Abstract
Virus-like particles (VLPs) have gained increasing interest for their use as vaccines due to their repetitive antigenic structure which is capable of efficiently activating the immune system. The efficacy of virus-like particle immunization may lie in its ability to traffic into draining lymph nodes while activating antigen-presenting cells in order to initiate the orchestration of signals required for the development of a robust immune response. Currently, there is no comprehensive study showing the correlation of different VLP vaccination routes to immune outcome. In this study, we took an optical imaging approach to directly visualize the trafficking of SHIV VLPs after immunization by commonly used routes and analyzed the corresponding humoral and cellular immune responses generated. We found that VLPs can easily enter the subcapsular sinus of draining lymph nodes with quantitative differences in the number of lymph node involvement depending on the immunization route used. Intradermal immunization led to the largest level of lymph node involvement for the longest period of time, which correlated with the strongest humoral and cellular immune responses. Flow cytometry analysis from extracted splenocytes showed that intradermal immunization led to the largest population of germinal center and activated B cells which translated into higher antibody levels and antigen-specific CTL responses. Our results indicate that VLPs traffic into lymph nodes upon immunization and can be directly visualized by optical imaging techniques. Intradermal immunization showed improved responses and might be a preferable delivery route to use for viral and cancer immunotherapeutic studies involving VLPs.
Introduction
Vaccine research is in the constant outlook for new and efficacious methods to induce strong immune responses that can protect individuals from the many maladies present in our era. Virus-like particles (VLP) have gained increasing interest due to their particulate nature which has been shown to act as strong immunogens capable of eliciting both humoral and cellular immune responses (1–4). VLPs are non-infectious particles consisting of viral structural proteins and lacking viral nucleic acid. Their repetitive, antigenic structure enables them to induce strong T-helper and CTL responses without the need for any adjuvants (5, 6). These particles can further activate dendritic cells (DCs) which become essential players in the initiation of an immune response by capturing and processing antigen, delivering them to secondary lymphoid organs and providing co-stimulatory signals (7). The efficacy of VLP immunization may lie in its ability to traffic into draining lymph nodes in order to bind and activate antigen presenting cells for the development of a robust immune response.
The humoral immune response is mounted in lymph nodes which act as filters for foreign particles. Recent work has shown that the humoral response can be initiated by soluble antigens that enter lymph nodes through the afferent lymphatic vessels into the subcapsular sinus where they are acquired by antigen-specific B cells in the follicles or by resident DCs (8). After entering the subcapsular sinus, antigen can diffuse into the follicular regions through small (0.1–1 µm) gaps in the sinus floor where they can interact with naïve B cells (8–10). Since these gaps are large enough to allow VLPs to easily flow-through, it is possible that VLPs enter the cortex region primarily in a free state form where they can reach the follicular regions or alternatively, processed by DCs, where they can reach the paracortex region. The end result is the initiation of an immune response and the development of effector mechanisms which confer clearance and protection to the host. For vaccine development, the induction of immune effector functions is a major determinant for the efficacy of a vaccine.
The protection conferred by a vaccine against infection or even cancer cells is in part dependent on the level and type of the immune response generated. For vaccination studies, the route of immunization becomes a major factor which can dictate the strength of the subsequent immune response. Deciding which immunization route to use in animal studies may signify the difference between observing a strong immune response or a weak effector action which might otherwise render a vaccine as ineffective. The route of immunization can therefore mask the potency of a vaccine by producing weak responses which are not due to the vaccine itself, but rather to the immunization route used.
With an increasing interest in the use of VLPs as vaccine agents, it is essential to comprehend their movements inside the body after immunization and the best delivery route to use when performing animal vaccination studies. Currently, there is no data showing in-vivo trafficking of VLPs nor the level and quantity of lymph node involvement by different immunization routes. Furthermore, there are no studies comparing the systemic immune responses generated in mice and thus no data showing an optimal immunization route to use when testing VLPs as vaccine agents. In this study, by using a near infrared (NIR) fluorescent dye coupled to simian-human immunodeficiency (SHIV) VLPs, we were able to track VLP movement inside SKH-1 mice following immunization by commonly used routes and observed clear differences in the range of lymph node involvement. Further immunization of C57BL/6 mice with SHIV VLPs was used to evaluate quantitative differences in the humoral immune response mounted, showing that intradermal immunization led to significant differences in antibody levels. Flow cytometry analysis from extracted splenocytes confirmed a larger population of germinal center and activated B cells and i.d. immunization also led to the highest percentage of antigen-specific CTLs. Taken together our data shows that intradermal immunization leads to a broader range of lymph node involvement with the generation of more robust cellular and humoral immune responses. These characteristics make it a preferable immunization route to use in animal vaccination studies using VLPs.
Material and Methods
VLP production and characterization
SHIV VLPs (SIV Gag + HIV SF162 Env) were produced and characterized as previously described (11). Briefly, Spodoptera frugiperda (Sf9) cells were co-infected with a recombinant baculovirus (rBV) expressing SIVmac239 gag at an m.o.i. of 2 and with a rBV expressing HIV env at an m.o.i. of 10. Infected cells were maintained in suspension in serum-free SF900 II medium (Gibco, Gaithersburg, MD) at 27°C for 3 days. The culture medium was then collected and centrifuged at 1000 rpm for 8 min (Beckman GPR desktop centrifuge). The supernatant was filtered through a 0.45 µM membrane and centrifuged through a 20% (wt/vol) sucrose cushion at 300,000 × g for 2 h at 4°C. VLPs were then resuspended in phosphate-buffered saline (PBS) and purified through a 20–60% continuous sucrose gradient at 100,000 × g for 16 h at 4°C. The VLP band was collected and dialyzed against PBS using a 10,000 MW cutoff membrane cassette (Pierce Biotechnology, Rockford, IL). VLPs were then pelleted at 300,000 × g for 1 h at 4°C and resuspended overnight in PBS. The efficient incorporation of Env protein was confirmed by western blot. Protein concentrations of purified SHIV VLPs were determined by using Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). The endotoxin level was quantitated using the Limulus amebocyte assay kit (Associates of Cape Cod, Inc., Woods Hole, MA) and was controlled below 0.0041 µg/ml. Electron microscopy was used to determine the integrity of the particles.
VLP dye and hapten conjugation
Conjugation of SHIV VLPs with IRDye800CW was performed according to the IRDye® 800CW labeling kit protocol (LI-COR Biosciences, Cambridge, UK). In brief, SHIV VLPs were reacted with the dye at a concentration ratio of 1:10 in PBS (pH 7.4). The labeling reaction was performed in the dark at RT for 2 h. To separate any excess, unreacted dye, dialysis was performed in PBS (pH 7.4) for 24 h with two buffer changes using a Slide-A-Lyzer dialysis cassette (Pierce Biotechnology, Rockford, IL) with a 10,000 molecular weight cutoff. To conjugate SHIV VLPs to Atto 590 (Sigma, St. Louis, MO) VLPs were added to a molar excess (4 fold) of activated dye. The reaction was carried for 1 h at RT in the dark. To separate excess, unreacted dye a similar dialysis step was performed. For the labeling of VLPs with the lipophilic tracer DiO (Invitrogen, Carlsbad, CA) 10 ug of stock solution in dimethylformamide (DMF) was incubated with 300 ug of VLPs for 2 h at RT in the dark. Following this incubation period dialysis was performed to remove unreacted dye. To conjugate VLPs with hapten, SHIV VLPs (dissolved in PBS) were allowed to incubate with NP-OSu (4-hydroxy-3-nitrophenylacetic acid active ester, BioSearch Technologies, Novato, CA) at a ratio of 20:1 for 2 h at RT. The hapten was added in a drop-wise fashion with constant stirring. The conjugated NP-VLPs were then dialyzed against PBS (pH 7.4) using a Slide-A-Lyzer dialysis cassette (Pierce Biotechnology, Rockford, IL) with a 10,000 molecular weight cutoff for 24 h to remove any unconjugated NP from the mixture. For the conjugation of BSA to hapten, used for the coating of ELISA plates, the same procedure was performed with ratio modifications to yield BSA proteins conjugated to either 5 or 25 hapten molecules.
In vivo NIR optical imaging
For NIR optical imaging, a planar fluorescence optical imaging system was used as previously described (12). In brief, in vivo imaging was accomplished by illuminating anesthetized animals with light from a laser diode (80-mW used for 785 nm light). A holographic notch-plus, band-rejection filter (785-nm center wavelength for IRDye800CW) used to reject any back-scattered and reflected excitation light as well as a bandpass filter (830-nm center wavelength for IRDye800CW) to transmit fluorescent emission light were used. Fluorescent light that was re-emitted was collected by an electron-multiplying charge coupled device (EMCCD) camera (model PhotoMax:512B, Princeton Instruments, Trenton, NJ). Image acquisition was obtained by using V++ software (Digital Optics, Auckland, New Zealand). Subsequent data processing and analysis was accomplished by using the ImageJ program (National Institutes of Health, Washington, DC).
Mice were imaged at various time points after immunization: 5 min, 30 min, 1 h, 3 h, 24 h, 48 h, 72 h and 6 days. All animals were under inhalational anesthesia by constant supply of an isoflurane/oxygen mixture. After 24 h, one mouse from each group was sacrificed and the lymph nodes were extracted and imaged. After six days, only mice that showed fluorescence in the lymph nodes were sacrificed and the nodes extracted for further imaging.
Immunohistochemistry and tissue slide imaging
Lymph nodes were extracted from mice and frozen in O.C.T media. The frozen samples were then processed into 8 µm tissue slides using a cryostat cutter (Leica Microsystems CMS, Manheim, Germany). For immunostaining, tissue slides were fixed in acetone and then incubated for 2 h in PBS with 10% calf serum (blocking buffer). After blocking, the slides were incubated in primary antibody for 2 h at RT. VLP-immunized macaque serum was used for direct detection of VLP particles. Anti-B220, anti-CD4, anti-IgD and anti-IgM labeled antibodies were also used (eBioscience, San Diego, CA). Following incubation, the slides were washed three times with PBS-T and prepared for fluorescent detection. To detect macaque antibodies, secondary goat anti-human IgG antibodies were used (Molecular Probes, Eugene, OR). All images from lymph node tissue slides were recorded using a Leica fluorescent microscope (Model: DM6000 B, Leica Microsystems GmbH, Ernst-Leitz-Strasse, Germany) equipped with a 100W xenon lamp and fluorescent filters.
Mice Immunization
For NIR imaging, 4–6 week old female SKH-1 mice (Charles River Laboratories, Raleigh, NC) were used. SKH-1 mice are hairless, immunocompetent, (albino background) mice allowing for clear NIR imaging. Two mice were used for each immunization group. Imaging was repeated once to confirm the observations. C57BL/6j mice (The Jackson Laboratory, Bar Harbor, ME) were used for immunization experiments involving hapten conjugated VLPs. Six mice were used for each immunization group. All mice were maintained in accordance with the animal protocol approved by Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC).
For the imaging experiments, SKH-1 mice were immunized with 30 µg of SHIV VLPs conjugated with IRDye800CW. This corresponds to a total dye concentration of approximately 730 nM. Four different immunization routes were used: subcutaneous (s.c.), intraperitoneal (i.p.) intramuscular (i.m.) and intradermal (i.d.). The s.c., i.m. and i.d. immunizations were performed in the left flank.
For the immune response experiments, C57BL/6j mice were immunized by the same route and location using 100 µg of SHIV VLPs. When performing i.d. immunization, the left flank was shaven to remove most of the hair and injection was done by using a 31-gauge needle under a stereomicroscope (model SZ61, Olympus, Japan). Blood was extracted by tail bleed at 3, 7, 14 and 21 days post-immunization. Murine spleens were harvested 12 days post-immunization and single-cell suspensions of whole spleens were made.
ELISA
Serum was separated from the extracted blood of immunized mice. Specific antibody levels in the blood serum were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Becton Dickinson, San Jose, CA). For detection of SHIV VLP specific antibodies, microtiter plates were coated with either 250 ng of SHIV VLPs/well or 250 ng of SIV gag protein/well. For SHIV VLP-induced antibody affinity analysis, microtiter plates were coated with 5 µg/ml of BSA conjugated with either 5 or 25 hapten fragments. The coated plates were incubated overnight at 4°C and then blocked in PBS containing 10% calf serum at RT for 1 h. Plates were then washed three times with PBS-T and 100-fold/serial diluted sera was added and incubated at RT for 2 h. After this incubation period, the plates were washed again three times and then treated with goat anti-mouse IgG, IgG1, and IgG2a-HRP conjugated antibody for 1 h at RT. The plates were then washed three times to remove any unbound conjugates, and then ABTS colorimetric substrate solution (Sigma, St. Louis, MO) was added to each well. The enzyme reaction was stopped by the addition of a 1% SDS solution and the ODs were read at 405-nm (reference at 490-nm) in an ELISA plate reader (EL800, Bio-Tek Instruments, Winooski, VT). Data was analyzed and results given as the arithmetic mean ± SD. A one-way analysis of variance (ANOVA) following Dunnett’s multiple comparison test based on i.d. results was used. P values less than 0.05 were considered significant.
Flow cytometry analysis
Murine splenocyte single cell suspensions were resuspended in PBS supplemented with 3% FBS and 0.08% NaN3. A total of 2 × 106 cells were added to 5 ml polystyrene round bottom tubes. Primary labeled antibody was diluted in 100 µl of PBS with 3% FBS and 0.08% NaN3 and added to each corresponding tube for a 1 h incubation period at RT. To determine the activation of B cells, anti-B220, anti-CD80 and anti-PD-1 antibodies were used. To determine the population of germinal center B cells, we used anti-B220, anti-fas and anti-GL-7 antibodies. Following this incubation period, the cells were washed with 5 ml of PBS containing 0.08% NaN3. The tubes were then centrifuged at 350 × g for 4 min followed by decanting. The cell pellet was resuspended in 300 µl of PBS supplemented with 0.08% NaN3 and run on a FACSCalibur (Becton Dickinson, San Jose, CA). Flow cytometry data was analyzed by using the FlowJo Software (Tree star, Ashland, OR).
CTL assay
To determine the levels of antigen-specific CTLs, the CytoTox 96® non-radioactive cytotoxicity assay (Promega, Madison, WI) was used following the manufacturer’s protocol. This assay quantitatively measures lactate dehydrogenase (LDH) release upon cell lysis. In brief, EL4 mouse lymphoma target cells were pulsed with SIV gag peptides (SIVmac239 Gag 15-mer peptides, NIH AIDS Research & Reference Reagent Program, Germantown, MD) for 1 h at 37°C (2 µM for every 2 million cells). Following this incubation period, the target cells were incubated with effector cells (from splenocyte suspension) for 4 h at 37°C. The effector cells (splenocytes) had been previously incubated for 6 days in RPMI1640 media supplemented with 10% FBS, 500 units/ml of IL-2 (Sigma-Aldrich, St. Louis, MO) and 10 µg/ml of SIV gag peptides. The appropriate spontaneous LDH release, maximum LDH release, volume correction and background controls were used. After this incubation period, the cells were centrifuged at 250 × g for 4 min. A total of 50 µl of supernatant was transferred to the enzymatic assay plate and 50 µl of reconstituted substrate mix was added to each well. The plate was covered and incubated for 30 min at RT. The enzymatic reaction was stopped by adding 50 µl of stop solution. The absorbance was then recorded at 490 nm by using a plate reader (EL800, Bio-Tek Instruments, Winooski, VT).
Results
IRDye® 800CW can be efficiently conjugated to VLPs without disrupting particle structure
To clearly visualize the in vivo trafficking of VLPs, a detection method that provides high sensitivity with low background fluorescence is required. Visible fluorophores are not convenient to use since some tissues have components that are intrinsically fluorescent leading to autofluorescence. By using an excitation wavelength in the near infrared region, it is possible to minimize autofluorescence while providing improved clarity and specificity while imaging. For our studies, we used a NIR fluorescent dye (IRDye® 800CW) containing an NHS reactive ester group which can easily couple to primary and secondary amino groups. This NIR dye provides great advantages for in vivo imaging due to its high sensitivity conferred by reduced scattering and low infrared background. To show that conjugation with this infrared dye did not affect particle structure, we performed electron microscopy on dye-conjugated VLPs (VLP-IRDye). SHIV VLPs contain an outer membrane envelope with incorporated HIV envelope proteins as well as some Spodoptera frugiperda (Sf9) cell membrane proteins that arise from the budding of gag particles during VLP production. This characteristic allows the dye to bind to several distinct membrane proteins. EM images show that VLPs remain intact (unbroken) after conjugation (Fig. 1A). Following dialysis, the dye conjugated VLPs were run in an SDS-polyacrylamide gel to separate the VLP proteins. After Coomasie blue staining, the gel was illuminated with a NIR wavelength of light to detect any fluorescence arising from dye conjugated protein bands. As shown in Fig.1B, the dye is capable of directly and stably binding to VLP proteins. Prior to utilization of the conjugated particles for immunization, quantification of the amount of dye present in the preparation was performed by using various dilutions of the conjugated VLPs and determined to be approximately 730 nM (Fig. 1C). Dye conjugated particles were kept for two weeks in the dark and dialyzed to determine the stability of the conjugation. Subsequent analysis showed that the concentration of dye was approximately 680 nM showing only a 7% reduction in dye concentration (data not shown). Together, these results indicate that the conjugation of dye to VLPs is quite stable and can be used for efficient particle tracking.
Figure 1.
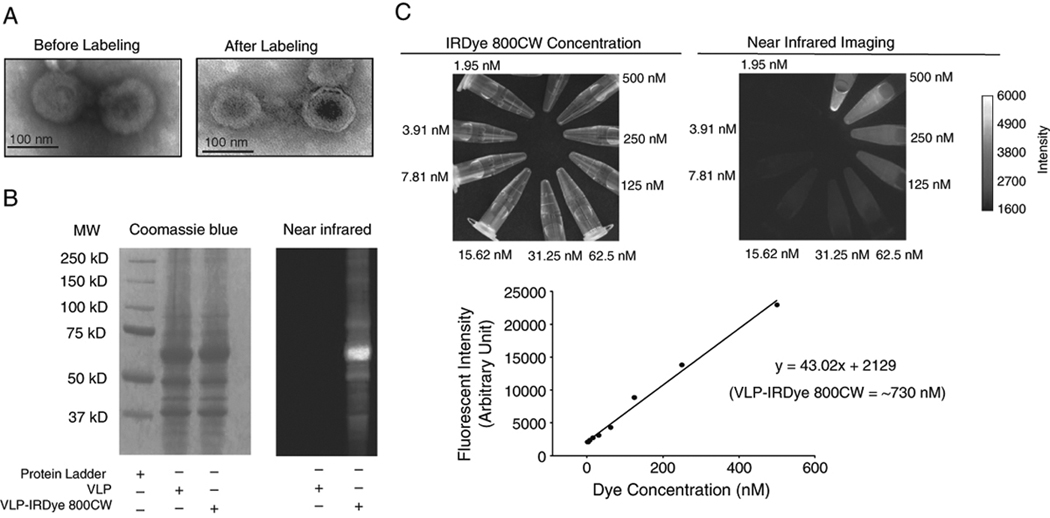
Characterization of dye conjugated SHIV VLPs. (A). Electron micrographs of SHIV VLPs before (left panel) and after conjugation (right panel) with IRDye800CW. IRDye® 800CW can directly conjugate to VLP proteins without having any detrimental effect on particle structure. (B). Direct conjugation of IRDye800CW to SHIV VLP proteins. Sodium dodecyl sulfate polyacrylamide gel electrophoresis of dye conjugated and control SHIV VLPs. VLP proteins were detected by Coomasie blue staining (left panel) and NIR fluorescence imaging (right panel). (C). Quantification of IRDye800CW present in the VLP preparation. Dye conjugated VLPs were diluted and the concentration of dye was determined at each dilution to obtain a standard curve.
Imaging of dye conjugated VLPs after immunization by different routes
Knowing that IRDye® 800CW is capable of stably binding to VLPs, these conjugated particles were used to visualize their movement in mice after immunization by different routes. Mice were imaged at various time points for up to six days. One mouse from each group was sacrificed at 24 h to extract the draining lymph nodes. A total of 30 µg of VLPs was used to immunize SKH-1 hairless, immunocompetent mice by commonly used vaccination routes: subcutaneous (s.c.), intraperitoneal (i.p.), intramuscular (i.m.) and intradermal (i.d.). As shown in Fig. 2A, after subcutaneous injection, most of the fluorescence remains confined to the left flank region. Three hours after injection, there are appreciable levels of fluorescence in the kidney possibly due to clearance of released fluorophores. After 24 h, there is some weak fluorescence in the inguinal and popliteal lymph nodes. Following lymph node extraction, we were able to confirm the weak fluorescence in these nodes. When imaged at subsequent time points, there was no longer any apparent fluorescence. With intraperitoneal injection, analysis of VLP lymphatic trafficking becomes more complicated since lymphatic uptake occurs via the diaphragm stomata. The injection site can not be covered as this would also hide the lymphatic uptake region. This leads to high fluorescent signal coming from the injection site which might blur or hide the accumulation of weaker fluorescence in proximal regions. As shown in Fig. 2B, after 30 min and 1 h, fluorescence is particularly concentrated at the injection site with another accumulation area in what appears to be the diaphragm region. After 3 h, there was vast diffusion of fluorescence throughout the body, and after 24 h, there was no longer any appreciable levels of fluorescence. Upon sacrifice there was no detection of fluorescence in the diaphragm region. Intramuscular injection led to a rapid dispersal of fluorescence after 30 min with high accumulation of fluorescence in the kidneys after 3 h and detectable fluorescence in the popliteal and lumbar lymph nodes after 24 h (Fig. 2C). Dissection and subsequent imaging confirmed the high fluorescence in these nodes. As shown in Fig. 2D, among all routes of administration, we found that intradermal injection led to the highest level of lymph node involvement for the longest period of time. After 5 min, there was detectable fluorescence in the inguinal, popliteal, lumbar and sciatic lymph nodes and it was possible to observe the lymphatic vessels connecting these nodes. After 24 h, the specific distribution of fluorescence in these nodes became rather apparent and signal remained in these nodes for up to six days (data not shown). Following dissection, we were able to confirm the fluorescence in these four draining lymph nodes. In all immunization groups mice were re-imaged after they were sacrificed and lymph nodes extracted. For all the groups we were unable to detect fluorescence in other organs except for the kidneys and some weak fluorescence in the liver.
Figure 2.
Imaging of SHIV VLP trafficking after immunization by different routes. SKH-1 mice were immunized with 30 µg of dye conjugated VLPs. All injection sites were covered and mice were imaged at 5 min, 30 min, 1 h, 3 h, 24 h, 48 h, 72 h, and 6 days post-immunization. Not all immunization routes led to fluorescence at all the time points analyzed and only images up to 24 h are included. At 24 h post-immunization, one mouse from each group was sacrificed for the extraction of lymph nodes and spleen in order to confirm the fluorescent signal at these sites. Different immunization routes were used: (A). subcutaneous (s.c.), (B). intraperitoneal (i.p.), (C). intramuscular (i.m.), and (D). intradermal (i.d.).
The tracking of dye conjugated VLPs after immunization by these commonly used routes showed a differential level and quantity of lymph node involvement. Differences in the amount of VLPs (antigen) that reaches the draining lymph nodes might have important effects in the strength of the immune response mounted. A stronger lymph node involvement might correlate with the development of more robust immune effector mechanisms. We further sought to determine whether the level of lymph node involvement had any effect on immune outcome.
VLPs can enter lymphatic vessels and drain into the subcapsular sinus of lymph nodes
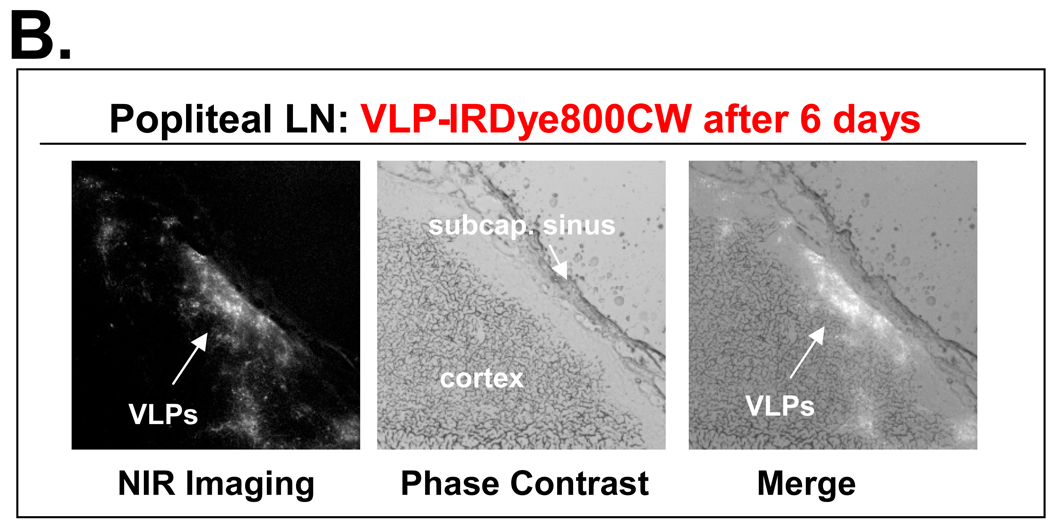
Following in vivo imaging of the conjugated VLPs, it was clear that upon immunization these particles have the capacity to enter the lymphatic vessels and reach the draining lymph nodes. This trafficking can take place either as intact particles flowing in the lymph or as processed particles taken up by DCs. Due to the particle size of approximately 90 nm, drainage of VLPs to the lymph nodes can take place primarily in an intact form. To investigate the distribution of VLPs inside lymph nodes, we used sciatic and popliteal lymph nodes extracted from i.d. immunized mice since these nodes showed the highest levels of fluorescence after 24 hours and 6 days respectively. Frozen tissues were used for the preparation of tissue slides which were subsequently used for NIR imaging and immunohistochemistry. After exposure to a NIR wavelength of light, there was high fluorescence inside the subcapsular sinus showing that VLPs can enter lymph nodes through the afferent lymphatic vessels (Fig. 3A). The distribution of fluorescence after 6 days showed that VLPs were no longer present in the subcapsular sinus but rather in the adjacent cortex region (Fig. 3B).
Figure 3.
VLP distribution inside draining lymph nodes. Lymph nodes were extracted from i.d. immunized mice, frozen and processed into tissue sections. Tissue slides were imaged using a fluorescent microscope with a NIR wavelength of light. (A). Visualization of dye conjugated VLPs inside the sciatic lymph node extracted 24 h post-immunization. Most of the fluorescence is concentrated in the subcapsular sinus. (B). Visualization of dye conjugated VLPs inside the popliteal lymph node extracted 6 days post-immunization. Most of the fluorescence is detected in the adjacent cortex region.
In order to directly confirm the presence of VLPs inside the nodes and demonstrate that the observed fluorescence arises from dye conjugated VLPs and not simply from free floating dye, serum from SHIV VLP immunized macaques was used for tissue staining. As shown in Fig. 4A, VLPs are majorly concentrated in the outer regions of the lymph node following the same distribution observed with NIR imaging. A lower amount of VLPs can be seen internalized in the B cell region with some fluorescence present in the T cell zone. When using pre-immunized macaque serum, there is no detection of VLPs, corroborating the specificity of the serum used. These results show that the observed fluorescence in the imaging experiment is indeed due to the presence of dye conjugated VLP proteins and not only to free floating dye which might become separated from VLP proteins after immunization.
Figure 4.
Detection of SHIV VLPs in local draining lymph nodes. (A). After i.d. immunization using sera from SHIV VLP immunized macaques. Sciatic lymph nodes extracted from i.d. immunized mice 24 h post-immunization were used for the preparation of tissue slides. Pre-immunized macaque serum was used as a negative control for VLP staining. (B) After i.d. immunization using Atto 590 and DiO conjugated VLPs for the detection of intact particles reaching the node. DiO is a lipophilic tracer with high fluorescence when incorporated into membranes. Conjugated BSA was used as a negative control for DiO fluorescence.
To confirm that VLPs enter lymph nodes primarily in an intact form we labeled the VLPs with either Atto 590 NHS ester or carbocyanine dye (DiO). Atto 590 is a red fluorescent compound which can easily bind to primary and secondary amino groups in a similar fashion to IRDye800CW. DiO is a green fluorescent lipophilic tracer that is highly fluorescent and photostable when incorporated into membranes. This lipophilic tracer can be used to label the membrane envelope of SHIV VLPs and determine the integrity of the particles as VLP degradation would result in the disruption of the membrane envelope and release of the carbocyanine dye. Once this dye is released from the phospholipid bilayer it loses its high fluorescent capacity. As shown in Fig. 4B, DiO fluorescence is observed only in lymph nodes from mice immunized with DiO-VLPs, but not in lymph nodes from control DiO-BSA immunized mice. These results indicate that VLPs can enter lymph nodes in an intact form without disruption of their membrane envelope as evidenced by the presence of DiO fluorescence.
Different VLP immunization routes lead to differences in antibody production levels
VLPs that reach a particular lymph node can initiate a humoral immune response by the activation of antigen presenting cells. Particles that enter through the subcapsular sinus have the potential of moving through small gaps present in the sinus floor into the follicular regions directly activating B cells. These gaps (0.1–1 µm) are large enough to allow SHIV VLPs (~90 nm) to flow through. Upon activation of B cells and T cells, a humoral immune response is mounted which can be quantitated by measuring the levels of antibody-mediated and cell-mediated effectors. To determine the level of antibody production against SHIV VLPs, these particles were used to immunize C57BL/6 mice. In order to measure the affinity of the antibodies produced, VLPs were conjugated with NP-OSu hapten (4-hydroxy-3-nitrophenylacetic active ester hapten). Sera from day 14 post-immunization was used to measure the levels of IgG, IgG1 and IgG2a antibodies against SHIV VLPs and gag protein. As shown in Fig. 5, i.d. immunization led to a significant increase in the level of anti-SHIV IgG antibodies as well as anti-Gag (VLP core protein) specific IgG1 and IgG2a antibodies when compared to the other groups (ANOVA post test, P<0.0001, P<0.001 and P<0.001 respectively). These results show that the immune response is capable of generating antibodies both to external VLP envelope proteins (SHIV VLP specific IgG) and to internal VLP proteins (gag specific IgG1/IgG2a) with differences depending on the immunization route used. For i.p and i.m. similar levels of anti-SHIV antibodies were observed and s.c. immunization led to the lowest levels of IgG antibodies. For SIV gag specific IgG1 and IgG2a, i.d. immunization led to the highest antibody levels and a similar pattern was observed for the other immunization routes except for IgG1 were i.p. immunization showed levels only slightly higher than the PBS control group. All immunization routes led to similar affinity levels for IgG1 and IgG2a antibodies (data not shown). The results indicate that different immunization routes can lead to variations in antibody production. The differences observed appear to follow the pattern of VLP trafficking to lymph nodes where higher antibody levels correspond to higher levels and range of lymph node involvement. The only exception is i.p. immunization for which specific lymphatic uptake was not readily observed with our imaging system.
Figure 5.

Comparison of antibody production levels after immunization of SHIV VLPs by different routes. For the detection of Ag-specific antibodies, microtiter plates were coated with either 250 ng of SHIV VLPs/well or 250 ng of SIV gag protein/well. Mouse sera was diluted to 1:2,500 and goat anti-mouse IgG, IgG1, and IgG2a-HRP conjugated antibodies were used to determine the level of antibody subtypes. Columns, mean O.D. value of five mice per group; bars, SD. *, P value of i.d. group compared with the other groups, one-way analysis of variance (ANOVA).
Intradermal immunization leads to the highest population of germinal center and activated B cells
Since antibody affinity arises from somatic hypermutation which takes place in germinal centers, we analyzed the population of germinal center B cells by flow cytometry. A key aspect for vaccine induced antibodies is the generation of high affinity antibodies which can have improved binding for a particular epitope. At 12 days post-immunization, splenocytes were extracted and incubated with anti-B220, anti-fas and anti-GL7 antibodies. Both fas and GL-7 proteins are expressed on activated germinal center B cells. Flow cytometry results showed that i.d. immunization led to the largest population of germinal center B cells positive for both fas and GL7 (Fig. 6A). CD80 is a co-stimulatory molecule required for T-cell activation and survival. Its expression is upregulated on activated B cells which can then provide T cell co-stimulation through CD28 signaling. The population of B220+CD80+ cells was also higher following i.d. immunization (Fig. 6B). When determining the expression of PD-1 (CD279), a protein that is upregulated on activated B cells, again i.d. immunization led to the highest population of B220+PD1+ cells (Fig. 6C). For the other immunization routes, there was no particular pattern observed. Taken together, these data shows that following i.d. immunization, there is a higher induction of activated and germinal center B cells. It is possible that a higher level of lymph node involvement results in the activation of a larger population of antigen specific B cells which can further provide co-stimulatory signals for T cell activation. Increasing the amount of germinal center formation could result in the development of a larger population of long-lived memory B cells as well as increased titers of higher affinity antibodies.
Figure 6.
Characterization of germinal center and activated B cell populations in the spleen after immunization by different routes. SHIV VLPs were used to immunize C57BL/6 mice by different immunization routes. PBS was used as a negative control immunized by i.d. route. At 12 days post-immunization, mice splenocyte single cell suspensions were harvested and the expression of cell surface markers was analyzed by flow cytometry. The percentage of positive cells is denoted. (A). Germinal center B220+GL-7+fas+ B cell population. Splenocytes were gated on B220+ cells and GL-7+fas+ cells encircled. (B). CD80+B220+ B cell population. Splenocytes were gated on B220+ cells and the percent of CD80 positive cells shown in numbers. (C). PD-1+B220+ B cell population. Splenocytes were gated on B220+ cells and the percent of PD-1 positive cells shown in numbers. The results shown are representative plots out of three independent analysis from each group.
Intradermal immunization leads to the highest percentage of antigen-specific cytotoxic T lymphocytes (CTLs)
Cytotoxic T lymphocytes are essential players in the cellular immune response. These cells are capable of inducing the death of virally infected or dysfunctional somatic cells as well as tumor cells. VLPs like the ones used in our experiments can be modified to express a variety of proteins such as tumor associated antigens on the membrane envelope. The effectiveness of such a versatile vaccine agent is highly dependent on the induction of antigen specific CTLs. In order to determine which immunization route led to an increase in the population of antigen specific CTLs, splenocytes were extracted 10 days post immunization, incubated for 6 days in the presence of SIV gag peptides and IL-2 and subsequently incubated with EL4 target cells pulsed with the same SIV gag peptide pool. A non-radioactive cytotoxicity assay was used to measure the lysis activity of gag-specific CTLs. As shown in Fig. 7, i.d. immunization led to the highest population of antigen specific cytotoxic T lymphocytes when compared to the other immunization routes at all the effector:target cell ratios. The activation of CTLs is dependent on the interaction of T cell receptors with peptide-bound MHC-I molecules as well as secondary signals including the interaction of CD28 with CD80/CD86 expressed on APCs. With more antigens reaching secondary lymphoid organs a larger population of antigen presenting cells can become activated which can then have the potential of increasing the activation of CD8+ T cells. This increase in antigen dispersal to different lymph nodes is particularly evident for intradermal immunization which not only led to the largest lymph node involvement, but also maintained antigen for the longest period of time.
Figure 7.
Induction of gag-specific CTLs in splenocytes from immunized mice. Cytotoxicity was detected using a non-radioactive colorimetric assay that measures the release of lactate dehydrogenase (LDH) upon cell lysis. 12 days post immunization splenocyte single cell suspensions were harvested. Effector cells were incubated for 6 days with 500 U/ml of mIL-2 and 10 µg/ml of SIV gag peptide pool. EL4 target cells were pulsed with 10 µg/ml of SIV gag peptide pool. Cytotoxicity was measured at the indicated effector:target cell ratios. The percent cytotoxicity is correlated to the amount of antigen specific CTLs. The data shown represents the mean value from each group (3 mice/group).
Discussion
The present study shows that upon immunization, VLPs enter the lymphatic system and reach the subcapsular sinus of draining lymph nodes. Different immunization routes lead to a differential level of lymph node involvement. After reaching the node, VLPs have the potential of diffusing through the sinus floor into the follicular regions or be captured by macrophages lining the subcapsular sinus. (8, 9) Alternatively, VLPs can be processed by DCs at the injection site and be transported into the draining lymph nodes. A majority of the particles can reach the nodes in an intact form due to their small size of approximately 90 nm (13). In this way VLPs can traffic from the injection site into lymph nodes in either an intact form or processed by DC to activate both T cells and B cells and mount a humoral immune response with the development of measurable immune effector mechanisms.
Since there is no previous study visualizing the trafficking of VLPs after immunization by commonly used routes or studies comparing the immune response elicited to VLPs by distinct administration routes, we believe that the information obtained from this study is highly valuable for the understanding of VLP movement and its capacity to generate an immune response. An important aspect in mounting an effective immune response is dependent on the movement of antigen into the draining lymph nodes and its maintenance in the lymphoid follicles. (14) We found that after s.c., i.m., and i.d. immunization, VLPs can effectively traffic to the draining lymph nodes. Following intraperitoneal injection, we were unable to detect any specific fluorescence which might correspond to lymphatic uptake. This does not signify that there was no movement of VLPs into the lymphatic vasculature. Inside the peritoneal mouse cavity, reabsorption of cellular, corpuscular and liquid contents takes place through the diaphragm stomata (15). The stomata maintains an open contact between the peritoneum and the lymphatic vessels (16). VLPs could potentially enter the lymphatic system through the diaphragm stomata in order to initiate an immune response even though our in vivo imaging system could not detect this precise uptake probably due to the high fluorescence signal emanating from the injection site and the fact that fluorescence is not bound to a confined area like a node.
On the other hand, the trafficking of VLPs to a greater number of lymph nodes upon i.d. immunization might be attributed to the lymphatic structure in the intradermal zone. In the skin, lymphatic vessels form two plexuses (17, 18). The superficial plexus contains branches that drain vertically into larger lymphatic vessels located in the lower dermis and the superficial zone of the subcutaneous tissue (18). These deep lymphatic vessels contain numerous valves through which antigen can be uptaken. For s.c., and i.m. immunization, the level of lymph node involvement was lower and fluorescence was not observed after 24 hours. This might signify that a lower quantity of VLPs entered the lymphatic vasculature while some might have degraded close to the injection site or in other parts of the body. Following VLP immunization, it is possible that some of the particles were uptaken by blood capillaries and transported into the spleen. For i.m. immunization, this might be a preferential uptake route since the lymphatic capillaries in voluntary muscle are only confined to the fascial planes and are not present in the muscle bundles like blood capillaries (18). Therefore, it is possible that some VLPs were transported into the spleen through blood vessels. However, after dissection of the spleen, we were unable to detect any signal in this organ, possibly attributed to degradation of the fluorophore. IRDye800, once released from a targeting moiety, clears quickly through the animal via the renal system. While we do not know if VLPs clear through the renal system, it is quite possible that dye is shed from the particles causing clearance and contrast in the kidneys as depicted at 3 hours and earlier time points. This effect is typical in fluorescence imaging, but since we know that the dye clears through the kidneys, then it shouldn’t impact our assessment of the labeled VLPs in the LNs. The differences in clearance should certainly depend upon the route of administration, thus differences in kidney fluorescence should also be expected. Nonetheless, this does not change the results and conclusion indicating that intradermal injection is the best administration route for VLP immunization.
By imaging lymph node tissue slides, we were able to observe the presence of dye conjugated VLP proteins in the subcapsular sinus of draining lymph nodes. We also observed a majority of the particles reaching the nodes in an intact form based on DiO fluorescence. Using direct labeling of VLPs with macaque serum, we were able to confirm the presence of VLPs inside the nodes. The fluorescence signals obtained in our imaging assays were therefore due to dye conjugated VLPs and not solely caused by free or dissociated dye entering the lymphatic vasculature. A higher fluorescence emanating from the nodes can therefore be translated into increased amounts of antigen uptake by the lymphatic vessels. With more antigen reaching the subcapsular sinus, a larger population of activated B cells can migrate to the border between the T cell zone and the follicle to receive proliferation signals from antigen-specific T helper cells. (19) This increase in B cell proliferation can lead to increased levels of antibody-secreting plasma cells as well as increased levels of germinal center formation. (20) In this way, an increase in antigen uptake by lymph nodes can result in the induction of a stronger immune response. This would signify that intradermal immunization, while showing an increase in lymph node involvement, should also lead to a stronger overall immune response due to the levels of antigen reaching the nodes.
When analyzing the antibody responses generated after immunization of VLPs by different routes, we observed a differential level of antibody production with a significant increase following i.d. immunization. Subcutaneous immunization led to the lowest level of antibody production overall, which seems to be correlated with a lower degree of lymph node involvement. For i.d. immunization, the high level of antibody production correlates to the higher and stronger involvement of lymph nodes. For i.p. immunization, the antibody levels can not be compared to lymph node involvement since we were unable to clearly visualize the in vivo uptake of VLPs. With respect to antibody affinity, we observed similar results for all immunization routes. Affinity maturation of antibodies is achieved by somatic hypermutation and clonal expansion which takes place in germinal centers. (21) This process becomes highly important for vaccination purposes where antibodies with high affinity for a particular antigen are desired. Flow cytometry analysis of germinal center B cells showed that intradermal immunization led to the largest population increase for GL7 and fas double positive cells. These two markers are upregulated and characteristic of germinal center B cells (22, 23). This increase in the population of germinal center B cells can lead to increased titers of high affinity antibodies as well as the generation of more long-lived plasma cells and memory B cells. There was also a larger population increase of B220+ cells expressing CD80 and PD-1 after i.d. immunization. CD80 (B7.1) is expressed on activated B cells and binds with CD28 expressed on the surface of T cells in order to provide costimulatory signals for activation and survival. PD-1 is a member of the CD28/CTLA4 family, which is upregulated on activated macrophages, T cells and B cells. This increase in the population of activated and germinal center B cells, as evidenced by the expression of specific surface markers, might be the reason why i.d. immunization leads to significantly higher levels of antibody production. Another possibility for the observed improvements in the humoral immune response might be due to the maintenance of antigen inside lymph nodes for longer periods of time. The presence of antigen after six days, as seen after i.d. immunization, can become an important factor for the continuation of clonal expansion and has great implications for the development of long-lived antibody responses. Antigen that reaches the lymphoid follicles can be kept for long periods of time in germinal centers by follicular DCs. (24) The maintenance of antigen in germinal centers can lead to increased antibody titers and long-lived germinal centers. (25) The presence of antigen specific memory B cells as well as long lived memory T cells has to be further evaluated.
The induction of antibody-mediated (humoral) immunity is highly desired for many vaccination purposes. In most instances, not only a humoral immune response, but also a cell-mediated response is wanted. The control of many infectious diseases and even tumor cells requires the activation of multiple effector mechanisms. Many protein or subunit based vaccines have failed due to their inability to activate a cell-mediated response. This response becomes indispensable for the clearance of virally infected cells and the destruction of cancer cells. Optimal cancer vaccines, for example, not only depend on the development of antigen specific antibodies, but also depend on the generation of antigen specific CTLs which can recognize particular antigens expressed on the surface of cancer cells and target them for destruction. Enveloped VLPs like the ones used in this study can be easily modified to express a variety of proteins on the membrane envelope. We have observed that different immunization routes lead to differential levels of lymph node involvement which appears to be correlated with the level of antibody production. When analyzing the cellular response, more specifically, the development of antigen specific CTLs, there is a clear difference between i.d. immunization and the other immunization routes.
We conclude that VLPs traffic into lymph nodes upon immunization and can be directly visualized by optical imaging techniques. We also conclude that i.d. immunization leads to an overall improved immune response when compared to the other immunization routes. This delivery route improves the level of antigen uptake by the lymphatic system and is the only route that produced a consistent pattern of results which led to the generation of more robust humoral and cellular responses. These characteristics make i.d. immunization a desirable immunization strategy to use when conducting immunotherapeutic studies with VLPs. Although our study focused only on SHIV VLPs, it is possible that the same effects can be observed with the use of other enveloped VLPs.
Acknowledgement
We would like to thank Ms. Terry Yin for generating the SHIV VLPs.
This work was supported in part by NIH grants DE15543, AT003094, and BCM Dan L Duncan Cancer Center pilot grant.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Reference
- 1.Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Expert Rev Vaccines. 2002;1:101–109. doi: 10.1586/14760584.1.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Wagner R, Teeuwsen VJ, Deml L, Notka F, Haaksma AG, Jhagjhoorsingh SS, Niphuis H, Wolf H, Heeney JL. Cytotoxic T cells and neutralizing antibodies induced in rhesus monkeys by virus-like particle HIV vaccines in the absence of protection from SHIV infection. Virology. 1998;245:65–74. doi: 10.1006/viro.1998.9104. [DOI] [PubMed] [Google Scholar]
- 3.Layton GT, Harris SJ, Myhan J, West D, Gotch F, Hill-Perkins M, Cole JS, Meyers N, Woodrow S, French TJ, Adams SE, Kingsman AJ. Induction of single and dual cytotoxic T-lymphocyte responses to viral proteins in mice using recombinant hybrid Ty-virus-like particles. Immunology. 1996;87:171–178. doi: 10.1046/j.1365-2567.1996.464539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, Tamms GM, Saah AJ, Barr E. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 5.Fromantin C, Jamot B, Cohen J, Piroth L, Pothier P, Kohli E. Rotavirus 2/6 virus-like particles administered intranasally in mice, with or without the mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, induce a Th1/Th2-like immune response. J Virol. 2001;75:11010–11016. doi: 10.1128/JVI.75.22.11010-11016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thones N, Muller M. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology. 2007;369:375–388. doi: 10.1016/j.virol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Li M, Chen C, Yao Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine. 2004;23:139–147. doi: 10.1016/j.vaccine.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Clark SL., Jr The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962;110:217–257. doi: 10.1002/aja.1001100303. [DOI] [PubMed] [Google Scholar]
- 10.Farr AG, Cho Y, De Bruyn PP. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat. 1980;157:265–284. doi: 10.1002/aja.1001570304. [DOI] [PubMed] [Google Scholar]
- 11.Yao Q, Kuhlmann FM, Eller R, Compans RW, Chen C. Production and characterization of simian--human immunodeficiency virus-like particles. AIDS Res Hum Retroviruses. 2000;16:227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- 12.Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, Sevick-Muraca EM. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007;48:1501–1510. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 13.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 14.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 15.Azzali G. The lymphatic vessels and the so-called "lymphatic stomata" of the diaphragm: a morphologic ultrastructural and three-dimensional study. Microvasc Res. 1999;57:30–43. doi: 10.1006/mvre.1998.2101. [DOI] [PubMed] [Google Scholar]
- 16.Li YY, Li JC. Ultrastructure and three-dimensional study of the lymphatic stomata in the costal pleura of the rabbit. Microsc Res Tech. 2003;62:240–246. doi: 10.1002/jemt.10388. [DOI] [PubMed] [Google Scholar]
- 17.Ryan T. The lymphatics of the skin. London: Academic Press; 1978. [Google Scholar]
- 18.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 21.Marinova E, Han S, Zheng B. Germinal center helper T cells are dual functional regulatory cells with suppressive activity to conventional CD4+ T cells. J Immunol. 2007;178:5010–5017. doi: 10.4049/jimmunol.178.8.5010. [DOI] [PubMed] [Google Scholar]
- 22.Cervenak L, Magyar A, Boja R, Laszlo G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett. 2001;78:89–96. doi: 10.1016/s0165-2478(01)00239-5. [DOI] [PubMed] [Google Scholar]
- 23.Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc Natl Acad Sci U S A. 1995;92:11628–11632. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tew JG, Phipps RP, Mandel TE. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 25.Gatto D, Martin SW, Bessa J, Pellicioli E, Saudan P, Hinton HJ, Bachmann MF. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]