Abstract
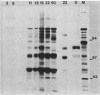
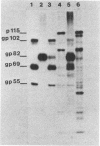
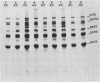
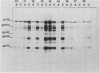
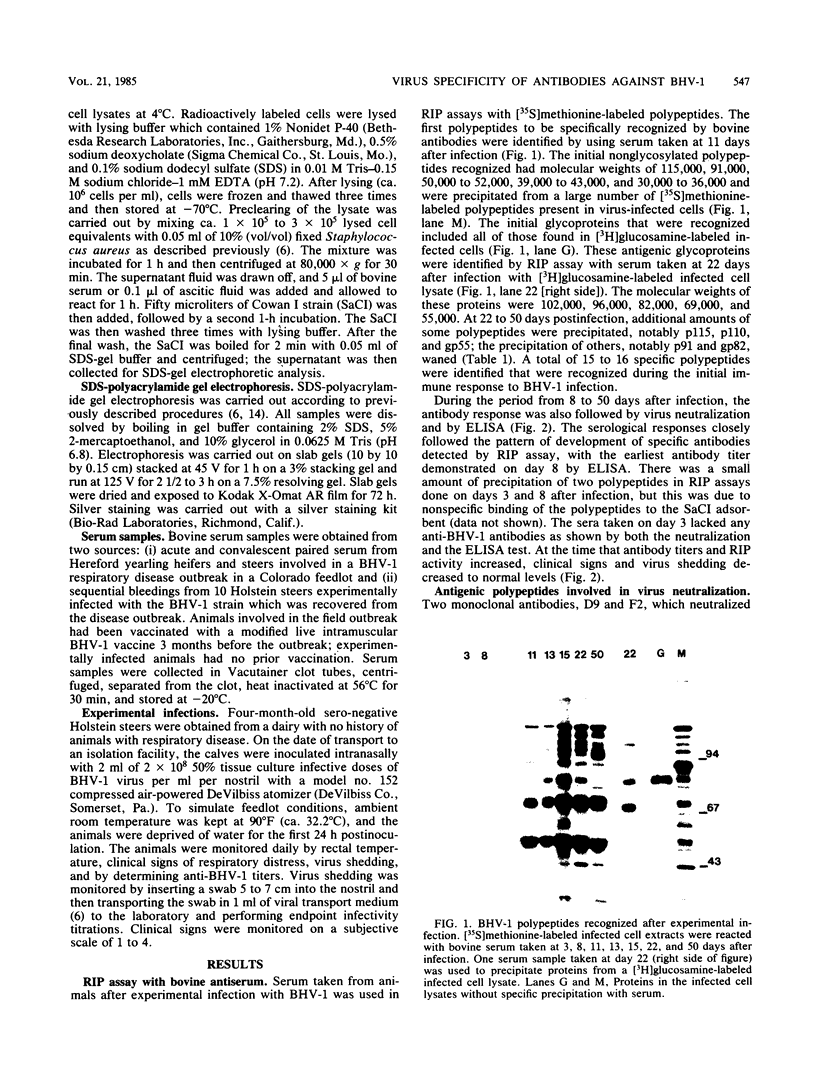
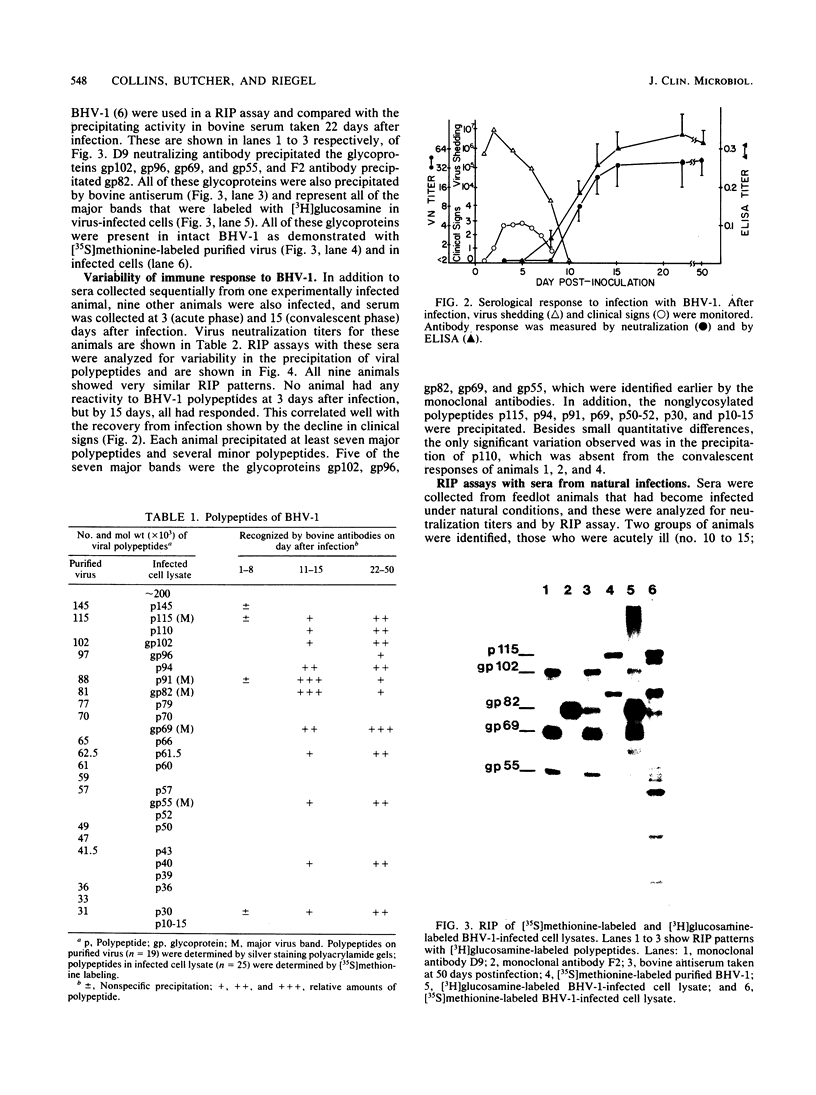
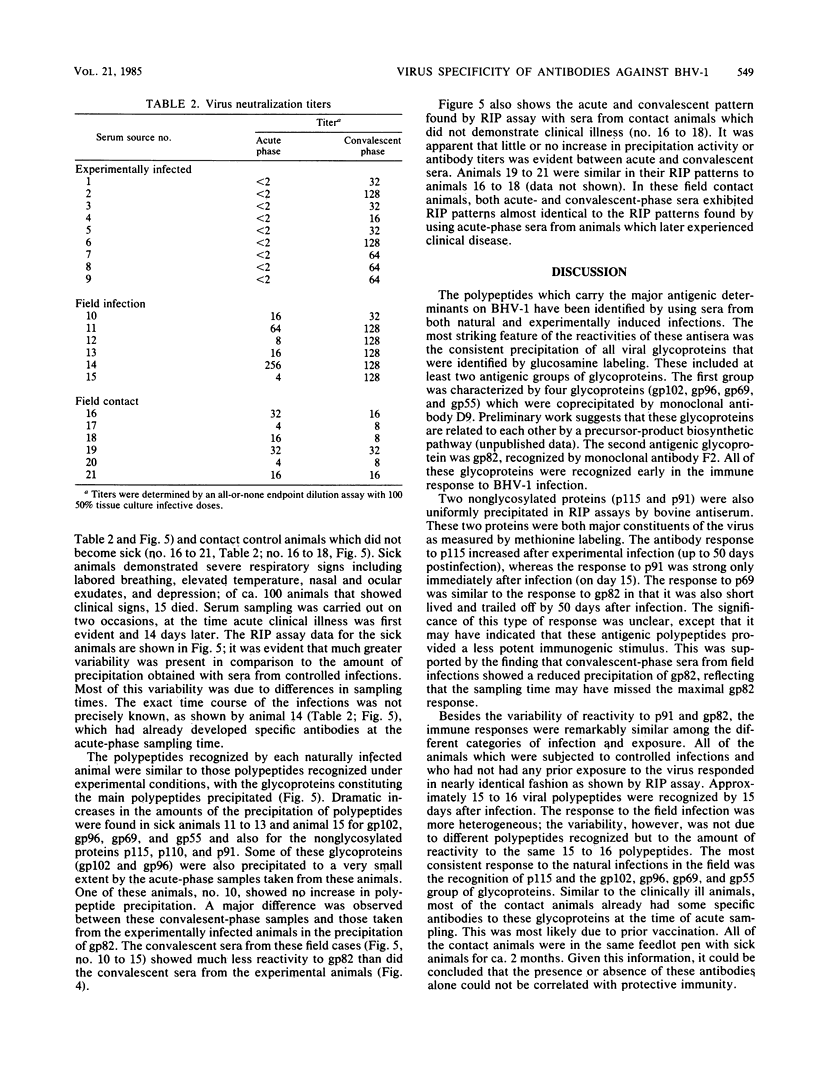
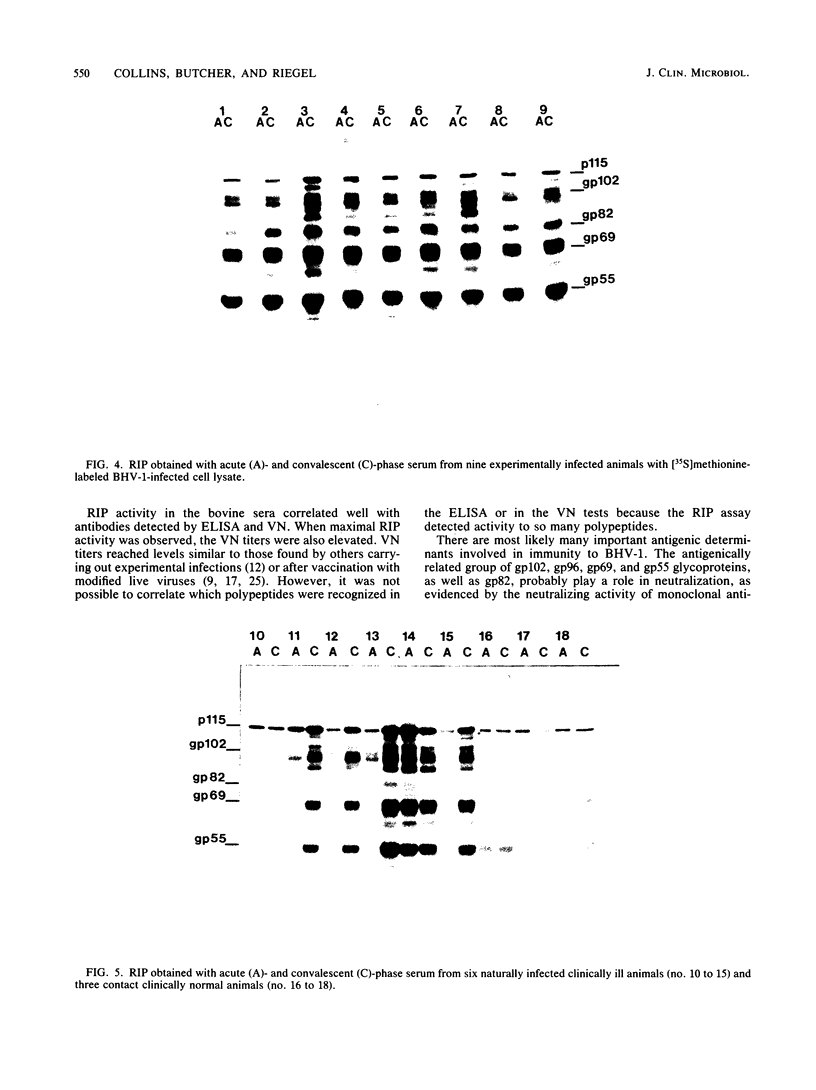
The virus specificity of antibodies against bovine herpes virus type 1 was determined with a radioimmunoprecipitation assay and serum collected from natural and experimentally induced infections. By using sequentially collected sera, the development of antibodies to 4 to 5 viral glycoproteins and 11 to 12 nonglycosylated proteins was followed for the first 50 days after infection. The major and most consistent responses in experimentally and naturally infected animals were to four glycoproteins with molecular weights of 102,000, 96,000, 69,000, and 55,000, as well as to a major virion 115,000-molecular-weight nonglycosylated protein. The four glycoproteins were all coprecipitated by a neutralizing monoclonal antibody and were probably involved as target antigens in virus neutralization. Another antigenically unrelated glycoprotein with a molecular weight of 82,000 and a nonglycosylated protein with a molecular weight of 91,000 were also precipitated, but the immune response to these two proteins was transient. Reactivity to gp82 was only weakly detected in serum from naturally infected animals. Contact control animals which did not contract a bovine herpes virus type 1 infection but were exposed to infected animals with signs of severe illness had antibodies which recognized gp102, gp96, gp69 and gp55 as well as p115. These antibodies were present in low amounts and, in contrast to infected animals, did not increase between acute and convalescent sampling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., Zee Y. C., Ardans A. A. Identification of envelope and nucleocapsid proteins of infectious bovine rhinotracheitis virus by SDS-polyacrylamide gel electrophoresis. Vet Microbiol. 1983 Feb;8(1):57–68. doi: 10.1016/0378-1135(83)90019-6. [DOI] [PubMed] [Google Scholar]
- Carter V. C., Rice P. L., Tevethia S. S. Intratypic and intertypic specificity of lymphocytes involved in the recognition of herpes simplex virus glycoproteins. Infect Immun. 1982 Jul;37(1):116–126. doi: 10.1128/iai.37.1.116-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Butcher A. C., Riegel C. A., McGrane V., Blair C. D., Teramoto Y. A., Winston S. Neutralizing determinants defined by monoclonal antibodies on polypeptides specified by bovine herpesvirus 1. J Virol. 1984 Nov;52(2):403–409. doi: 10.1128/jvi.52.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. H., Carmichael L. E. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infect Immun. 1973 Oct;8(4):510–518. doi: 10.1128/iai.8.4.510-518.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Russell R. G., Rouse B. T. Cell-mediated immunity to herpes simplex virus: recognition of type-specific and type-common surface antigens by cytotoxic T cell populations. Infect Immun. 1981 Dec;34(3):795–803. doi: 10.1128/iai.34.3.795-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. D., Marron A. E., Kucera C. J. Local and systemic cellular and antibody immune responses of cattle to infectious bovine rhinotracheitis virus vaccines administered intranassally or intramuscularly. Am J Vet Res. 1978 May;39(5):753–760. [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- House J. A., Baker J. A. Bovine herpesvirus IBR-IPV. The antibody virus neutralization reaction. Cornell Vet. 1971 Apr;61(2):320–335. [PubMed] [Google Scholar]
- Jericho K. W., Babiuk L. A. The effect of dose, route and virulence of bovine herpesvirus 1 vaccine on experimental respiratory disease in cattle. Can J Comp Med. 1983 Apr;47(2):133–139. [PMC free article] [PubMed] [Google Scholar]
- Kahrs R. F. Infectious bovine rhinotracheitis: a review and update. J Am Vet Med Assoc. 1977 Nov 15;171(10):1055–1064. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton H. W., Reed D. E. Evaluation of experimental subunit vaccines for infectious bovine rhinotracheitis. Am J Vet Res. 1980 Mar;41(3):383–390. [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Gilchrist J. E., Weinmaster G., Qualtiere L., Van den Hurk S., Babiuk L. A. Herpesvirus-induced "early" glycoprotein: characterization and possible role in immune cytolysis. J Virol. 1982 Sep;43(3):1046–1054. doi: 10.1128/jvi.43.3.1046-1054.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. The direct antiviral cytotoxicity by bovine lymphocytes is not restricted by genetic incompatibility of lymphocytes and target cells. J Immunol. 1977 Feb;118(2):618–624. [PubMed] [Google Scholar]
- Savan M., Angulo A. B., Derbyshire J. B. Interferon, antibody responses and protection induced by an intranasal infectious bovine rhinotracheitis vaccine. Can Vet J. 1979 Aug;20(8):207–210. [PMC free article] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. Antibody dependent cytotoxicity mediated by neutrophils: a possible mechanism of antiviral defense. J Reticuloendothel Soc. 1976 May;19(5):323–332. [PubMed] [Google Scholar]