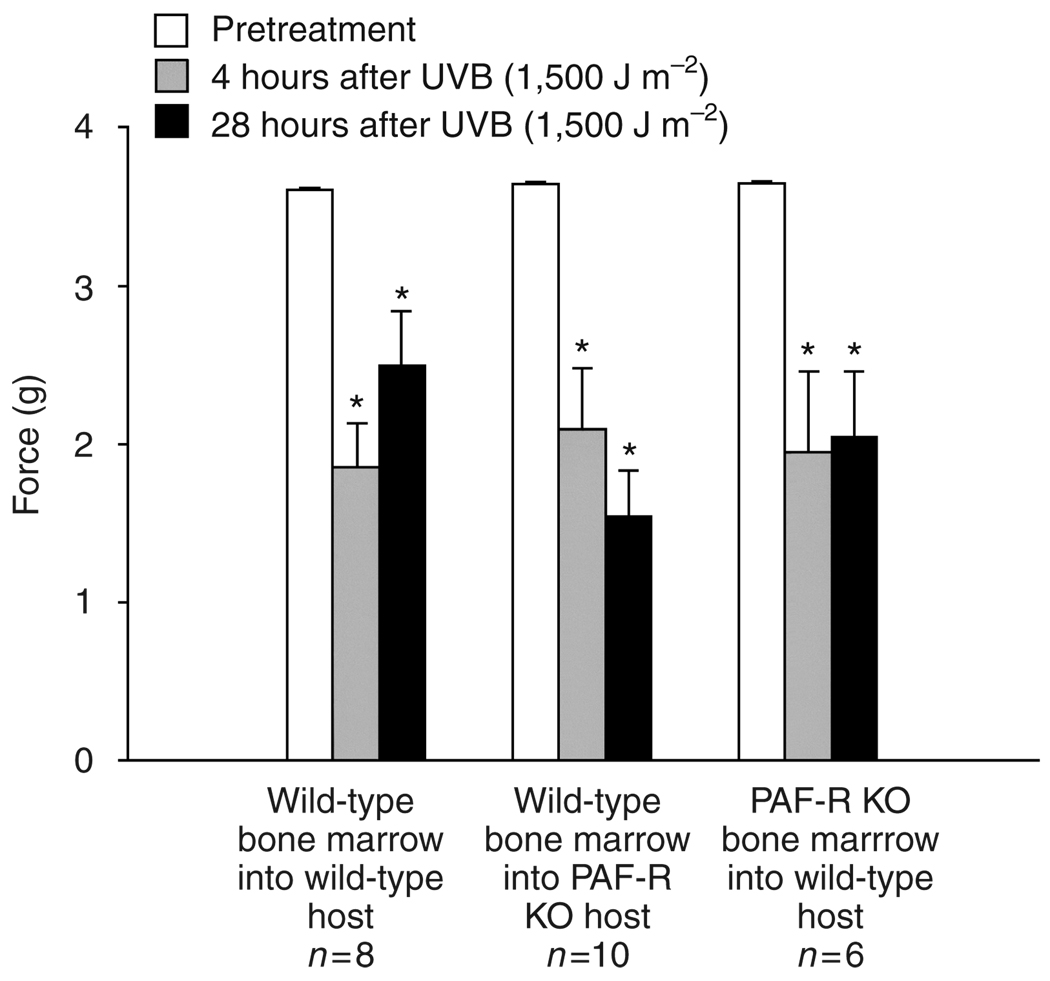
Figure 5. Ultraviolet B-induced mechanical hyperalgesia requires PAF-R in either bone marrow-derived or non-bone marrow-derived cells.
Columns represent the mean ± SEM (n = 6–10 mice per group) force of Von Frey hair that caused paw withdrawal before (open columns), and 4 hours (gray columns) and 28 hours (black columns) after exposure to 1,500 J m−2 UVB radiation. An asterisk indicates a statistically significant difference from pretreatment levels using a repeated-measures ANOVA with Dunnet’s analysis (*P<0.05).
Figure 6. Ultraviolet B-induced thermal hyperalgesia is inhibited in the absence of PAF-R in bone marrow-derived cells.
Columns represent the mean ± SEM (n = 6–10 mice per group) paw withdrawal latency 4 hours (gray columns) and 28 hours (black columns) after exposure to 1,500 J m−2 UVB radiation. An asterisk indicates a statistically significant difference between genotypes at a particular time point using a repeated-measures ANOVA with Dunnet’s analysis (*P<0.05).
As demonstrated in Figure 5, UVB irradiation caused a significant reduction in the mechanical force needed to induce paw withdrawal in all groups. The responses were similar regardless of which cell compartments contained PAF-R. These data suggest that while the presence of PAF-R is necessary for the full development of mechanical hyperalgesia after UVB irradiation, it is likely that there are many PAF-R positive cells that can function in this response. In contrast, UVB-induced thermal hyperalgesia was inhibited in the absence of PAF-R in bone marrow-derived cells (Figures 6), suggesting that cells of the hematological system must contain PAF-R for UVB radiation to have its full effect on thermal hyperalgesia.
DISCUSSION
It has been demonstrated that PAF has significant proinflammatory effects, including the synthesis of various inflammatory cytokines and stimulation of numerous signal transduction systems. In this study, we first investigated whether PAF and PAF-R system are involved in UVB-induced hyperalgesia in mice. It was shown that activation of skin PAF-R by intradermal injection of CPAF, a metabolically stable PAF-R agonist, induced mechanical hyperalgesia in a time- and dose-related manner in wild-type mice. In contrast, there was no hyperalgesic effect observed in PAF-R KO mice after CPAF injection, confirming that PAF-R is necessary for CPAF-mediated mechanical sensitization. No change in thermal sensitivity was observed with CPAF treatment. Dallob et al. (1987) also observed an increase in mechanical sensitivity in rats treated with a subplantar injection of synthetic PAF. Thermal stimulation was not examined in that study. Interestingly, intrathecal injection of a PAF analog induced both mechanical and thermal hyperalgesia (Morita et al., 2004). It is likely that this difference in thermal response to CPAF is related to the site of administration, and it suggests that the actions of PAF on thermal hyperalgesia are not necessarily mediated by PAF-R activation on primary sensory neurons.
The role of PAF in inflammatory pain has not been well established. PAF-Rs mediate synthesis of various inflammatory mediators, including TNF-α and eicosanoids (Poubelle et al., 1991; Ruis et al., 1991), which have been identified to be involved in nociception and inflammatory hyperalgesia (Richardson and Vasko, 2002). The hyperalgesic response to PAF can be inhibited by agents that interfere with the lipoxygenase pathway of arachidonic acid metabolism, but appear to be resistant to inhibition of cyclooxygenase pathway that produces prostaglandins (Dallob et al., 1987). Therefore, it is possible that PAF and PAF-R activation plays a role in the complex cascade involved in inflammatory hyperalgesia. A recent report using PAF-R KO mice also demonstrated that absence of PAF-R does not change baseline responses to noxious mechanical and thermal stimulation (Tusada et al., 2007). However, they demonstrated that pain-like behavior in response to capsaicin or formalin injection, which initiate an inflammatory response, were diminished in PAF-R KO mice. The inflammatory response itself, as measured by paw thickness, was not affected by a lack of the PAF-R, suggesting differing mechanisms behind the plasma extravasation of inflammation and hyperalgesic responses.
Ultraviolet B irradiation, as a potent oxidative stressor, has profound biological activities. Increased pain sensitivity of skin is one of the major symptoms of acute UVB exposure. Irradiation of the paw with UVB induced mechanical and thermal hyperalgesia in PAF-R-expressing wild-type mice. UVB irradiation of epidermal cells or cellular phospholipids produces PAF-mimetics, through oxidative fragmentation of the glycerophosphocholines. Exposure of human keratinocyte-derived cell lines to UVB irradiation in the range of 400–2,000 J m−2 can produce 120–160 pg of PAF/106 cells (Marathe et al., 2005). These PAF-like species can activate PAF-Rs in a variety of tissues. It is reasonable to speculate that PAF-R agonists were chemically synthesized from cellular phospholipids in mice skin exposed to UVB. The subsequent activation of the PAF-R could stimulate the production of inflammatory mediators, including TNF-α and eicosanoids. Indeed, UVB irradiation of epidermal cells induces TNF-α expression, and this process is mediated by the PAF-R receptor (Dy et al., 1999). Administration of TNF-α can induce both thermal and mechanical hyperalgesia (Junger and Sorkin, 2000; Sorkin and Doom, 2000), providing a possible mechanism for how the PAF-R could play a role in UVB-induced inflammatory hyperalgesia. Exposure of the rat paw to UVB irradiation also induced mechanical and thermal hyperalgesia (Bishop et al., 2007). The alteration of thermal and mechanical thresholds in the rats occurred at doses of UVB exposure as low as 2,500 J m−2, and no significant tissue damage was observed even days after the exposure. Although these investigators did not look at time points less than 24 hours after exposure, they did observe threshold changes of similar magnitude to those reported here in mice 24 hours after exposure. Interestingly, the UVB-induced hyperalgesia reported by Bishop et al. (2007) was significantly reduced by treatment with topical or systemic ibuprofen, a cyclooxygenase inhibitor, suggesting a significant role for prostaglandin production in UVB-mediated hyperalgesia.
In the PAF-R KO mice, there was a small, yet statistically insignificant, increase in mechanical sensitivity following UVB irradiation of the paws of PAF-R KO mice. This suggests that the PAF system is a necessary component in the UVB-induced mechanical hyperalgesia, but probably is not the only mediator involved. As CPAF induced a robust mechanical hyperalgesia when injected into the paw of wild-type mice, it is possible that the PAF-R-dependant component of UVB-induced mechanical sensitization is secondary to a direct action of PAF on nociceptive sensory neurons. Although there is mRNA for PAF-R in the dorsal root ganglia, where the cell bodies of these primary sensory neurons reside (Morita et al., 2004), it is not known which population of these neurons express PAF-R. Different subclasses of nociceptive sensory neurons mediate mechanical and thermal stimuli (Besson and Chaouch, 1987), and the difference in expression of PAF-R on these subclasses of neurons may explain why CPAF treatment elicited a mechanical hyperalgesia, but not a thermal hyperalgesia.
Although CPAF injection did not induce thermal sensitization, UVB irradiation did elicit thermal hyperalgesia in wild-type mice but not in mice lacking the PAF-R. This suggests that PAF-R signaling is an important component of UVB-induced increase in thermal sensitivity. As intrathecal injection of a PAF analog induced thermal hyperalgesia (Morita et al., 2004), it is possible that the actions of PAF on thermal hyperalgesia are not mediated by PAF-R activation on primary sensory neurons, but that the PAF-R is necessary for mediation of this response at the level of the spinal cord. Indeed, a 20 minute application of 250 ng of CPAF to isolated wild-type mouse sensory neurons maintained in culture did not alter capsaicin-stimulated release of peptide transmitters from the cells (Hingtgen et al., unpublished data). In addition, although CPAF induced calcium influx in isolated sensory neurons from rat and wild-type mice, there was no evidence that CPAF could initiate transmission of signal between the central terminals of the primary sensory neurons and the second-order neurons of the sensory pathway in the spinal cord slices from wild-type mice (Tusada et al., 2007). As the TRPV1 receptor that mediates capsaicin responses on these neurons is also important in the signaling of noxious thermal stimulation (Caterina et al., 2000), these observations suggest that CPAF may not have actions to directly sensitize sensory neurons to thermal stimuli. Instead, PAF and the PAF-R on other cells in the paw or spinal cord may be important in the production and maintenance of other inflammatory substances, such as TNF-α and eicosanoids, that do elicit thermal hyperalgesia. It is intriguing to speculate that administration of selective PAF antagonists, such as rupatadine (Guadano et al. 2004), might be a strategy worthy of further investigation in the treatment of acute and chronic inflammatory pain.
As the inflammatory response to UVB irradiation is complex and as the PAF-R is present in so many cells, it is difficult to determine where the presence of PAF-R may be needed for the development of hyperalgesia. The experiments in chimeric mice help to address these issues in the in vivo model. We have recently reported that the presence of PAF-R in bone marrow-derived cells is necessary for UVB-induced IL-10 production through a cyclooxygenase-dependant mechanism likely to involve production of prostaglandins (Zhang et al., 2008). Here, we present that the presence of PAF-R in bone marrow-derived cells is necessary for UVB-induced thermal hyperalgesia. As prostaglandins elicit thermal hyperalgesia (Treede et al., 1992), it seems likely that UVB-induced thermal hyperalgesia dependant on PAF-R expression may be the result of prostaglandin production in bone marrow-derived cells. In contrast, the mechanical hyperalgesia induced by UVB was not dependant on the expression of PAF-R in bone marrow-derived cells; it was sufficient for PAF-R to be present in non-bone marrow-derived cells. This suggests that there is a different compliment of cells that can account for UVB-induced mechanical hyperalgesia. Indeed, as CPAF itself was able to illicit mechanical hyperalgesia when injected into the paw, PAF-mimetic substances produced by UVB may act directly on sensory neuron endings in the skin.
The biological effects of UVB on skin are of particular interest because of the importance of UV-induced injury in the development of photoaging, skin cancer, and inflammation, and the role of UV irradiation in the therapy of skin disease (reviewed by Trautinger, 2001; Ichihashi et al., 2003; Schwarz, 2002). Determining the cellular and molecular mechanisms and finding novel factors that modulate inflammatory responses to acute UV absorption in epidermal cells are important for understanding the regulation of these processes. In this study, it was demonstrated that PAF-R is involved in UVB-induced mechanical and thermal hyperalgesia. Skin hyperalgesia is one of the major pathological responses to acute UV exposure and can be measured accurately by established behavioral methodology. Thus, the utilization of hyperalgesia measurement in PAF-R-expressing and KO mice models could provide an experimental strategy to better understand the role of PAF-R as a mediator of noxious stimulation in UVB-induced pathological conditions.
MATERIALS AND METHODS
Chemicals
Platelet-activating factor receptor agonist, CPAF, and all other chemicals were obtained from Sigma (St Louis, MO), unless indicated otherwise.
Animals
Platelet-activating factor receptor KO mice on a C57BL6 background were originally and generously provided by Dr Ishii (Department of Biochemistry and Molecular Biology, Faculty of Medicine, The University of Tokyo). They were generated as described previously (Ishii et al., 1998). The mice were bred in the Indiana University Laboratory Animal Resource Center. Age-matched PAF-R-expressing C57BL6 wild-type mice were used as controls. Mice were 8–12 weeks old at the time of testing. All mice were housed in a pathogen-free environment and studies were approved by the Animal Care and Use Committee of Indiana University School of Medicine.
Mice hind paw intradermal injection of CPAF
Platelet-activating factor KO and age-matched wild-type mice were randomly selected to receive vehicle control or CPAF injections in the left hind paw. Behavioral testing was conducted by investigators blinded to mouse genotype and treatment group. The baseline mechanical and thermal sensitivity of the left hind paw was determined. Approximately 1 hour after baseline testing, an intradermal injection with 50 µl of 0.25% fatty acid-free BSA vehicle control or 50 µl CPAF (250 or 50 ng) was administered into left hind paw, using 30G1/2 needle (Becton Dickson, Franklin Lakes, NJ).
Mice hind paw UVB irradiation
Platelet-activating factor KO and age-matched wild-type mice were randomly selected, and baseline thermal and mechanical sensitivity were determined as outlined below. Approximately 1 hour later, the mice were restrained and the left hind paw was irradiated with UVB at the dosage of 1,500 J m−2. The UVB source was a Philips F20T12/ UV-B lamp (270–390 nm, containing 2.6% UVC, 43.6% UVB, and 53.8% UVA). The intensity of the UVB irradiation was measured using an IL1700 radiometer and a SED240 UVB detector (International Light, Newburyport, MA).
Mechanical and thermal stimulation
The thresholds to mechanical stimulation were determined as described previously using the “up-down” method (Chaplan et al., 1994; Evans et al., 2000). Animals were placed on a wire mesh platform (3 mm mesh spacing) and covered with a 2,000 ml clear glass beaker. Von Frey hairs were introduced from below. Care was taken to make sure that the animals had equilibrated to the testing environment and that they had returned to normal activity between introduction of stimuli. Only Von Frey hairs producing a force of 0.005 g (the smallest fiber) to 3.63 g were used. Hairs producing force greater than 3.63 g lift the paw of the mouse. After testing for mechanical threshold, the threshold for thermal stimuli was determined by placing the animal in a Plexiglas box and exposing the paw to heat from a focused projection bulb (Hargreaves et al., 1988). The intensity of the bulb was adjusted to elicit paw withdrawal after approximately 10seconds in wild-type mice. Exposure time was limited to 30 seconds to prevent tissue damage. A mean of time to paw withdrawal was determined from three trials of testing the same hind paw in each mouse.
Bone marrow transplantation studies
Chimeric mice were generated as described previously (Kreklau et al., 2003). Briefly, bone marrow cells from donor mice were harvested and transplanted into lethally irradiated recipient mice at 4 × 106 cells per mouse. Irradiation was delivered as a split dose of 700 cGy followed by 400 cGy delivered 4 hours later. The peripheral blood was harvested 4 months later via the tail vein and the contribution of the PAF-R KO cells (CD45.2+) and the wild-type (BoyJ mice, CD45.1+) cells were analyzed by flow cytometry. A phycoerythrin-conjugated anti-mouse CD45.1 antibody (clone A20) and a PerCp-Cy5.5-conjugated anti-mouse CD45.2 antibody (clone 104) were purchased from BDPharmingen (Auburn, CA) for detection of CD45.1+ and CD45.2+ murine cells.
Statistical analysis
Results are expressed as the mean±standard error of the mean (SEM). Differences were determined by repeated-measures ANOVA with Dunnett’s post-hoc analysis. The significance level for all statistical tests was P<0.05.
ACKNOWLEDGMENTS
We thank Qiaofang Yi for her technical assistance and Dejane Duarte for her advice about behavioral testing. This research was supported in part by grants from the Riley Memorial Association, the National Institutes of Health grants HL62996 (J.B.T.) and U19 AI070448 (J.B.T.), and Veteran’s Administration Merit Award (J.B.T.).
Abbreviations
- ANOVA
analysis of variance
- CPAF
1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine
- KO
Knockout
- PAF
platelet-activating factor
- PAF-R
PAF receptor
- SEM
standard error of the mean
- TNF-α
tumor necrosis factor-α
- UVB
ultraviolet B
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Aepfelbacher M, Ziegler-Heitbrock H, Lux I, Weber P. Bacterial lipopolysaccharide up-regulates platelet-activating factor-stimulated Ca2+ mobilization and eicosanoid release in human Mono Mac 6 cells. J Immunol. 1992;148:2186–2193. [PubMed] [Google Scholar]
- Autier P, Doré J-F, Reis AC, Grivegnée A, Ollivaud L, Truchetet F, et al. Sunscreen use and intentional exposure to ultraviolet A and B radiation: a double blind randomized trial using personal dosimeters. Br J Cancer. 2000;83:1243–1248. doi: 10.1054/bjoc.2000.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber LA, Spandau DF, Rathman SC, Murphy RC, Johnson CA, Kelley SW, et al. Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J Biol Chem. 1998;273:18891–18897. doi: 10.1074/jbc.273.30.18891. [DOI] [PubMed] [Google Scholar]
- Besson J-M, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bishop T, Hewson DW, Yip PK, Fahey MS, Dawbarn D, Young AR, et al. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Prgrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Dallob A, Guindon Y, Goldenberg MM. Pharmacological evidence for a role of lipoxygenase products in platelet-activating factor (PAF)-induced hyperalgesia. Biochem Pharmacol. 1987;36:3201–3204. doi: 10.1016/0006-2952(87)90633-2. [DOI] [PubMed] [Google Scholar]
- Dy L, Pei Y, Travers JB. Augmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptor. J Biol Chem. 1999;274:26917–26921. doi: 10.1074/jbc.274.38.26917. [DOI] [PubMed] [Google Scholar]
- Evans AR, Junger H, Southall MD, Nicol GD, Sorkin LS, Broome JT, et al. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. J Pharmacol Exp Ther. 2000;293:912–920. [PubMed] [Google Scholar]
- Guadano EM, Serra-Batlles J, Meseguer J, Castillo JA, De Molina M, Valero A, et al. Rupatadine 10mg and ebastine 10mg in seasonal allergic rhinitis: a comparison study. Allergy. 2004;59:766–771. doi: 10.1111/j.1398-9995.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves A, Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hellewell PG, Williams TJ. Antagonism of Paf-induced oedema formation in rabbit skin: a comparison of different antagonists. Br J Pharmacol. 1989;97:171–180. doi: 10.1111/j.1476-5381.1989.tb11939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, et al. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Kreklau EL, Pollok KE, Bailey BJ, Liu N, Hartwell JR, Williams DA, et al. Hematopoietic expression of O(6)-methylguanine DNA methyltransferase-P140K allows intensive treatment of human glioma xenografts with combination O(6)-benzylguanine and 1, 3-bis-(2-chloroethyl)-1-nitrosourea. Mol Cancer Therap. 2003;2:1321–1329. [PubMed] [Google Scholar]
- Leverkus M, Year M, Eller MS, Tang EH, Gilchrest BA. Posttranscriptional regulation of UV induced TNF-alpha expression. J Invest Dermatol. 1998;110:353–357. doi: 10.1046/j.1523-1747.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- Morita K, Morioka N, Abdin J, Kitayama S, Nakata Y, Dohi T. Development of tactile allodynia and thermal hyperalgesia by intrathecally administered platelet- activating factor in mice. Pain. 2004;111:351–359. doi: 10.1016/j.pain.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Norins AL. Free radical formation in the skin following exposure to ultraviolet light. J Invest Dermatol. 1962;39:445–448. doi: 10.1038/jid.1962.137. [DOI] [PubMed] [Google Scholar]
- Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, et al. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J Invest Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- Poubelle PE, Gingras D, Demers C, Dubois C, Harbour D, Grassi J, et al. Platelet-activating factor enhances the concomitant production of TNF- and IL-1 by subsets of human monocytes. Immunology. 1991;72:181–187. [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharm Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- Roth M, Nauck M, Yousefi S, Tamm M, Blaser K, Perruchoud AP, et al. Platelet-activating factor exerts mitogenic activity and stimulates expression of interleukin 6 and interleukin 8 in human lung fibroblasts via binding to its functional receptor. J Exp Med. 1996;184:191–201. doi: 10.1084/jem.184.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis NM, Rose JK, Valone FH. Tumor necrosis factor release by human monocytes stimulated with platelet-activating factor. Lipids. 1991;26:1060–1064. doi: 10.1007/BF02536502. [DOI] [PubMed] [Google Scholar]
- Schwarz T. Photoimmunosuppression. Photodermal Photoimmunol Photomed. 2002;18:141–145. doi: 10.1034/j.1600-0781.2002.180307.x. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripheral Nervous System. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Stewart MS, Cameron GS, Pence BC. Antioxidant nutrients protect against UVB-induced oxidative damage to DNA of mouse keratinocytes in culture. J Invest Derm. 1996;106:1086–1089. doi: 10.1111/1523-1747.ep12339344. [DOI] [PubMed] [Google Scholar]
- Teather LA, Magnusson JE, Wurtman RJ. Platelet-activating factor antagonists decrease the inflammatory nociceptive response in rats. Psychopharmacology. 2002;163:430–433. doi: 10.1007/s00213-002-1039-9. [DOI] [PubMed] [Google Scholar]
- Teixeira CF, Cury Y, Oga S, Jancar S. Hyperalgesia induced by Bothrops jararaca venom in rats: role of eicosanoids and platelet activating factor (PAF) Toxicon. 1994;32:419–426. doi: 10.1016/0041-0101(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin aging. Clin Exper Dermatol. 2001;26:573–577. doi: 10.1046/j.1365-2230.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- Travers J, Pei Y, Morin SM, Hood AF. Antiinflammatory activity of the platelet-activating factor receptor antagonist A-85783. Arch Dermatol Res. 1998a;290:569–573. doi: 10.1007/s004030050353. [DOI] [PubMed] [Google Scholar]
- Travers JB, Huff JC, Rola-Pleszczynski M, Gelfand EW, Morelli JG, Murphy RC. Identification of functional platelet-activating factor receptors on human keratinocytes. J Invest Dermatol. 1995;105:816–823. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- Travers JB, Murphy RC, Johnson CA, Pei Y, Morin SM, Clay KL, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998b;56:305–324. doi: 10.1016/s0090-6980(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tusada M, Ishii S, Masuda T, Hasegawa S, Nakamura K, Nagata K, et al. Reduced pain behaviors and extracellular signal-related protein kinase activation in primary sensory neurons by peripheral tissue injury in mice lacking platelet-activating factor receptor. J Neurochem. 2007;102:1658–1668. doi: 10.1111/j.1471-4159.2007.04796.x. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochem Photobiol. 1995;62:389–401. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings SD, Perkins SM, et al. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J Clin Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yao Y, Konger RL, Sinn A, Cai S, Pollok KE, et al. Ultraviolet B radiation-mediated inhibition of contact hypersensitivity reactions is dependent upon the platelet-activating factor system. J Invest Dermatol. 2008 doi: 10.1038/sj.jid.5701251. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet- activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Critical Care Medicine. 2002;30:S294–S301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]