Abstract
Although many animal species sense gravity for spatial orientation, the molecular bases remain uncertain. Therefore, we studied Drosophila melanogaster, which possess an inherent upward movement against gravity-negative geotaxis. Negative geotaxis requires Johnston's organ, a mechanosensory structure located in the antenna that also detects near-field sound. Because channels of the transient receptor potential (TRP) superfamily can contribute to mechanosensory signaling, we asked whether they are important for negative geotaxis. We identified distinct expression patterns for 5 TRP genes; the TRPV genes nanchung and inactive were present in most Johnston's organ neurons, the TRPN gene nompC and the TRPA gene painless were localized to 2 subpopulations of neurons, and the TRPA gene pyrexia was expressed in cap cells that may interact with the neurons. Likewise, mutating specific TRP genes produced distinct phenotypes, disrupting negative geotaxis (painless and pyrexia), hearing (nompC), or both (nanchung and inactive). Our genetic, physiological and behavioral data indicate that the sensory component of negative geotaxis involves multiple TRP genes. The results also distinguish between different mechanosensory modalities and set the stage for understanding how TRP channels contribute to mechanosensation.
Keywords: Drosophila, transient receptor potential, geotaxis
The primary mechanosensory organ that detects gravity in Drosophila appears to be Johnston's organ (1). This organ is located in the second antennal segment. It consists of over 200 scolopidia arrayed in a bowl shape (2), with each scolopidium containing mechanosensory chordotonal neurons and their support cells (3–5) (Fig. 1A). Johnston's organ is well known as a detector of near-field sound (3–6). Air particle displacement vibrates the third antennal segment, deforming the cuticle at the joint between segments 2 and 3 where the sensory units of Johnston's organ attach. It was proposed that the third segment may also be deflected by gravity (7), and the geometry of Johnston's organ suggests it could respond to gravity irrespective of head orientation (2). Indeed, recent work indicates that Johnston's organ can also respond to gravity, as well as to wind (1, 8). Thus, Johnston's organ may detect multiple different mechanosensory stimuli, and investigations of specific molecular mechanisms underlying these sensory functions may benefit our understanding of other polymodal sensory structures such as the inner ear and dorsal root ganglion in mammals.
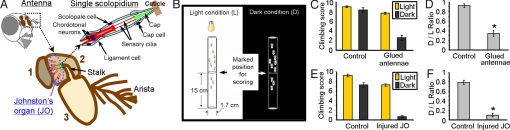
Fig. 1.
Johnston's organ is required for negative geotaxis in Drosophila. (A) Schematic showing the fly head, antenna, Johnston's organ, and a single scolopidium of Johnston's organ. The numbers 1, 2, and 3 refer to the 3 antennal segments. (B) Schematic of the tube-climbing test. In each trial, a group of 10 flies were tapped to the bottom of the tube, and we counted the number of flies crossing the 15-cm threshold line within 15 s as the climbing score. (C) Climbing scores in Light (L) and Dark (D) conditions of control Canton-S (CS) flies (n = 10 trials) and CS flies with the third antennal segment glued to head (n = 12 trials). (D) Ratios of climbing scores (D/L Ratios) for flies in C. (E) Climbing scores of control w1118-WLS flies (n = 10 trials) and w1118-WLS flies with injured Johnston's organ because of second segment pinching (n = 10 trials). (F) D/L Ratios for flies in E. Data are mean ± SEM. *, P < 0.05 by unpaired t test.
Almost 50 years ago, Hirsch and colleagues demonstrated that negative geotaxis is genetically encoded in Drosophila (9, 10). Since then, several genes influencing this behavior have been identified (11–13). However, those genes are expressed in both central and peripheral nervous systems, and the nature of their role in the sensory organ that detects gravity remains unknown. The goal of this work was to identify genes involved in sensory aspects of negative geotaxis and in so doing to obtain genetic data to discriminate between the structural and functional components of Johnston's organ involved in negative geotaxis and hearing.
Results
Johnston's Organ Is Essential for Negative Geotaxis.
To verify that Johnston's organ is essential for negative geotaxis, we restricted movement of the third antennal segments by fixing them to the head with nontoxic glue. To assess negative geotaxis, we used a tube-climbing assay. Flies were tapped to the bottom of a vertical tube, and the number of flies climbing above a 15-cm mark within 15 s was counted as a climbing score (Fig. 1B). This assay was done first with illumination and then repeated in the dark. The “Light” condition tests general locomotion driven by both phototaxis and negative geotaxis, and the “Dark” condition tests locomotion due to negative geotaxis only. Wild-type flies showed upward movement in both Light (L) and Dark (D) conditions, producing similar climbing scores and a D/L Ratio close to 1 (Fig. 1 C and D). This behavior reflects negative geotaxis. Gluing the antenna reduced the preference for upward movement. Injuring Johnston's organ by pinching the second segment with fine forceps had a similar effect (Fig. 1 E and F). An independent assay that does not depend on tapping-initiated locomotion (the vertical choice maze) also showed that gluing the antenna disrupted the preference for upward movement (Fig. S1). These data suggest that Johnston's organ is essential for negative geotaxis and are consistent with a recent publication (1).
TRP Channels Are Expressed in Specific Populations of Johnston's Organ Cells.
Previous data indicate that TRP superfamily ion channels may be involved in mechanosensation (14–18). Of note, the TRPN gene no mechanoreceptor potential C (nompC) (19, 20) and the TRPV genes nanchung (nan) and inactive (iav) (21, 22) are expressed in Johnston's organ chordotonal neurons and are required for normal hearing. Moreover, a nan mutant reduced the Ca2+ elevations in Johnston's organ neurons that occur with antennal movement (1). The TRPA genes painless (pain) (23) and pyrexia (pyx) (24) are also expressed in Johnston's organ, although their function there is unknown. Outside Johnston's organ, Pain channels are required for avoidance behaviors in response to harsh touch, noxious heat (≥38 °C), and aversive chemicals (23, 25–27), and Pyx channels contribute to protection from noxious heat (≥40 °C) (24).
We hypothesized that some of these TRP channels are involved in gravity sensing, and we therefore tested their expression in Johnston's organ using TRP gene promoters linked to Gal4 to drive UAS-fluorescent reporters. With nan, iav, nompC, and pain promoters, the GFP reporter filled sensory dendrites and cilia of chordotonal neurons (Fig. 2A Upper). With a nuclear-localized DsRed as the reporter, we localized the cell bodies of these neurons (Fig. 2A Lower). nan-Gal4 and iav-Gal4 expressed in chordotonal neurons throughout Johnston's organ. In contrast, nompC-Gal4 expressed in a cluster of ≈60–70 medial chordotonal neurons, and painGal4 expressed in ≈100 chordotonal neurons arranged in a bent ring. Identification of distinct populations of Johnston's organ neurons expressing nompC and pain are consistent with recent reports that Johnston's organ contains different cell types that project to discrete portions of the brain (1, 2, 8).
Fig. 2.
TRP genes have distinct expression patterns in Johnston's organ. (A) Expression of nan-Gal4, iav-Gal4, nompC-Gal4, painGal4, and pyx-Gal4 in Johnston's organ visualized by UAS-GFP, which labels the cytoplasm (Upper, scale bars, 10 μm, except for pyx-Gal4 where scale bar, 30 μm), and UAS-Nuclear DsRed, which labels cell nuclei (Lower, fluorescent images are overlaid on DIC images, scale bars, 30 μm). In the second antennal segment, all labeling appeared to be in Johnston's organ; nan-Gal4, painGal4 and pyx-Gal4 also labeled some cells in the third segment. White dashed arrows indicate direction from cell body to dendrite and cilium. (B) Confocal reconstruction of Johnston's organ expressing both painGal4 and pyx-Gal4 visualized by UAS-Nuclear DsRed (red). Inset shows a different view of the structure with pain and pyx expressing cell nuclei indicated. D, dorsal; A, anterior; M, medial; P, posterior. (C) Expression of both painGal4 and pyx-Gal4 visualized by UAS-myr-mRFP (red), which labels the plasma membrane. Scolopale rods are stained with Alexa633-phalloidin (blue). Dendritic caps are labeled with transgenic GFP-NompA (green). (Scale bar, 20 μm.) The boxed region highlights 2 painGal4-expressing neurons projecting their cilia to pyx-Gal4-expressing cap cells. Three enlarged views of this region are displayed on the right. Arrows indicate cilia and arrowheads indicate a cluster of cap cells.
In contrast to the neuronal expression patterns, pyx-Gal4 expressed in ≈50 Johnston's organ cells, with the cell bodies forming a ring (Fig. 2A Lower) that was located at the distal end of the scolopidium array (Fig. 2A Upper). This site receives projections of the apical tips of the chordotonal cilia and includes the caps that cover the ciliary tips (Fig. 1A). In flies carrying both painGal4 and pyx-Gal4 driving the UAS-Nuclear-DsRed reporter, the labeled nuclei formed 2 concentric rings (Fig. 2B and Movie S1); the outer ring was in the position of painGal4, and the inner ring was in the position of pyx-Gal4. Using a cell membrane tethered mRFP (UAS-myr-mRFP) as a reporter, we were able to trace the projection of painGal4-expressing chordotonal cilia to pyx-Gal4-expressing cells (Fig. 2C). These observations suggest that painGal4-labeled neurons and pyx-Gal4-labeled cap cells might assemble in the same scolopidia (4).
Specific TRP Channels Are Required for Normal Negative Geotaxis Behavior.
To determine whether any of these TRP channels contribute to gravity sensing, we tested mutants (Table S1) for negative geotaxis. We found that a nompC mutant (nompCf00642, Note S1 and Fig. S2), behaved like wild-type controls, showing a preference for upward movement in the tube-climbing assay (Fig. 3 A and B and Fig. S3) and vertical choice maze (Fig. S1). These data suggest that this TRP channel is not required for negative geotaxis behavior and are consistent with the prediction that gravity detection would be independent of nompC (1). In contrast, mutants of pain and pyx were impaired in negative geotaxis (Fig. 3 A and B and Fig. S1). Likewise, nan and iav mutants showed defective negative geotaxis, although their general locomotion was reduced as indicated by a reduced climbing score in light conditions (Fig. 3 A and B, Note S2, and Fig. S1).
Fig. 3.
Specific TRP mutants impair negative geotaxis behavior in the tube-climbing test. (A) Climbing scores of TRP mutants. nompCf00642 was backcrossed to the w1118-WLS control strain. pain1, painGal4, and pyx3 were backcrossed to the Canton-S (CS) control strain. nan36a and iav3621 were in their original genetic backgrounds. (B) D/L Ratios for flies in A. w1118-WLS (n = 14 trials) and nompCf00642 (n = 27 trials) were compared with unpaired t test. CS (n = 25 trials) and mutants (pain1, n = 25 trials; painGal4, n = 10 trials; pyx3, n = 9 trials; nan36a, 10 trials; iav3621, n = 10 trials) were compared by ANOVA (α level = 0.05) and post hoc test of Games-Howell. (C and D) pyxDf4 flies (n = 22 trials) had normal behavior, but pyxDf9 (n = 24 trials) flies were defective compared with the w1118-WLS control (n = 15 trials). D/L Ratios were analyzed by ANOVA (α level = 0.05) and post hoc test of Games-Howell. Data are mean ± SEM. *, significant difference from the control.
The pyx gene has at least 2 transcripts of differing length—both encode channel subunits, but only the long transcript encodes 9 ankyrin repeats at the N terminus (24). The pyx3 mutation eliminates both transcripts and disrupted negative geotaxis (Fig. 3 A and B). pyxDf9 selectively eliminates the long transcript disrupted negative geotaxis (Fig. 3 C and D). In contrast, the pyxDf4 mutation, which only eliminates the short transcript, had normal behavior. These data suggest that the Pyx channel requires the ankyrin repeats for its role in negative geotaxis.
We tested whether the geotaxis defect of pyx and pain mutants arose from loss of their function in Johnston's organ. When pyx-Gal4 drove expression of a dominant-negative pyx (UAS-pyxFAP, Note S3), it mimicked the geotaxis defect of pyx3 mutants (Fig. 4A). Of note, pyx-Gal4 labeled cap cells in Johnston's organ (Fig. 2 A and C), but it did not label the CNS (Fig. 4B). In contrast, expressing pyxFAP with the pan-neuronal Appl-Gal4 driver failed to alter negative geotaxis (Fig. 4C). These results imply that Pyx channel function in Johnston's organ cap cells is important for negative geotaxis. To manipulate pain function specifically in the sensory system, we used iav-Gal4 whose expression was restricted to chordotonal neurons of Johnston's organ (Fig. 2A) and femoral chordotonal organs (Fig. 4D). These sensory neurons project their axons to the CNS (Fig. 4E), but we did not detect iav-Gal4 activity in CNS neurons with the UAS-Nuclear DsRed reporter. When iav-Gal4 drove expression of UAS-pain (encoding the pain cDNA), we rescued the pain1 geotaxis defect (Fig. 4F). Although these data cannot exclude a role for pain in femoral chordotonal organs, they indicate that the geotaxis defect of pain mutants is due to abnormal transduction in the sensory system.
Fig. 4.
Function of pain and pyx in peripheral sensory tissues is key for negative geotaxis. (A) Expression of the dominant negative UAS-pyxFAP driven by pyx-Gal4 disrupted negative geotaxis in the climbing assay. Note that in the 3 genotypes each transgene was in homozygous state. *, significant difference from pyx-Gal4 based on ANOVA (α level = 0.05) and post hoc test of Games Howell (ANOVA). n = 10 trials in each group. (B) pyx-Gal4 did not drive mCD8::GFP reporter expression in the brain or thoracico-abdominal ganglion. CNS tissues were stained with the nc82 antibody to visualize neuropil (red) and anti-GFP antibody to visualize the reporter. (Scale bars, 100 μm.) (C) Expression of UAS-pyxFAP driven by Appl-Gal4 (a pan-neuronal Gal4 driver) had no effect on negative geotaxis. n = 9 trials in each group. (D) In the leg, nuclear DsRed expression driven by iav-Gal4 was only detected in femoral chordotonal organs. Inset shows a cluster of nuclei of chordotonal neurons. (E) iav-Gal4 did not drive mCD8::GFP expression in central neurons, but anti-GFP staining was present in projections of sensory afferents from Johnston's organ and leg chordotonal neurons (green). (Scale bars, 100 μm.) (F) Expression of UAS-pain under the control of iav-Gal4 restored negative geotaxis. *, significant difference from each of the 2 controls, based on ANOVA (α level = 0.05) and post hoc test of Games Howell. n = 39 trials for pain1; iav-Gal4/+, n = 35 trials for pain1; UAS-pain/+, and n = 39 trials for pain1; iav-Gal4/UAS-pain. Data are mean ± SEM.
TRP Mutations Impaired the Electrophysiological Response of the Antenna to Changes in Body Position.
To more directly test whether these TRP channels contribute to gravity detection in Johnston's organ, we developed an electrophysiological assay. Prior studies in insects have shown that controlled body rotations mimic gravity-sensing experiences (28, 29). We therefore recorded neural activity of Johnston's organ while the fly body was rotated (Fig. 5A and Fig. S4). The extracellular recording electrode was placed at the junction between the first and second antennal segments (Fig. 5A Inset), a position allowing us to capture most of the action potentials generated by Johnston's organ chordotonal neurons (19). Rotating the fly in all 3 orthogonal axes—pitch, roll, and yaw—triggered spiking activity in wild-type flies (Fig. 5 B–D). Reverse movements that returned the body to its original orientation evoked similar spiking activity. The responses were transient, coincided with the rotation event (≈0.5 s), and were reproducible with repeated rotations (Fig. 5B). A pitch of 90° from horizontal to an upward vertical orientation evoked ≈20 spikes in Canton-S flies (Fig. 5 B expanded trace and G). When we restricted antennal movements by gluing the third antennal segment, we abolished rotation-evoked spikes (Fig. 5E). Removing the glue partially reversed the effect.
Fig. 5.
Specific TRP mutants show defective antennal nerve responses to rotation. (A) Schematic of the recording apparatus. The recording electrode and fly were both mounted on a rotatable platform. Rotation of the platform changes the orientation of the fly body, but the electrode position remains constant relative to the fly. The recording electrode was positioned between the first and second antennal segment where axons of Johnston's organ fasciculate (Inset). (B–D) Bidirectional 90° pitch (B), roll (C), and yaw (D) all induced transient spiking responses in the antennal nerve of Canton-S (CS) wild-type flies. The response was reproducible with repetitive stimulations. Expanded trace in B shows quantification of the response. We counted the number of spikes with amplitudes greater than 2-fold baseline activity. The threshold is represented with the horizontal line in cyan. The counted spikes are marked with blue dots at their negative peaks and represented by a temporally aligned raster above the trace. Variability in the amplitude of individual spikes suggests that multiple units (neurons) were recorded. (E) Spiking responses to bidirectional 90° pitches were abolished by gluing the third antennal segment to the head to prevent its movement, and the response partially recovered after removing the glue. (F) Sample traces for nan36a, iav3621, pain1, painGal4, and pyx3 mutants in response to bidirectional 90° pitches. Each trace represents an example that had a spike number (forward pitch) at the median for all specimens of that genotype. (G) Quantification of spiking responses in the mutants. For nan36a, iav3621, and painGal4. *, significant difference from CS by ANOVA (α level = 0.05) and post hoc test of Games-Howell. *, P < 0.05 by unpaired t test for pyx3. The number (n) of antennae recorded in each group is shown. Data are mean ± SEM.
iav3621 and nan36a mutations eliminated most of the electrophysiological response to a 90° pitch (Fig. 5 F and G). These results suggest that Iav and Nan channels may serve an essential function in the response of chordotonal neurons to gravity. Likewise, body rotations elicited fewer spikes in painGal4 and pyx3 flies than in congenic controls (Fig. 5 F and G). pain1 flies were statistically indistinguishable from Canton-S flies, consistent with the observation that they were also less defective than painGal4 flies in the climbing tube assay (Fig. 3 A and B).
pain and pyx Mutations Do Not Impair Hearing.
Earlier data showing that nan and iav mutations impair hearing (21, 22) combined with our results indicate that these channels are essential for both geotaxis and hearing. To learn whether the other TRP channels that are expressed in Johnston's organ also influence both mechanosensory modalities, we assayed hearing (Fig. 6A). The nompCf00642 mutation greatly reduced sound-evoked antennal responses (Fig. 6B). These results were expected based on data from other nompC mutants (19) and serve as a positive control. Conversely, whereas pain mutants manifest defective negative geotaxis, their sound-evoked potentials were normal. Likewise, the pyx3 mutation had only minimal effects on sound-evoked potentials. These results suggest that specific TRP channels play distinct roles in signaling sound and gravity.
Fig. 6.
The nompC but not the pain and pyx mutations disrupted auditory responses. (A) Schematic of the auditory recording method. Near-field sound, mimicking the Drosophila courtship song was delivered to the antenna. An extracellular electrode positioned as in Fig. 5A recorded sound-evoked potentials (SEP) in the axons of Johnston's organ. A sample trace from a wild-type fly is shown. (B) Amplitude of SEPs in pain1, painGal4, pyx3, and nompCf00642 flies. nompCf00642 heterozygous (het.) and homozygous (homo.) flies are shown. *, difference from control by ANOVA (α level = 0.05) and post hoc test of Games-Howell. The number (n) of antennae recorded in each group is shown underneath the corresponding genotype. Data are mean ± SEM.
Discussion
Our data show that several TRP superfamily channels in Johnston's organ are required for Drosophila to respond to gravity. nan and iav are expressed in chordotonal neurons throughout Johnston's organ, and their mutation disrupted both hearing and geotaxis. Previous studies of hearing suggested that Nan and Iav form a TRPV channel that acts downstream of the primary mechanotransducer and enhances the relay of excitatory signals toward the cell body (20). We speculate that they may serve a similar role in gravity sensing.
In contrast to nan and iav, pain expression was limited to a subset of chordotonal neurons, and its mutation disrupted negative geotaxis, but not hearing. Might the Pain channel be a mechanosensor? This possibility is intriguing because the Pain channel also mediates mechanical nociception in fly larvae (25). However, pain also contributes to thermal and chemical nociception in larvae (25) and heat-induced currents when expressed in heterologous cells (30), and thus a specific role in detecting mechanical stimuli remains speculative.
The contribution of pyx to geotaxis was distinct from the other TRP subunits by its expression in cap cells rather than neurons. Cap cells form structural links between chordotonal neurons and the moving joint between second and third antennal segments. Interestingly, in the cap cells of the katydid Caedicia simplex auditory sensilla, acoustic stimuli generate a slow, graded hyperpolarizing membrane potential and a train of fast, biphasic spikes, and the spikes correlated temporally with depolarizing spikes in the chordotonal sensory neurons (31). Those results raise the possibility that in Drosophila gravity perception cap cells and their Pyx channels might actively respond to motion and participate in mechanosensory signaling. In addition, cap cells in Drosophila larval chordotonal organs contain numerous aligned microtubules (6, 32–34), and motility of those microtubules is thought to modulate tension in the chordotonal organ (4). Thus, another possibility is that Pyx channels trigger contraction or microtubule motility in Johnston's organ cap cells to influence gravity signals.
We found that pitch, roll, and yaw in either direction triggered action potential firing from Johnston's organ. Once the rotation stopped, the electrical activity returned to basal levels. Interestingly, recent data indicated that continuous mechanical displacement of the arista to mimic the effect of gravity (or wind) induced a tonic increase in intracellular Ca2+ concentration, [Ca2+]i, in some Johnston's organ neurons (1, 8). Methodological factors likely explain the apparent difference in phasic vs. tonic responses. First, the dynamic level of [Ca2+]i may not precisely predict the timing of action potentials in a neuron, and thus phasic action potential firing is not necessarily inconsistent with a tonic elevation of [Ca2+]i. Second, in our study body rotations mimicked real-life experience of a fly moving in the gravitational field, and the antennal receiver (third segment including the arista) was free to respond to transient angular accelerations accompanying body rotations. Elastic properties of the antenna and muscle control of antennal movement might have played a role in our studies. In studies measuring [Ca2+]i (1, 8), a probe statically controlled the position of the antennal receiver, and the first and second antennal segments were immobilized to prevent muscle-based antennal movement. In the future, a comparison between [Ca2+] signals and action potential firing in the same experimental setting may yield a better understanding of how Johnston's organ codes sensory information of gravity, acceleration, and orientation.
Our study also has limitations. First, a caveat to our gene expression data are use of promoter-Gal4 transgenic constructs. A putative enhancer/promoter fragment may not contain the complete information to precisely reproduce endogenous gene expression. Moreover, the location of a transgene in the fly genome may affect expression patterns and levels. In the future, it will be desirable to examine the anatomical and subcellular localization of these TRP channels with specific antibodies. Second, the nompC and pain lines were hypomorphs rather than nulls. Third, although our rotating device for electrophysiological recordings has the advantage that it may mimic gravitational changes, it could introduce unappreciated vibration of the antennal receiver. In addition, programmable control of movement would be desireable.
Our data combined with recent studies (1, 8) indicate that geotaxis, hearing, and wind detection use the same general structures, Johnston's organ scolopidia and its chordotonal neurons and support cells. They also indicate that subsets of these structures are specialized for distinct senses. Based on the observations that the promoter-Gal4 constructs of pain, pyx, and nompC label different cell populations in Johnston's organ and that their mutants display specific defects in either geotaxis or auditory tests, we speculate that the expression and function of these different TRP channels may be key for Johnston's organ to distinguish between gravity and sound.
Materials and Methods
Tube-Climbing Test.
Each fly strain or genetic cross was kept in an environmental chamber at 25 °C with 12-h light–dark cycles. Four- to seven-day-old male progeny were collected with light CO2 anesthesia into groups of 10 and allowed to recover for at least 1 day. The behavioral tests were performed between 9 and 11 AM or 6 and 8 PM. A climbing tube was made from a 50 mL Costar Stripette by removing both ends and plugging them with a cotton ball. To restrict movements of the antenna, the Glue-All MultiPurpose glue (nontoxic, Elmer's Products) was applied with forceps to the antenna and adjacent head cuticle. Once it solidifies, the glue is resistant to grooming and remains stably on the fly head for at least 3 days.
Electrophysiological Recordings.
Recordings of auditory responses in the antennal nerve were performed as described in ref. 19. Briefly, computer-generated sound, which mimics the pulse phase of the Drosophila courtship song, was delivered frontally to the fly's head through Tygon tubing from a loudspeaker. An electrolytically sharpened tungsten electrode was inserted dorso-medially between the first and second antennal segments to record extracellularly from the antennal nerve. A similar, reference electrode was inserted into the dorsal side of the head. Raw differential signals were amplified and digitized as previously described. Each antenna was given 10 consecutive auditory stimuli. Amplitudes are the average of the 10 recordings. Flies of different genotypes were recorded in alternate order to minimize systematic variations.
Responses of Johnston's organ to body rotations were also recorded extracellularly (see Fig. 5A for a diagram for the recording apparatus). A living female fly (≈1 week old) was placed into a cut plastic pipette tip, with the fly head protruding from the tip. A piece of wet cotton was placed next to the fly abdomen and connected to an AgCl-coated silver wire as the reference electrode. A sharp tungsten electrode was placed between the first and second antennal segments. Voltage differences between the reference and recording electrodes were amplified by an Axopatch-1D (Axon Instruments). Clampfit 9.0 software (Axon Instruments) was used to analyze spike frequency. The amplitude threshold was set at 2 times the baseline noise level and the number of spikes was counted automatically. Each antenna was stimulated with multiple, manually controlled 90° rotations. The spiking responses occurred immediately after initiation of the rotation and stopped as soon as the rotation ended. The number of spikes was scored as the average of the 2 strongest responses of ≈6 trials. Flies of different genotypes were recorded in alternate order. The experimenter was blinded to the genotypes during the recording and subsequent data analysis. To control for possible stimulation by airflow, we placed a chamber over the fly and electrode; we observed no difference in electrophysiological response in the presence or absence of the chamber. In addition, the manually controlled rotation is not accompanied by noise, so contribution of an auditory response to the recording should be negligible (Note S4).
See SI Text for additional materials and methods.
Supplementary Material
Acknowledgments.
We thank T. Kitamoto (University of Iowa) for valuable discussions and sharing fly strains, C. Reddy for help with electrophysiology, Y. Li for invaluable support, and T. O. Moninger for assistance with microscopy. We are also grateful to the following investigators for fly strains: C. Kim (Chonnam National University, Gwangju, Korea) for nan36a, iav3621, and nan-Gal4; J. Kim (Korea Advanced Institute of Science and Technology, Daejeon, Korea) for pyx3, pyxDf4, pyxDf9, and pyxGe4 pyx3; S. Benzer (California Institute of Technology, Pasadena, CA) W. D. Tracey, Jr. (Duke University, Durham, NC) and B. Al-Anzi (California Institute of Technology, Pasadena, CA) for pain1, painGal4, and UAS-pain; M. J. Kernan (Stony Brook University, Stony Brook, NY) for GFP-nompA; and R. S. Hewes (University of Oklahoma, Norman) for Appl-Gal4. We thank J. Hoang, K. Crose, and J. Berge for help with fly husbandry and general laboratory assistance. This work was partially supported by National Institutes of Health Grant DC04848 (to D.F.E.). Y.B.S. was an Associate and M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906377106/DCSupplemental.
References
- 1.Kamikouchi A, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 2.Kamikouchi A, Shimada T, Ito K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 2006;499:317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- 3.Yack JE. The structure and function of auditory chordotonal organs in insects. Microsc Res Tech. 2004;63:315–337. doi: 10.1002/jemt.20051. [DOI] [PubMed] [Google Scholar]
- 4.Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech. 2004;63:388–399. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 6.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 7.Beckingham KM, Texada MJ, Baker DA, Munjaal R, Armstrong JD. Genetics of graviperception in animals. Adv Genet. 2005;55:105–145. doi: 10.1016/S0065-2660(05)55004-1. [DOI] [PubMed] [Google Scholar]
- 8.Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlenmeyer-Kimling L, Hirsch J. Measurement of the relations between chromosomes and behavior. Science. 1961;134:1068–1069. doi: 10.1126/science.134.3485.1068. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch J, Erlenmeyer-Kimling L. Sign of taxis as a property of the genotype. Science. 1961;134:835–836. doi: 10.1126/science.134.3482.835. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong JD, Texada MJ, Munjaal R, Baker DA, Beckingham KM. Gravitaxis in Drosophila melanogaster: A forward genetic screen. Genes Brain Behav. 2006;5:222–239. doi: 10.1111/j.1601-183X.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 12.Mertens I, et al. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Toma DP, White KP, Hirsch J, Greenspan RJ. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002;31:349–353. doi: 10.1038/ng893. [DOI] [PubMed] [Google Scholar]
- 14.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liedtke W. TRPV channels' role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007;179:473–487. doi: 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- 16.Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 17.Myers BR, Saimi Y, Julius D, Kung C. Multiple unbiased prospective screens identify TRP channels and their conserved gating elements. J Gen Physiol. 2008;132:481486. doi: 10.1085/jgp.200810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen AP, Corey DP. TRP channels in mechanosensation: Direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 19.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 21.Gong Z, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 23.Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 25.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 26.Xu SY, et al. Thermal nociception in adult Drosophila: Behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat Neurosci. 2008;11:676–682. doi: 10.1038/nn.2119. [DOI] [PubMed] [Google Scholar]
- 28.Bischof H-J. Club-shaped hairs in the cerci of the cricket Gryllus bimaculatus acting as gravity receptors. J Comp Physiol. 1975;98:277–288. [Google Scholar]
- 29.Hartman HB, Walthall WW, Bennett LP, Stewart RR. Giant interneurons mediating equilibrium reception in an insect. Science. 1979;205:503–505. doi: 10.1126/science.205.4405.503. [DOI] [PubMed] [Google Scholar]
- 30.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldfield BP, Hill KG. Functional organization of insect auditory sensilla. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;158:27–34. [Google Scholar]
- 32.Matthews KA, Miller DF, Kaufman TC. Functional implications of the unusual spatial distribution of a minor alpha-tubulin isotype in Drosophila: A common thread among chordotonal ligaments, developing muscle, and testis cyst cells. Dev Biol. 1990;137:171–183. doi: 10.1016/0012-1606(90)90018-e. [DOI] [PubMed] [Google Scholar]
- 33.Dettman RW, Turner FR, Hoyle HD, Raff EC. Embryonic expression of the divergent Drosophila beta3-tubulin isoform is required for larval behavior. Genetics. 2001;158:253–263. doi: 10.1093/genetics/158.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mech Dev. 2001;108:171–178. doi: 10.1016/s0925-4773(01)00487-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.