Abstract
Background & Aims
Hedgehog signaling is critical in gastrointestinal patterning. Mice deficient in Hedgehog signaling exhibit abnormalities that mirror deformities seen in the human VACTERL (vertebral, anal, cardiac, tracheal, esophageal, renal, limb) association. However, the direction of Hedgehog signal flow is controversial and the cellular targets of Hedgehog signaling change with time during development. We profiled cellular Hedgehog response patterns from embryonic day 10.5 (E10.5) to adult in murine antrum, pyloric region, small intestine and colon.
Methods
Hedgehog signaling was profiled using Hedgehog pathway reporter mice and in situ hybridization. Cellular targets were identified by immunostaining. Ihh-overexpressing transgenic animals were generated and analyzed.
Results
Hedgehog signaling is strictly paracrine from antrum to colon throughout embryonic and adult life. Novel findings include: mesothelial cells of the serosa transduce Hedgehog signals in fetal life; the hindgut epithelium expresses Ptch but not Gli1 at E10.5; the two layers of the muscularis externa respond differently to Hedgehog signals; organogenesis of the pyloric sphincter is associated with robust Hedgehog signaling; dramatically different Hedgehog responses characterize stomach and intestine at E16; after birth, the muscularis mucosa and villus smooth muscle (SM) consist primarily of Hedgehog responsive cells and Hh levels actively modulate villus core SM.
Conclusions
These studies reveal a previously unrecognized association of paracrine Hedgehog signaling with several gastrointestinal patterning events involving the serosa, pylorus and villus smooth muscle. The results may have implications for several human anomalies and could potentially expand the spectrum of the human VACTERL association.
Introduction
Organogenesis of the gut relies on soluble signals that pass bi-directionally between endodermal and mesodermal layers (reviewed in 1). The Hedgehog (Hh) signaling pathway participates in this process at multiple sites along the developing gut2. Indeed, Hedgehog signaling is part of an ancient gut sculpting program, as components of this pathway in gut tissues of Drosophila3, Amphioxus4, leech5, sea urchin6, zebrafish7, Xenopus8, chicken9 and mouse10 11 coordinate morphogenic patterning events that are specific to each regional address along the anterior/posterior axis of the gut tube.
In vertebrates, Hh ligands include Sonic Hedgehog (Shh), Indian Hedgehog (Ihh) and Desert Hedgehog (Dhh). All three are expressed in the developing gut tube. Shh and Ihh are epithelially expressed and do not overlap with Dhh, which is expressed in Schwann cells, peripheral nerves and endotheial cells10. The three ligands bind to the receptors, Patched-1 (Ptch-1) and Patched-2 (Ptch-2). In the absence of ligand, the unoccupied Ptch receptor inhibits another membrane protein, Smoothened (Smo), deactivating the pathway. Hh ligand binding to Ptch relieves this repression, activating the pathway as measured by transcriptional modulation of target genes. The Gli transcription factors (Gli1, Gli2 and Gli3) represent the downstream effectors of Hh signaling in vertebrates (reviewed in 12) All three of these factors are expressed in the gastrointestinal tract13.
Significant GI pathology results from reduction of Hh ligand levels. Shh-/- or Ihh-/- mice exhibit malrotation of the GI tract, decreased muscularis propria and enteric neuron abnormalities14, 15. Other aspects of the phenotypes of these two ligand knockouts are distinct and include: esophageal atresia with tracheal esophageal fistula, gastric overgrowth and imperforate anus in Shh deficient animals; and Hirschprung's-like dilation of the colon as well as epithelial stem cell defects in Ihh null mice16. Reducing the combined (Shh+Ihh) Hh signal from the epithelium either by expression of a soluble form of the Hedgehog inhibitor protein, Hhip 17 or by injection of an anti-Hedgehog antibody18, results in a distinct phenotype that includes branched villi and vacuolated epithelium as well as disrupted mesenchymal patterning.
Loss of any of the Gli factors also has pathological consequences. Gli2 null animals exhibit malformations of the esophagus and hindgut while Gli3 deficient mice present with anal stenosis and overgrowth of the distal stomach, without apparent small intestinal phenotype 19. Gli1-/- mice show no apparent gut abnormalities 20, but a full complement of Gli1 activity is important in coping with inflammatory stress: a Gli1 variant in the human population (E1100Q) is implicated in inflammatory bowel disease and the Gli1+/- mouse is highly sensitive to chemically-induced colitis21.
Likewise, in humans, perturbed Hedgehog signaling is implicated in malformations of the GI tract. Pallister-Hall syndrome, which includes limb defects, hypothalamic hamartomas and imperforate anus, is due to a frameshift in the Gli3 protein22. In large part, however, the association of human deformities with the Hedgehog pathway has been based on the similarity of these malformations to those described in mouse Hh pathway mutants: the human VACTERL association (which includes vertebral, cardiac, tracheo-esophageal and/or anorectal malformations) mirrors foregut and/or hindgut phenotypes seen in Shh, Gli2 or Gli3 null mice14, 23.
Despite the importance of the Hedgehog signaling program to gut development and disease, conflicting reports exist as to the direction of Hh signals. Some studies support a strictly paracrine Hh signal (from epithelium to mesenchyme)10, 14, 17, while others suggest that the epithelium can also participate in an autocrine signaling program, especially during later development and adulthood24-26. Indeed, autocrine Hh signals have been proposed to mediate Paneth cell differentiation26, control colonic epithelial cell differentiation25 and promote epithelial regeneration in a setting of chronic inflammation24.
In this study, we mapped regional expression of Hh signaling molecules (Shh, Ihh, Ptch1, Gli1, Gli2, Gli3) throughout development in the antrum, small intestine and large intestine. The data reveal that the Hh signaling pathway is strictly paracrine at all times. The dynamic expression domains of Hh pathway activators suggest that in addition to previously assigned roles, Hh signaling might be involved in formation of the pylorus, patterning of antral epithelium, emergence of intestinal cell identity, and development of the serosal layer. Analysis of transgenic mice that overexpress Ihh revealed that Hh levels control SM populations in the cores of the villi.
Material and methods
Mice
Gli1+/LacZ, Gli2 +/LacZ, Ptch1+/LacZ mice have been described20, 27-29. Shh+/LacZ mice were used in a previous study30; their derivation will be described elsewhere (Gonzalez and Kottman, in preparation). Heterozygous mice were mated with C57Bl/6 mice and the morning of vaginal plug was counted as E0.5. Genotyping was performed as previously described13, 17, 31, 32. Protocols for X-gal staining, immunostaining, in situ hybridization, Q-RT-PCR and generation of Ihh transgenic mice are detailed in Supplementary Methods (www.gastrojournal.org).
Results
Although Gli1, Gli2 and Ptch1 are all components of the Hh pathway, they reveal different aspects of the Hh signaling network. Gli1 is a direct target of Hh and its expression is dependent upon active Hh signaling27. Ptch1 is also a Hh target gene, but its transcription is not solely dependent upon Hh. Finally, Gli2 is an important mediator of activation27, but its expression is not transcriptionally regulated by Hh. Thus, Ptch1LacZ/+ and Gli2LacZ/+ mice indicate cells capable of responding to Hh signals while Gli1LacZ/+ mice reveal the cells that are actively responding. We used these reporters to map Hh pathway components in the developing and adult GI tract. In situ hybridization was used to confirm the reporter findings and examine additional Hh pathway molecules (Gli3, Ptch2).
E10.5: Ptch1, but not Gli1 is expressed in hindgut epithelium
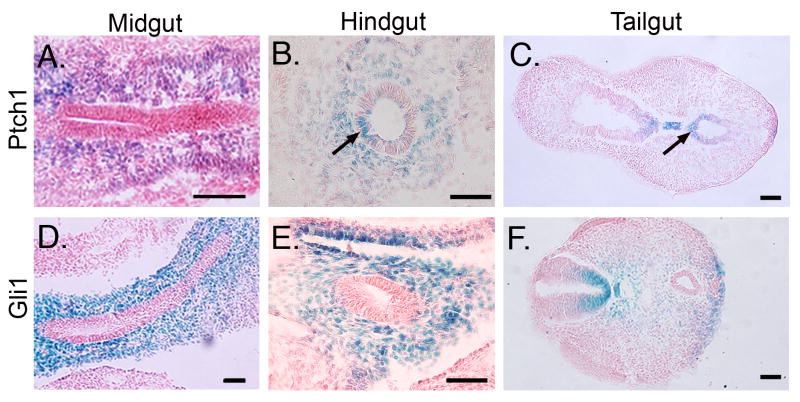
Both Ihh and Shh are expressed robustly in the multilayered E10.5 endoderm throughout midgut and hindgut as documented previously10. Ptch1LacZ/+ is expressed in mesenchymal cells of the presumptive antrum (data not shown) and midgut (Figure 1A). However, on the dorsal side of the hindgut epithelium and post-anal portion of the tail gut, Ptch1LacZ/+ staining is clearly seen in the epithelium (Figure 1B,C). In contrast, Gli1LacZ/+ staining is strictly mesenchymal in all of these tissues (Figure D-F). Since Gli1 expression requires Hh ligands while Ptch1 may be expressed independently33, 34, we conclude that Hh signaling is paracrine in these tissues at this time. Gli3 is also highly expressed in the early intestinal mesenchyme and is progressively down-regulated during fetal development (Supplementary Information 1).
Figure 1. Epithelial expression of Ptch1 in E10.5 hindgut.
X-gal staining of Ptch1LacZ/+ (A-C) and Gli1LacZ/+ (D-F) mice reveals mesenchymal expression of both reporters. Dorsal epithelium of the hindgut (B, arrow) and tailgut (C, arrow), but not the midgut (A) is Ptch1 positive. All epithelial tissues are Gli1 negative. Bars = 200 μm.
E14.5: Novel aspects of Hh signaling in stomach, pyloric sphincter, muscularis externa, enteric neurons and serosa
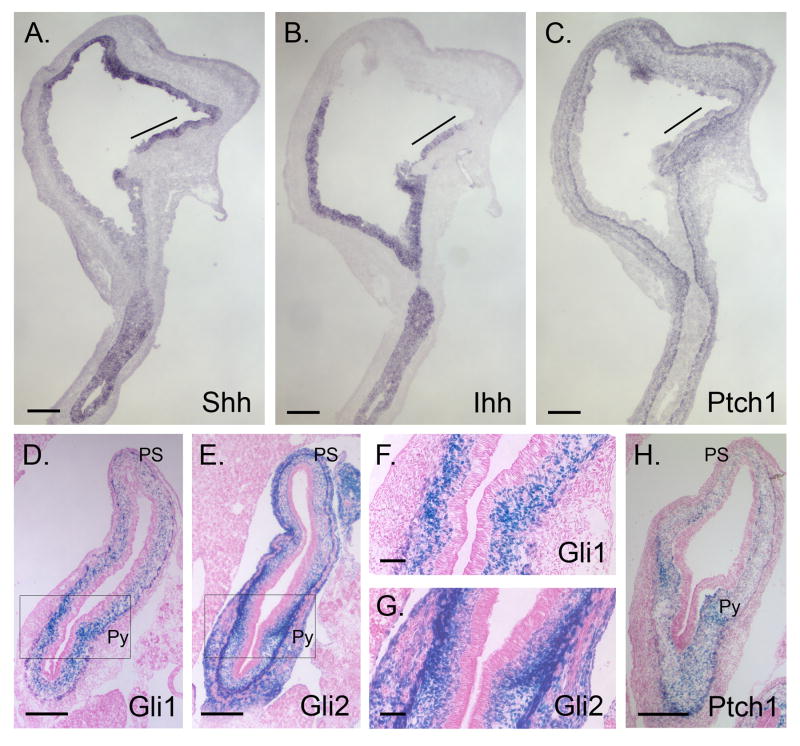
An opposing gradient of Shh and Ihh expression has been previously documented in E11 stomach10. We detected three different patterns of Hh expression in E14.5 stomach: in forestomach epithelium, Shh is expressed strongly while Ihh is undetectable; in distal stomach that will give rise to corpus and antrum, Ihh expression is strong, and overlaps with the weaker, but detectable expression of Shh (Figure 2A, B); finally, on the lesser curvature near the esophagus, a small region of epithelium expresses both Shh and Ihh at high levels (line, Figure 2A,B). Ptch1 expression is also high in this region (Figure 2C), as observed previously35. The lesser curvature of the stomach derives from the ventral gut tube36 and is clinically important since it is a common site for the development of gastric tumors37.
Figure 2. Expression of Hh pathway components in E14.5 stomach.
(A-C) In situ hybridization of serial sagittal sections from E14.5 stomachs using Shh (A), Ihh (B) and Ptch1 (C) probes. Note reciprocal expression gradients of Shh and Ihh in the epithelium. Shh and Ihh expression overlaps at the position marked with a line; Ptch1 expression is prominent in mesenchyme of this area. (D-H) X-gal staining of transverse stomach sections from E14.5 Gli1LacZ/+ (D, F), Gli2LacZ/+ (E, G) and Ptch1LacZ/+ (H) mice demonstrates paracrine Hh signaling, with intense Hh response near the forming pylorus. (F,G) Higher magnification of the boxed areas in D and E. PS, proximal stomach; Py, pyloric border. Bars: A-E = 200μm; F-H = 50μm.
Ptch1, Gli1 and Gli2 are all exclusively mesenchymal at E14.5 in stomach and proximal duodenum (Figure 2C-H). The inner layer of the muscularis externa (ME) is positive for Gli1 (Figure 2D, F), while both layers are positive for Gli2 (Figure 2E,G). The signals for Ptch1 (Figure 2C, H), Gli1 (Figure 2D) and Gli2 (Figure 2E) are prominent surrounding the pyloric border. The forming pyloric sphincter is seen as a spur of Gli1, Gli2 and Ptch1 positive nuclei that extends inward from the inner circular layer of the ME. Interestingly, enteric neurons in the stomach and small intestine are unstained in all three reporter models (Supplementary Information 2).
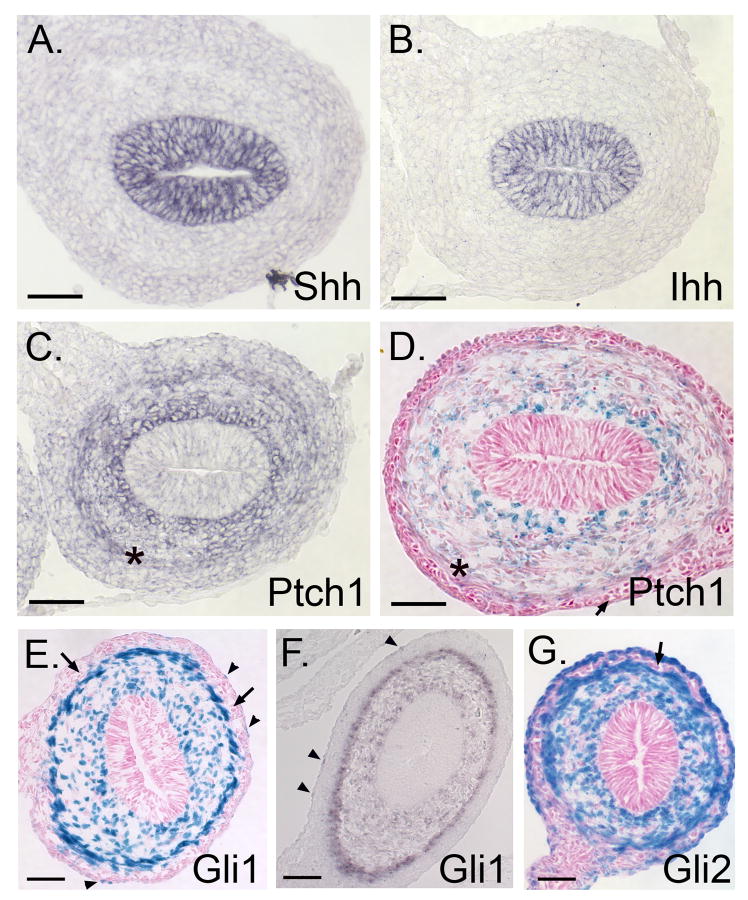
As previously reported10, both Shh and Ihh are highly expressed in the intestinal epithelium (Figure 3A,B). Ptch1, Gli1 and Gli2, are expressed only in the mesenchyme (Figure 3C-G). Both in situ hybridization using a Ptch1 specific probe (Figure 3C) and X-gal stained sections from the Ptch1LacZ/+ reporter mice (Figure 3D) reveal that Ptch1 expression is high near the epithelium, falls off sharply in mesenchymal cells that lie 8-10 cell diameters away from the epithelium, and then rises again at the inner side of the ME (Figure 3C,D, asterisks). Gli1 is expressed throughout the loose mesenchyme, with particularly robust expression at the epithelial surface and at the inner layer of the ME (Figure 3E,F). Gli1 expression is also apparent in some cells of the serosal layer (arrowheads).
Figure 3. Expression of Hh pathway components in E14.5 small intestine.
In situ hybridization reveals expression of Shh (A) and Ihh (B) mRNA in epithelium while Ptch1 (C) and Gli1 (F) are confined to the mesenchyme. All LacZ stained cells in Ptch1LacZ/+ (D), Gli1LacZ/+ (E) and Gli2LacZ/+ (G) reporter mice are also mesenchymal. In the ME, expression domains of Ptch1 (C,D - asterisk) and Gli1 are restricted to the inner muscular layer; Gli2 is expressed in both layers. Both Gli1 and Gli2 are seen in serosal cells (arrowheads). Enteric neurons are negative for LacZ (D-F, arrows). Expression patterns for Ptch1 (C,D) and Gli1 (E,F) are concordant in reporter mice and in situ hybridizations. Bars = 50μm.
The pattern of Gli2 expression is similar to that of Gli1, but more cells are stained. In addition, both inner and outer muscle layers are strongly positive for Gli2 (Figure 3G), while Gli1 is expressed only in the inner muscular layer (Figure 3E, F). Most, but not all cells of the serosa are also positive for Gli2. Enteric neurons are strikingly negative (Figure 3G, arrow). Gli3 expression is much weaker at E14.5 than at E12.5, but remains detectable in the ME (Supplemental Information 1E,F). In E14.5 colon, staining patterns for Gli1, Gli2 and Ptch1 are entirely similar to those of the small intestine; no epithelial expression is detectable (data not shown).
E16.5: Dynamic alterations in Hh signal transduction in stomach and intestine
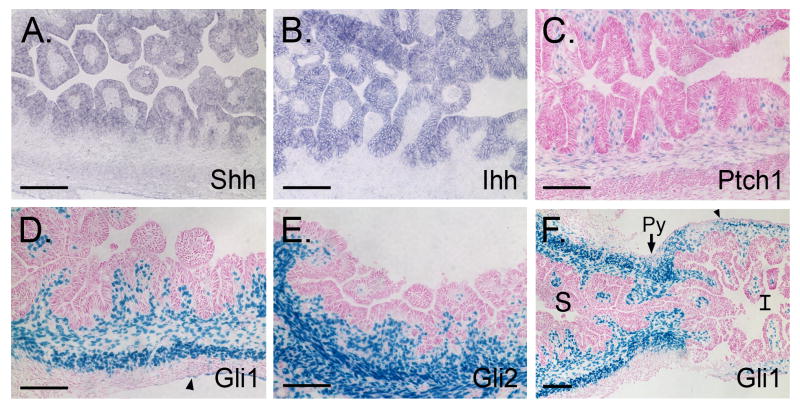
As antral gland morphogenesis begins, both Shh and Ihh mRNA are detected throughout the epithelium of the antral villus-like structures (Figure 4A,B). Ptch1, Gli1 and Gli2 are restricted to the underlying mesenchyme (Figure 4C-F). Ptch1 is expressed between the invaginating epithelial folds, in the submucosa and in the innermost cells of the inner circular layer of the ME (Figure 4C). Gli1 is expressed similarly, but is additionally found within entire circular muscle as well as some cells of the serosa (Figure 4D). Gli2 is detected throughout the lamina propria and in both layers of the ME (Figure 4E). In cross sections of the pyloric border region, Gli1 expression and, by implication, active Hh signaling, is now much more prominent in the antrum and pyloric border region itself than in the adjacent duodenal tissue (Figure 4F). This dramatic difference in Hh signaling between antrum and duodenum was not present at E14.5.
Figure 4. Hh pathway activity across the E16.5 pyloric border.
(A, B) In situ hybridization of antrum using Shh and Ihh specific probes. (C-F) X-gal stained antral sections from E16.5 Ptch1LacZ/+, Gli1LacZ/+, and Gli2LacZ/+ antrum. (F) Cross section of the forming pyloric border in a Gli1 LacZ/+ mouse reveals a drastic difference in active Hh signaling between the stomach (S, left) and intestine (I, right); Py = pylorus. Arrowheads = Gli1 positive serosal cells. Bars = 100μm.
Intestinal villus morphogenesis is initiated at approximately E15.5 and proceeds in an anterior to posterior wave. As villi grow, proliferating epithelial cells become concentrated at the intervillus base (Figure 5A). Shh expression resolves to encompass only the region occupied by the proliferative cells at the base of forming villi (Figure 5B); this differs from the pattern seen in antral epithelium (Figure 4A). In contrast, Ihh is expressed throughout the epithelium in newly formed villi; as villi lengthen, Ihh message is reduced slightly at the tips (Figure 5C).
Figure 5. Hh signal transduction during villus emergence in E16.5 small intestine.
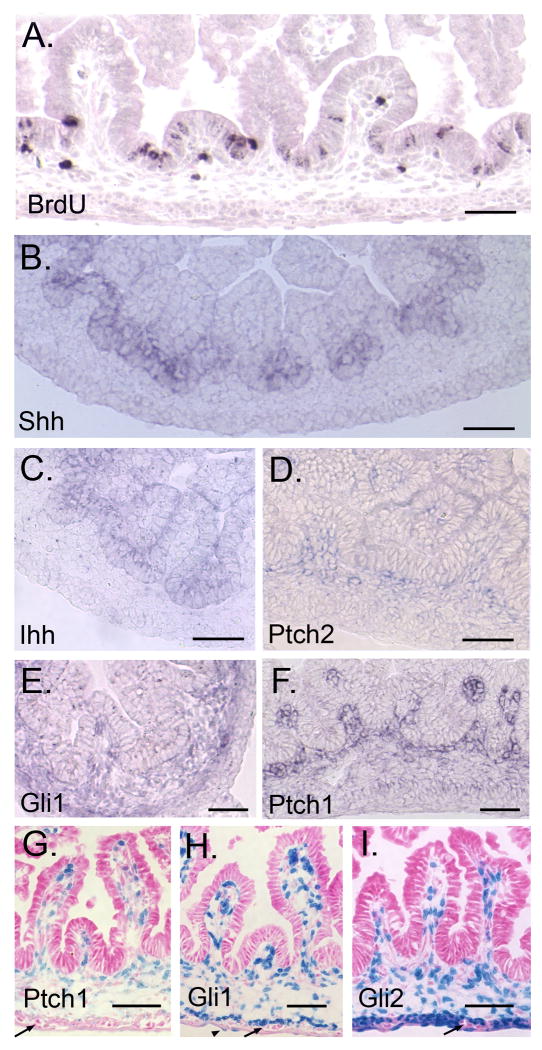
(A) BrdU staining. Epithelial proliferation is restricted to the inter-villus area. (B-F) In situ hybridization. Shh (B), but not Ihh (C) expression correlates with the restriction in epithelial proliferation. Ptch2 (D), Gli1 (E) and Ptch1 (F) are mesenchymally expressed. (G-I) X-gal staining of E16.5 PtchLacZ/+, GliLacZ/+ and Gli2LacZ/+ small intestine. All reporters are expressed in villus cores. Gli1 and Gli2 expression is also seen in ME and some serosal cells (H arrowhead). Enteric neurons are negative (arrows). Bars = 100μm (A-F) or 50μm (G-I).
Ptch2, Gli1, Ptch1 and Gli2 are expressed in the cores of growing villi (Figure 5D-I). Ptch2 (Figure 5D) and Gli3 (Supplementary Information 1H) are weakly expressed in mesenchyme. Gli1 is expressed in the circular SM of the ME (Figure 5E,H) while Gli2 is expressed in both externa layers (Figure 5I). Both Gli1 and Gli2 are expressed in some serosal cells. Enteric neurons of Auerbach's plexus are negative for Hh signal transduction components (Figure 5G-I, Supplementary Information 2). Colonic expression of all reporters at E16.5 is also mesenchymal and is similar to the pattern seen in small intestine (Supplementary Information 3).
P0-P10: Muscularis mucosa (MM) and intervillus muscle contain Hh responsive cells
At birth (P0), Ptch1, Gli1 and Gli2 are robustly expressed in mesenchymal cells, with prominent activity in antrum and pylorus. At postnatal day 10, a distinct muscularis mucosa, containing many PtchLacZ/+ and Gli1LacZ/+ stained cells also becomes clearly visible (Supplementary Information 4). By P0, Gli3 activity is undetectable in antral stomach (Supplementary Information 1I,J).
In newborn intestine, a layer of Gli1 positive cells lies just below the intervillus epithelium prior to crypt formation (Figure 6A, arrows). Ten days later, the intervillus epithelium has started to reshape to form crypt like structures. A nearly continuous layer of X-gal positive cells with elongated nuclei is seen immediately beneath the emerging crypts; this is the developing MM (Figure 6B, arrowhead). Some Hh responsive cells within the villus cores are connected with this layer; these cells express desmin and αSMA and represent the developing SM cores within the villi (Figure 6C-E). Budding crypts remain in close contact with a cuff of Hh responsive cells (Figure 6F-H). Enteric neurons are devoid of Gli1 and Gli2, but for the first time, a subset of these cells is positive for Ptch1 (Supplementary Information 2). Gli3 is undetectable after P0 (Supplementary Information 1K,L).
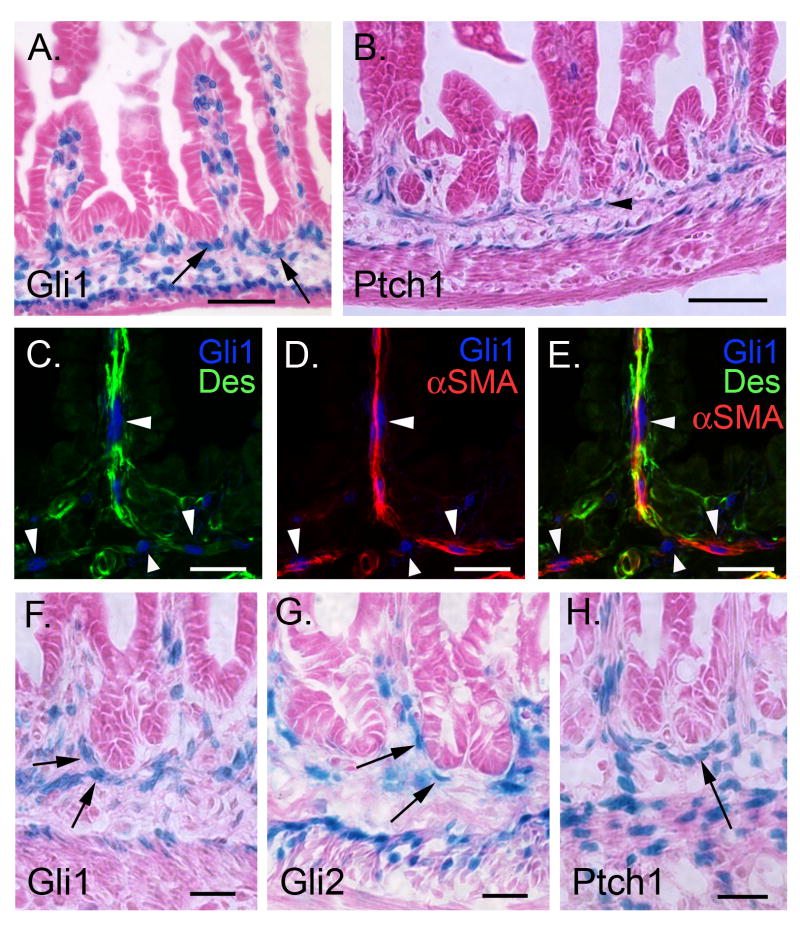
Figure 6. P0 and P10: Hh responsive cells in developing muscularis mucosa.
(A) At birth, p0, Gli1 expressing cells are found in villus cores and in close contact with the presumptive crypt epithelium (arrows). (B) At p10, the developing MM contains Ptch1 positive cells (arrowhead). (C-E) Gli1 positive cells of the MM and villus cores (blue nuclei, arrowheads) are positive for αSMA (red) and desmin (green), identifying them as SM cells. (F-H) Gli1, Gli2 and Ptch1 expressing cells (arrows) are in close contact with the tips of the forming crypts at P10. Bars: 50μm for A and B; 20μm for C-H.
Adult mice: paracrine signaling in antrum, small intestine and colon
As in the fetus, analysis of adult mice revealed no evidence for autocrine Hh signaling in these portions of the gut tube (Supplementary Information 5-8). Though antral stomach was previously thought to be devoid of Hh signaling38, we found consistent evidence of Shh expression and Hh signal transduction in this tissue (Supplementary Information 5 and 6). In adult intestine (Figure 7) and colon (Supplementary Figure 7), Hh responsive cells included SM precursors, differentiated SM cells of the villus cores and MM, myofibroblasts and pericytes. Gli3 was not detectable in adult tissues, though high background in colon could have obscured weak expression (data not shown).
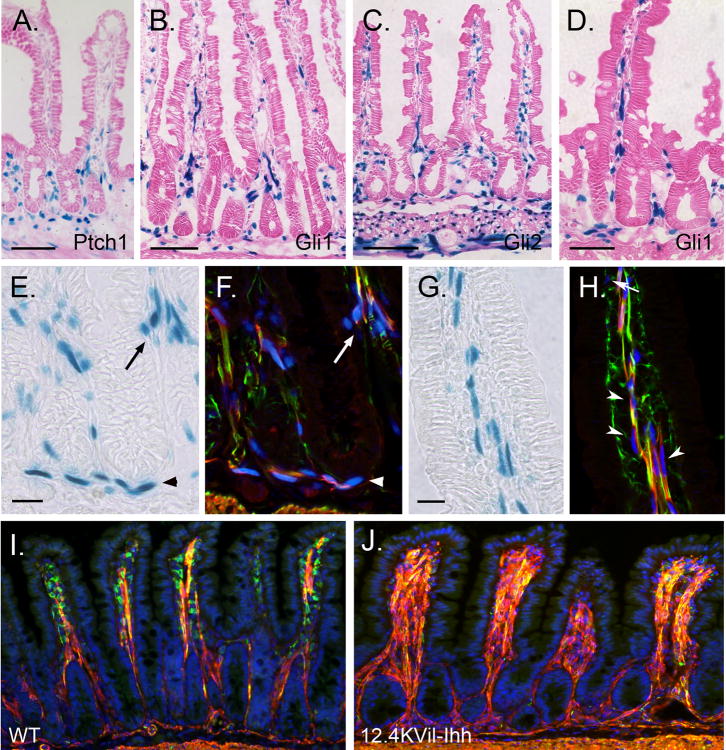
Figure 7. Hedgehog controls smooth muscle development in postnatal mice.
X-gal staining of jejunum from adult Ptch1 LacZ/+ (A), Gli1 LacZ/+ (B,D-J), and Gli2 LacZ/+ (C) mice. (A-D) Hh responsive cells are located within villus cores, at the crypt villus junction, in the submucosa and in the muscularis externa. (E-H) Sections were stained with X-gal (E,G) and then co-stained with antibodies against α-SMA (red) and desmin (green). Immunostained images taken on the confocal were overlaid (F,H) with images of the same field taken under bright field illumination (X-gal pattern, E,G). Hh responsive cells included desmin and α-SMA double positive smooth muscle cells (yellow) in the villus cores (arrowheads in H), at the crypt villus junction (arrows in E,F) and in the MM (arrowheads in E,F). Gli1 expressing cells are also detected among the desmin positive, α-SMA negative muscle precursors (green) located inside the villi close to the epithelium (arrow in H) and among the α-SMA positive, desmin negative (red) subepithelial myofibroblasts that line the crypts (not shown). (I,J) One month old wild type (WT) mice (I) and their littermates transgenic for 12.4KVil-Ihh (J). Transgenic animals show dramatic increase in villus smooth muscle and reduced smooth muscle precursors. Scale bars: (A-C, I,J) = 100μm; (D) = 50μm; (E-H) = 20μm.
Overexpression of Hh results in amplification of villus core smooth muscle
The studies above indicated that SM precursors and differentiated SMCs in the MM and villus cores transduce Hh signals, suggesting that hedgehog might be important in the development or maintenance of these populations. Indeed, our earlier studies of a mouse model of Hh inhibition indicated that reduced Hh signaling results in loss of differentiated SMC from villus cores17. To examine whether increased levels of Hh ligand would promote SM differentiation, we used the mouse villin promoter31 to generate transgenic mice that overexpress Ihh in small intestinal epithelium. Figure 7J shows that 12.4KVil-Ihh mice exhibit expanded smooth muscle in villus cores. Together, these findings indicate that Hh signals control the size of the smooth muscle population in the villus cores.
Discussion
This analysis of Hh signal response in the developing and mature GI tract provides a cellular basis for Hh function in this tissue and suggests new avenues for exploration. Novel findings include: a) shortly after gut tube formation, epithelial cells of the hindgut and tailgut express Ptch1, but not Gli1; b) serosal cells respond to Hh signals during fetal life; c) the developing MM contains Hh responsive cells at P10 and continues to receive Hh signals during adult life; d) Hh levels control the amount of smooth muscle in villus cores; e) enteric neurons are not responsive to Hh signals in fetal life; they express Ptch1, but not Gli1 in neonatal and adult life; f) Hh responding cells are concentrated at the pyloric border during formation of that sphincter and are prominent in the antrum, and much less so in the intestine after E16.5; g) epithelial cells of the antrum, small intestine and colon do not express Gli1 and therefore do not respond to Hh signals at any point during embryonic, fetal or adult life. This last finding is in conflict with previous reports that propose autocrine epithelial hedgehog signaling25, 26 (discussed in Supplementary Information 6-8). Here, we further enlarge upon the potential implications of these data in the context of gastrointestinal development and disease.
It is interesting that transient expression of Ptch1, but not Gli1 is detectable in E10.5 hindgut and tailgut epithelium as well as in postnatal enteric neurons. Ptch1 expression in the absence of Gli1 has also been seen in neural tissue33, 34. Although Ptch1 is normally a Hh target gene, it can also act as a dependence receptor, to promote apoptosis in the absence of a Hh signal. It has been proposed that such apoptotic activity may help to shape the neural tube during development34. Perhaps transient expression of Ptch1 in these hindgut cells that clearly are not transducing Hh signals (since they are Gli1 negative) plays a role in preventing the apoptosis of these cells.
Hh signal transduction has not previously been demonstrated in the MM, but our data indicate that the cells of the forming MM are Hh responsive. Morphological studies indicate that the MM arises from the inner circular layer of ME39. Indeed, we detected apparent connections between the MM and cells of the inner circular layer of ME (Figure 6) and the SM cells of the villus core. It will be important to lineage trace this developing SM network to confirm these apparent origins. Our transgenic studies further reveal that villus core SM is highly sensitive to the level of Hh ligand. Though MM did not appear amplified, this might be due to the fact that the strength of the villin promoter is greater in villus tips than in crypts31. Alternatively, MM cells might be under separate control, even though these cells are also responsive to Hh signals as measured by their expression of Gli1.
Interestingly, from an early time point, the signaling properties of the inner circular and outer longitudinal muscles of the ME are different: only the former is Gli1 positive, indicative of active Hh signaling. Such different properties could potentially play a role in the differential response of these two muscles in disease. For example, familial type IV visceral myopathy presents with hypertophy of the inner circular and atrophy of the outer longitudinal muscle of the small bowel and is a rare cause of chronic intestinal pseudoobstruction40, 41. Given the ability of Hh signaling to modulate villus core smooth muscle as shown above and in our earlier studies17, it will be important to examine whether increased Hedgehog signaling plays any role in the etiology of this rare but usually fatal pathological condition.
The finding that the serosal mesothelium responds to Hh signals throughout development suggests that a source of Hh ligand may lie in the peritoneal cavity and/or that these cells receive Hh signals as they migrate onto the surface of the gut tube at E11.542. Recent studies indicate that mesothelial cells undergo epithelial to mesenchymal transition and migrate into the gut tube, differentiating into endothelial cells, vascular SM cells and pericytes42. Whether the vasculogenic activity of gut serosal mesothelium requires Hh signaling has not been directly tested, but it is noteworthy that Smo null embryos, which lack Hh response, have major vascular defects43 and administration of Shh blocking antibodies or a chemical inhibitor of Hh signaling produces vascular malformations and impaired vascular remodeling44, 45. Confirmation of the connection between Hedgehog signaling and the serosal mesothelium of the gut that is suggested here could potentially have therapeutic implications in the context of a rare developmental anomaly called “apple peel bowel”, a syndrome of intestinal wasting that is associated with loss of the serosa and its associated blood vessels46, 47.
Our data also suggest a possible indirect role for Hh in the establishment of intestinal vs. stomach epithelial identity. Exactly at E16.5, the precise time when a clear-cut epithelial boundary is being generated between stomach and intestine32, a dramatic difference in Hh signaling is generated in the mesenchyme: Hh signal transduction is robust in the antrum and much less prominent in small intestine. This difference is not visible at E14.5. This finding fits well with previous functional studies in Xenopus, where Hh signaling is also downregulated during differentiation of the intestine48. Indeed, constitutive activation of Hh signaling in the midgut results in arrested cytodifferentiation and poor growth of that tissue49. Thus, there may be an evolutionarily conserved requirement for downregulation of Hh signaling to permit intestinal cell differentiation.
We show here that in contrast to earlier conclusions50, Hh signal transduction is quite active in the antrum. Though its function in this adult tissue is not clear, a role for Hh in patterning the perinatal antral epithelium was previously suggested by the antral overgrowth phenotype seen in Gli3 and Shh null mice14, 19. Since signal transduction in the antrum is exclusively paracrine, this effect must represent an altered relay from epithelium to mesenchyme and back to epithelium. In this context, it is of interest to consider a common developmental abnormality of the pyloric stomach known as hypertrophic pyloric stenosis (HPS). In a study of over 100 infants with HPS, Hernanz-Schulman et al. found that robust overgrowth of the antral mucosa was responsible for obstruction of the pyloric opening51. Interestingly, a survey of the literature shows that HPS is often associated with other anomalies51, 52, including: abdominal malrotation or volvulus53, 54, cardiac anomalies55, imperforate anus, tracheal esophageal fistula56-58 and hydronephrosis of the kidney52, 59. Each of these malformations is seen in the VACTERL association, a constellation of abnormalities involving vertebral, anal, tracheal, esophageal, renal and limb development that are thought to be linked to defects in Hh signaling23. Since both Shh null mice and humans with HPS exhibit antral epithelial overgrowth, HPS might represent yet another associated abnormality within the VACTERL spectrum.
Supplementary Material
Acknowledgments
Grant support: NIH P01 DK62041, NIH R01 DK065850, T32-HD007505, T32-HL07622
The authors acknowledge support from NIH R01 DK065850 (DG), NIH P01 DK62041 (DG, JM, AD); the Organogenesis Training Program, T32-HD007505 (WZ and AG and ÅK); and the Hematology Training Program, T32-HL07622 (KW). Excellent technical support was provided by the Organogenesis Morphology Core and the Microscopy and Image Analysis Laboratory.
Abbreviations
- VACTERL
vertebral, anal, cardiac, tracheal, esophageal, renal, limb
- Hh
Hedgehog
- Shh
Sonic Hedgehog
- Ihh
Indian Hedgehog
- Dhh
Desert Hedgehog
- Ptch-1
Patched-1
- Ptch-2
Patched-2
- Smo
Smoothened
- Hhip
Hedgehog inhibitor protein
- ME
muscularis externa
- MM
muscularis mucosa
- α-SMA
alpha smooth muscle actin
- HPS
hypertrophic pyloric stenosis
- SM
Smooth Muscle
Footnotes
Financial Disclosures: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–20. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Lees C, Howie S, Sartor RB, et al. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129:1696–710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–71. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- 4.Shimeld SM. The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev Genes Evol. 1999;209:40–7. doi: 10.1007/s004270050225. [DOI] [PubMed] [Google Scholar]
- 5.Kang D, Huang F, Li D, et al. A hedgehog homolog regulates gut formation in leech (Helobdella) Development. 2003;130:1645–57. doi: 10.1242/dev.00395. [DOI] [PubMed] [Google Scholar]
- 6.Walton KD, Croce JC, Glenn TD, et al. Genomics and expression profiles of the Hedgehog and Notch signaling pathways in sea urchin development. Dev Biol. 2006;300:153–64. doi: 10.1016/j.ydbio.2006.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strahle U, Blader P, Ingham PW. Expression of axial and sonic hedgehog in wildtype and midline defective zebrafish embryos. Int J Dev Biol. 1996;40:929–40. [PubMed] [Google Scholar]
- 8.Ekker SC, McGrew LL, Lai CJ, et al. Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis. Development. 1995;121:2337–47. doi: 10.1242/dev.121.8.2337. [DOI] [PubMed] [Google Scholar]
- 9.Sukegawa A, Narita T, Kameda T, et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–80. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 10.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–38. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 11.Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 12.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Madison BB, Zacharias W, et al. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 15.Litingtung Y, Lei L, Westphal H, et al. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 16.Chiang C, Litingtung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 17.Madison BB, Braunstein K, Kuizon E, et al. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 18.Wang LC, Nassir F, Liu ZY, et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–82. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Huang Z, Mo R. Gli3 null mice display glandular overgrowth of the developing stomach. Dev Dyn. 2005;234:984–91. doi: 10.1002/dvdy.20542. [DOI] [PubMed] [Google Scholar]
- 20.Park HL, Bai C, Platt KA, et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 21.Lees CW, Zacharias WJ, Tremelling M, et al. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008;5:e239. doi: 10.1371/journal.pmed.0050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston JJ, Olivos-Glander I, Killoran C, et al. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–22. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim PC, Mo R, Hui Cc C. Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36:381–4. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen CM, Williams J, van den Brink GR, et al. Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest. 2004;84:1631–42. doi: 10.1038/labinvest.3700197. [DOI] [PubMed] [Google Scholar]
- 25.van den Brink GR, Bleuming SA, Hardwick JC, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–82. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 26.Varnat F, Heggeler BB, Grisel P, et al. PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–53. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bai CB, Auerbach W, Lee JS, et al. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 28.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–72. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 29.Goodrich LV, Milenkovic L, Higgins KM, et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 30.Jeong J, Mao J, Tenzen T, et al. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–51. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 32.Braunstein EM, Qiao XT, Madison B, et al. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 33.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6:229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Thibert C, Teillet MA, Lapointe F, et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 35.Spencer-Dene B, Sala FG, Bellusci S, et al. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology. 2006;130:1233–44. doi: 10.1053/j.gastro.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist's perspective. Am J Gastroenterol. 2005;100:1853–67. doi: 10.1111/j.1572-0241.2005.50096.x. [DOI] [PubMed] [Google Scholar]
- 38.van den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 39.Masumoto K, Nada O, Suita S, et al. The formation of the chick ileal muscle layers as revealed by alpha-smooth muscle actin immunohistochemistry. Anat Embryol (Berl) 2000;201:121–9. doi: 10.1007/pl00008232. [DOI] [PubMed] [Google Scholar]
- 40.Kansu A, Ensari A, Kalayci AG, et al. A very rare cause of intestinal pseudoobstruction: familial visceral myopathy type IV. Acta Paediatr. 2000;89:733–6. doi: 10.1080/080352500750044115. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs E, Ardichvili D, Perissino A, et al. A case of familial visceral myopathy with atrophy and fibrosis of the longitudinal muscle layer of the entire small bowel. Gastroenterology. 1979;77:745–50. [PubMed] [Google Scholar]
- 42.Wilm B, Ipenberg A, Hastie ND, et al. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–28. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 43.Byrd N, Becker S, Maye P, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–72. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 44.Nagase T, Nagase M, Yoshimura K, et al. Defects in aortic fusion and craniofacial vasculature in the holoprosencephalic mouse embryo under inhibition of sonic hedgehog signaling. J Craniofac Surg. 2006;17:736–44. doi: 10.1097/00001665-200607000-00026. [DOI] [PubMed] [Google Scholar]
- 45.Kolesova H, Roelink H, Grim M. Sonic hedgehog is required for the assembly and remodeling of branchial arch blood vessels. Dev Dyn. 2008;237:1923–34. doi: 10.1002/dvdy.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickson JA. Apple peel small bowel: an uncommon variant of duodenal and jejunal atresia. J Pediatr Surg. 1970;5:595–600. doi: 10.1016/s0022-3468(70)80002-1. [DOI] [PubMed] [Google Scholar]
- 47.Pumberger W, Birnbacher R, Pomberger G, et al. Duodeno-jejunal atresia with volvulus, absent dorsal mesentery, and absent superior mesenteric artery: a hereditary compound structure in duodenal atresia? Am J Med Genet. 2002;109:52–5. doi: 10.1002/ajmg.10309. [DOI] [PubMed] [Google Scholar]
- 48.Bailey TJ, El-Hodiri H, Zhang L, et al. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol. 2004;48:761–70. doi: 10.1387/ijdb.041878tb. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Rosenthal A, de Sauvage FJ, et al. Downregulation of Hedgehog signaling is required for organogenesis of the small intestine in Xenopus. Dev Biol. 2001;229:188–202. doi: 10.1006/dbio.2000.9953. [DOI] [PubMed] [Google Scholar]
- 50.van den Brink GR, Hardwick JC, Nielsen C, et al. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–33. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernanz-Schulman M, Lowe LH, Johnson J, et al. In vivo visualization of pyloric mucosal hypertrophy in infants with hypertrophic pyloric stenosis: is there an etiologic role? AJR Am J Roentgenol. 2001;177:843–8. doi: 10.2214/ajr.177.4.1770843. [DOI] [PubMed] [Google Scholar]
- 52.Bidair M, Kalota SJ, Kaplan GW. Infantile hypertrophic pyloric stenosis and hydronephrosis: is there an association? J Urol. 1993;150:153–5. doi: 10.1016/s0022-5347(17)35420-4. [DOI] [PubMed] [Google Scholar]
- 53.Anagnostara A, Koumanidou C, Vakaki M, et al. Chronic gastric volvulus and hypertrophic pyloric stenosis in an infant. J Clin Ultrasound. 2003;31:383–6. doi: 10.1002/jcu.10182. [DOI] [PubMed] [Google Scholar]
- 54.Oguzkurt P, Senocak ME, Hicsonmez A. A rare coexistence of two gastric outlet obstructive lesions: infantile hypertrophic pyloric stenosis and organoaxial gastric volvulus. Turk J Pediatr. 2000;42:87–9. [PubMed] [Google Scholar]
- 55.Mehta AV, Ambalavanan SK. Infantile hypertrophic pyloric stenosis and congenital heart disease: an under-recognized association. Tenn Med. 1997;90:496–7. [PubMed] [Google Scholar]
- 56.Kilic N, Gurpinar A, Kiristioglu I, et al. Association of oesophageal atresia and hypertrophic pyloric stenosis. Acta Paediatr. 2000;89:118–9. [PubMed] [Google Scholar]
- 57.Magilner AD. Esophageal atresia and hypertrophic pyloric stenosis: sequential coexistence of disease (case report) AJR Am J Roentgenol. 1986;147:329–30. doi: 10.2214/ajr.147.2.329. [DOI] [PubMed] [Google Scholar]
- 58.Oguzkurt P, Tanyel FC, Haliloglu M, et al. An uncommon association of H-type tracheoesophageal fistula with infantile hypertrophic pyloric stenosis. Turk J Pediatr. 1999;41:143–6. [PubMed] [Google Scholar]
- 59.Atwell JD, Levick P. Congenital hypertrophic pyloric stenosis and associated anomalies in the genitourinary tract. J Pediatr Surg. 1981;16:1029–35. doi: 10.1016/s0022-3468(81)80870-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.